
Am Fam Physician. 2000;61(4):1027-1032
A more recent article on peripheral artery disease is available.
See related patient information handout on claudication, written by the authors of this article.
Peripheral arterial occlusive disease occurs in about 18 percent of persons over 70 years of age. Usually, patients who have this disease present with intermittent claudication with pain in the calf, thigh or buttock that is elicited by exertion and relieved with a few minutes of rest. The disease may also present in a subacute or acute fashion. Symptoms of ischemic rest pain, ulceration or gangrene may be present at the most advanced stage of the disease. In most cases, the underlying etiology is atherosclerotic disease of the arteries. In caring for these patients, the primary care physician should focus on evaluation, risk factor modification and exercise. The physician should consider referral to a vascular subspecialist when symptoms progress or are severe. While the prognosis for the affected limb is quite good, patients with peripheral arterial occlusive disease are at increased risk of myocardial infarction and stroke. Therefore, treatment measures should address overall vascular health. .
Arterial occlusive diseases, such as coronary artery disease, cerebrovascular disease and peripheral arterial occlusive disease (PAOD), are common in the primary care setting. These diseases often coexist in the same patient. Treatment of these diseases, which typically affect older adults, will consume a greater percentage of health care costs as the elderly population in the United States increases.
Natural History
The prognosis of the diseased extremity is generally favorable. Without specific therapy, the distance that affected persons are able to walk generally remains stable, worsening in 26 percent of persons and improving in 27 percent.1 Over five years, approximately 4 to 8 percent of affected persons require arterial reconstruction, and 2 to 4 percent require amputation.1–3
However, patients with PAOD are at risk for other atherosclerotic diseases. Up to 20 percent of asymptomatic patients may have carotid artery stenosis greater than 50 percent, and 12 to 17 percent have stenosis greater than 75 percent.4,5 Finding PAOD should increase the physician's suspicion of carotid artery stenosis, because the absence of a bruit is not sufficient to rule out significant stenosis.
The ankle-brachial index, which measures the ratio of lower and upper extremity blood pressure, is a significant predictor of cardiac events. The overall rate of cardiovascular morbidity at five years is 12 percent, and the rate is 60 percent in patients with an ankle-brachial index greater than 0.70 and less than 0.50.1 After lower extremity bypass, the five-year risk of nonfatal cardiac events is 28 to 50 percent.6,7
PAOD is associated with a significant increase in mortality2,6–10; a major contributor to this is cardiac death. In the Bypass Angioplasty Revascularization Investigation Trial,9 the five-year survival rate was 77 percent in patients with coronary artery disease and PAOD, compared with 90 percent in patients who had isolated coronary disease. Other studies have demonstrated a cumulative mortality of approximately 30 percent at five years and 47 to 61 percent at 10 years.6,7,10 Given these associated risks, it would seem reasonable for asymptomatic patients with PAOD to be screened for coronary artery disease and carotid artery stenosis; however, the most appropriate and cost-effective course of action remains unclear.
Evaluation
It is important to take a complete history that identifies symptoms of and risk factors for systemic atherosclerosis. Patients usually inform physicians of the signs and symptoms of coronary artery disease or cerebrovascular disease, but the presentation of PAOD may be subtle, particularly in sedentary patients. The most common complaint is intermittent claudication with pain of the calf, thigh or buttock occurring with exertion and relieved after several minutes of rest. Other conditions that may need to be distinguished from PAOD are listed in Table 1.
| Neurologic |
| Lumbar canal stenosis (pseudoclaudication) |
| Radiculopathy/plexopathy |
| Peripheral neuropathy |
| Musculoskeletal |
| Baker's cyst |
| Muscle strain |
| Ligament/tendon injury |
| Arthritis/connective tissue disorder |
| Vascular |
| Intermittent claudication/ischemia |
| Arterial thromboembolism |
| Cholesterol embolism |
| Deep venous thrombosis vasculitis |
Examination of the patient with PAOD may reveal bruits over the abdominal aorta, iliac, femoral, carotid or subclavian arteries, and absent or decreased peripheral pulses. Physical findings that further support the diagnosis of PAOD include decreased skin temperature, shiny, hairless skin over the lower extremities, dystrophic toenails, pallor on elevation of the extremity and rubor when the limb is dependent (Figure 1).
