Am Fam Physician. 2024;109(1):10-12
Author disclosure: No relevant financial relationships.
Screening for and treatment of sexually transmitted infections (STIs) is a key aspect of prenatal care. Family physicians have a responsibility to initiate open and informative discussions about sexual health with pregnant patients. Obtaining a sexual history is detailed in a previous issue of American Family Physician (AFP).1
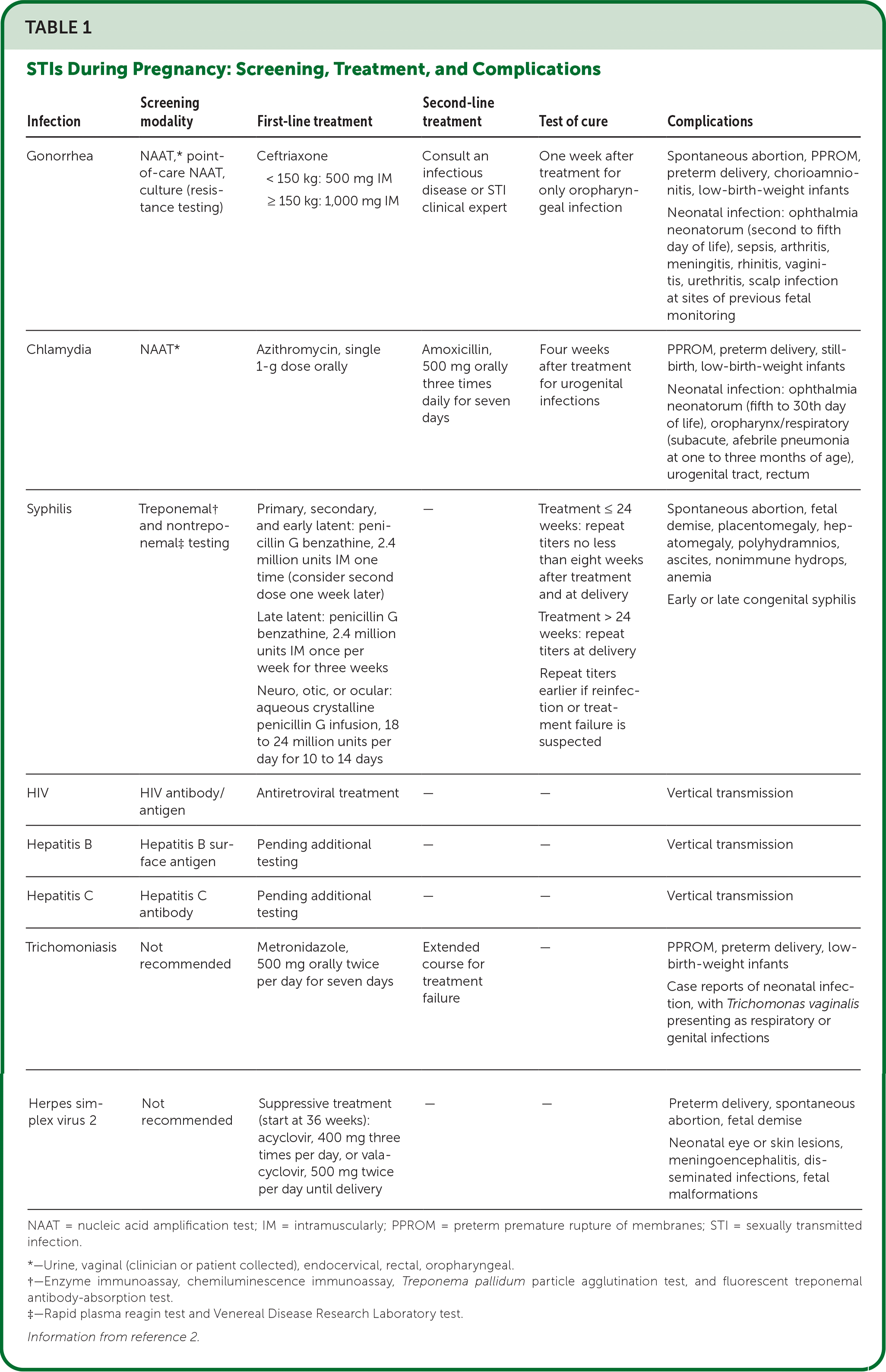
The Centers for Disease Control and Prevention (CDC) and U.S. Preventive Services Task Force (USPSTF) recommend screening for HIV, syphilis, hepatitis B, and usually hepatitis C at the first prenatal visit.2–5 Current guidelines were developed based on data from cis women, but no available data suggest that recommendations should be different for any pregnant person. Key points of diagnosis, treatment, test of cure, and pregnancy complications for STIs are summarized in Table 1.2
| Infection | Screening modality | First-line treatment | Second-line treatment | Test of cure | Complications |
|---|---|---|---|---|---|
| Gonorrhea | NAAT,* point-of-care NAAT, culture (resistance testing) | Ceftriaxone < 150 kg: 500 mg IM ≥ 150 kg: 1,000 mg IM | Consult an infectious disease or STI clinical expert | One week after treatment for only oropharyngeal infection | Spontaneous abortion, PPROM, preterm delivery, chorioamnionitis, low-birth-weight infants Neonatal infection: ophthalmia neonatorum (second to fifth day of life), sepsis, arthritis, meningitis, rhinitis, vaginitis, urethritis, scalp infection at sites of previous fetal monitoring |
| Chlamydia | NAAT* | Azithromycin, single 1-g dose orally | Amoxicillin, 500 mg orally three times daily for seven days | Four weeks after treatment for urogenital infections | PPROM, preterm delivery, still-birth, low-birth-weight infants Neonatal infection: ophthalmia neonatorum (fifth to 30th day of life), oropharynx/respiratory (subacute, afebrile pneumonia at one to three months of age), urogenital tract, rectum |
| Syphilis | Treponemal† and nontreponemal‡ testing | Primary, secondary, and early latent: penicillin G benzathine, 2.4 million units IM one time (consider second dose one week later) Late latent: penicillin G benzathine, 2.4 million units IM once per week for three weeks Neuro, otic, or ocular: aqueous crystalline penicillin G infusion, 18 to 24 million units per day for 10 to 14 days | — | Treatment ≤ 24 weeks: repeat titers no less than eight weeks after treatment and at delivery Treatment > 24 weeks: repeat titers at delivery Repeat titers earlier if reinfection or treatment failure is suspected | Spontaneous abortion, fetal demise, placentomegaly, hepatomegaly, polyhydramnios, ascites, nonimmune hydrops, anemia Early or late congenital syphilis |
| HIV | HIV antibody/antigen | Antiretroviral treatment | — | — | Vertical transmission |
| Hepatitis B | Hepatitis B surface antigen | Pending additional testing | — | — | Vertical transmission |
| Hepatitis C | Hepatitis C antibody | Pending additional testing | — | — | Vertical transmission |
| Trichomoniasis | Not recommended | Metronidazole, 500 mg orally twice per day for seven days | Extended course for treatment failure | — | PPROM, preterm delivery, low-birth-weight infants Case reports of neonatal infection, with Trichomonas vaginalis presenting as respiratory or genital infections |
| Herpes simplex virus 2 | Not recommended | Suppressive treatment (start at 36 weeks): acyclovir, 400 mg three times per day, or valacyclovir, 500 mg twice per day until delivery | — | — | Preterm delivery, spontaneous abortion, fetal demise Neonatal eye or skin lesions, meningoencephalitis, disseminated infections, fetal malformations |
Chlamydia and Gonorrhea
Chlamydia trachomatis infection is the most common reportable STI in the United States, followed by Neisseria gonorrhoeae infection.2 These two infections are usually asymptomatic.6–8 The CDC recommends testing for chlamydia and gonorrhea at the first prenatal visit in most women younger than 25 years and in women 25 years and older who are at high risk of infection.2 Testing should be repeated in the third trimester for individuals at high risk.
