
Am Fam Physician. 2025;112(3):320-321
Author disclosure: No relevant financial relationships.
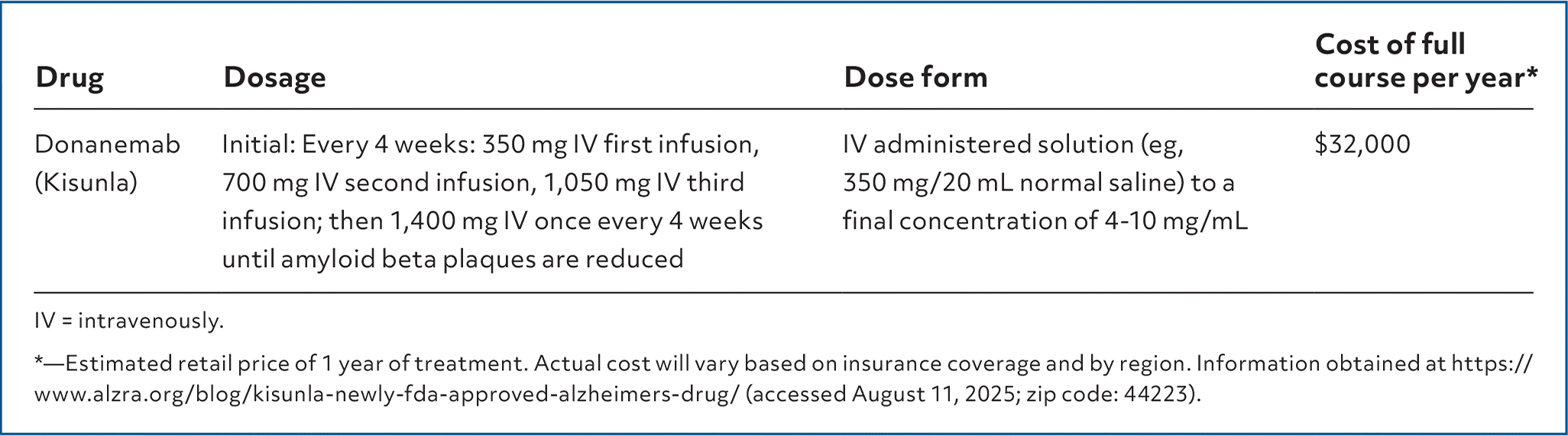
| Drug | Dosage | Dose form | Cost of full course per year* |
|---|---|---|---|
| Donanemab (Kisunla) | Initial: Every 4 weeks: 350 mg IV first infusion, 700 mg IV second infusion, 1,050 mg IV third infusion; then 1,400 mg IV once every 4 weeks until amyloid beta plaques are reduced | IV administered solution (eg, 350 mg/20 mL normal saline) to a final concentration of 4–10 mg/mL | $32,000 |
Donanemab (Kisunla) is labeled for the treatment of Alzheimer disease in patients with mild cognitive impairment or who are in the mild stage of dementia (eg, have a Mini-Mental State Examination score of 20–28 of 30) with confirmed amyloid beta pathology. Donanemab is a humanized monoclonal antibody that targets and reduces accumulations of amyloid beta plaques, which can be found in patients diagnosed with Alzheimer disease.1
Subscribe
From $180- Immediate, unlimited access to all AFP content
- More than 125 CME credits/year
- Print delivery available
Issue Access
$59.95- Immediate, unlimited access to this issue's content
- CME credits
- Print delivery available
