
Am Fam Physician. 2025;111(6):549-550
Author disclosure: No relevant financial relationships.
Lecanemab (Leqembi) is labeled for the treatment of Alzheimer disease in adults with mild cognitive impairment or who are in the mild dementia stage of Alzheimer disease (eg, have a Mini-Mental State Examination [MMSE] score of 20–28 points) with confirmed amyloid beta pathology. Lecanemab is a humanized monoclonal antibody that targets and reduces accumulations of amyloid beta plaques, which can be found in those diagnosed with Alzheimer disease.
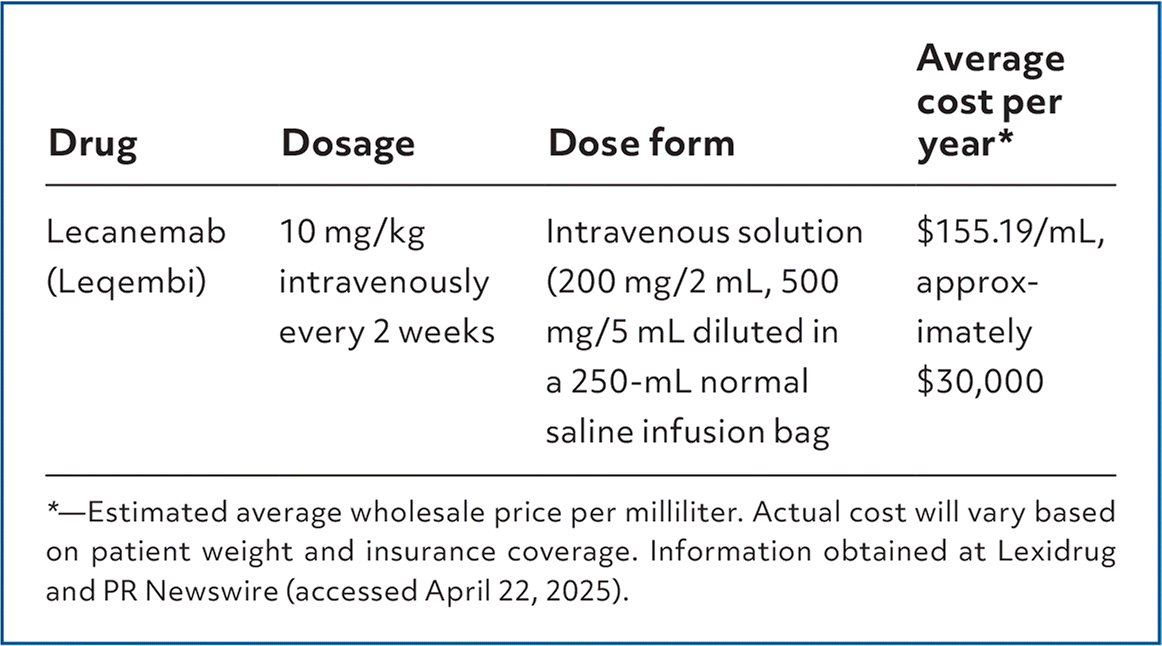
| Drug | Dosage | Dose form | Average cost per year* |
|---|---|---|---|
| Lecanemab (Leqembi) | 10 mg/kg intravenously every 2 weeks | Intravenous solution (200 mg/2 mL, 500 mg/5 mL diluted in a 250-mL normal saline infusion bag | $155.19/mL, approximately $30,000 |
Subscribe
From $180- Immediate, unlimited access to all AFP content
- More than 125 CME credits/year
- Print delivery available
Issue Access
$59.95- Immediate, unlimited access to this issue's content
- CME credits
- Print delivery available