A nucleic acid amplification test (NAAT) for chlamydia and gonorrhea can be performed using vaginal or endocervical swab samples or first-catch urine.2 Self collection of vaginal swabs is well validated.9 An NAAT using liquid-based cytology specimens from Papanicolaou smears have lower sensitivity than using endocervical or vaginal swabs.10 In patients with cervicitis, urine samples may miss 10% of infections compared with vaginal or endocervical samples.11 Oropharyngeal and rectal testing for chlamydia and gonorrhea should be considered based on the patient's sexual practices. Of note, urogenital testing is not a substitute for anorectal testing because there is poor concordance in test results between sites.12,13 Expedited partner treatment regulations vary by state but should be used, when possible, due to high reinfection rates. For gonorrhea and chlamydia, all sex partners in the past 60 days should be treated.2
Syphilis
Syphilis rates have been increasing since 2000, especially recently, with methamphetamine use contributing significantly to incident cases among women.14 From 2015 to 2019, reported cases of primary and secondary syphilis in women increased 179%, and congenital syphilis cases increased 291%.14,15 The dramatic rise in congenital syphilis can further be attributed to insufficient access to prenatal care and poor adherence to national screening guidelines, as discussed in a 2020 AFP editorial.16,17 All pregnant patients should be screened in the first trimester, and patients at high risk or living in high-prevalence communities should receive repeat testing at 28 weeks and delivery.18
Diagnosing syphilis requires a low threshold of suspicion and a thorough history and physical examination. Although the classic syphilitic chancre is a single, sharply demarcated, painless ulcer, only 30% of patients have this presentation.19 Chancres may be hidden (e.g., in the cervix or rectum) or absent.19 Opt-out screening is essential because a large National Institutes of Health study found that 49% of pregnant women with syphilis from 2012 to 2016 had no identifiable risk factors.15
Adverse pregnancy outcomes occur in 50% to 80% of pregnancies affected by syphilis.20 Treatment decreases fetal risk. Penicillin is the only appropriate treatment for syphilis in pregnancy; desensitization is required for patients allergic to penicillin.2 Most pregnant women will not have time to achieve the fourfold decrease in rapid plasma reagin titer that indicates successful treatment; the absence of this decrease does not suggest treatment failure. However, a four-fold increase sustained for more than two weeks raises concerns for reinfection or treatment failure.
When syphilis is diagnosed during pregnancy, congenital syphilis is presumed.17 Treatment should be provided immediately in conjunction with maternal-fetal medicine. Appropriate treatment does not guarantee that the fetus will be unaffected. Infants born to mothers with syphilis during pregnancy should receive a serum quantitative nontreponemal test and an HIV viral load measurement and be referred to a pediatric infectious disease specialist for long-term monitoring, because most neonates are not born with overt signs of congenital syphilis. For syphilis, expedited partner treatment recommendations are expanded to include any sex or needle-sharing partners in the past 90 days.2
HIV
The CDC and USPSTF guidelines recommend discussing preexposure prophylaxis (PrEP) with patients at risk of HIV, especially those with a recent bacterial STI.21,22 Preconception, during pregnancy, and the first six months postpartum are particularly high-risk times for HIV acquisition. PrEP with combination emtricitabine/tenofovir disoproxil fumarate (Truvada) is safe during pregnancy and breastfeeding.