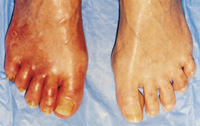
PAOD is classified using the Fontaine Staging System (Table 2). The initial claudication distance (distance at which the patient first experiences pain with exertion) and the absolute claudication distance (distance at which the patient can no longer ambulate) are usually constant. With advancing disease or acute ischemia, patients may complain of a sudden decrease in the initial claudication distance, disabling claudication, or rest pain, or on examination may be found to have ulceration or tissue loss. Any of these complaints or findings warrants immediate referral to a vascular subspecialist.
| Stage I | Asymptomatic, decreased pulses, ABI < 0.9 |
| Stage II | Intermittent claudication |
| Stage III | Daily rest pain |
| Stage IV | Focal tissue necrosis |
The ankle-brachial index is an effective screening tool. The tools required to obtain an ankle-brachial index include a blood pressure cuff and a continuous wave Doppler. Blood pressure is measured in both upper extremities, and the highest systolic reading—the first return of Doppler sound as the cuff is deflated—is recorded. The ankle systolic pressure is similarly measured using the dorsalis pedis or posterior tibial arteries. The ankle-brachial index is calculated by dividing the ankle pressure (the higher of the posterior tibial artery pressures) by the brachial systolic pressure (the higher of the two arm pressures). An ankle-brachial index below 0.95 at rest or following exercise is considered abnormal. An ankle-brachial index between 0.8 and 0.5 is consistent with intermittent claudication, and an index of less than 0.5 indicates severe disease.11 In patients with an abnormal ankle-brachial index, testing with segmental arterial pressures and a pulse volume recording before and after exercising to the point of absolute claudication are indicated.
Segmental arterial pressures are obtained in a similar fashion to the ankle-brachial index. Blood pressure cuffs are placed on the proximal and distal thigh, below the knee and above the ankle. The proximal lower extremity pressures should be equal to or greater than the upper extremity pressures, and the drop in Doppler pressure between segments no greater than 20 mm Hg. The pulse volume recording waveform is dampened, and the amplitude is decreased in the presence of arterial disease.11 These studies help physicians predict the location and severity of the disease (Figure 2).
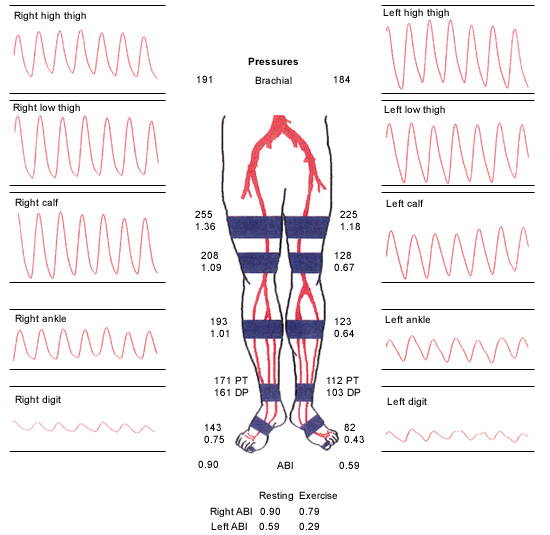
Arteriography or magnetic resonance angiography is required to delineate the extent of the disease when intervention is anticipated. These studies should not be used to make the initial diagnosis.
Management
Patients with intermittent claudication should receive conservative treatment. Aggressive risk factor modification, smoking cessation, antiplatelet therapy and a walking program are essential. In addition, medical treatment of the symptoms of claudication may benefit some patients.
RISK FACTOR MODIFICATION
The goals of risk factor modification in patients with PAOD are the same as those in patients with coronary artery disease. Unfortunately, many patients with PAOD are under-treated.12,13 All classes of antihypertensive agents are suitable in the treatment of PAOD; the type of therapy is influenced by coexisting disease. Vasodilators provide no symptomatic relief and are not indicated over other agents. Historically, beta blockers have been avoided; however, the literature does not support worsening of symptoms with their use.14 Many patients may have underlying coronary artery disease and could benefit from treatment with beta blockers.
Lipid abnormalities must be recognized and treated. High levels of low-density lipoprotein (LDL) cholesterol, low levels of high-density lipoprotein (HDL) cholesterol and high levels of triglycerides are associated with the development and progression of atherosclerosis. Patients should be treated in accordance with the guidelines of the National Cholesterol Education Program,15 which recommend a target LDL cholesterol level of less than 100 mg per dL (2.60 mmol per L) in patients with symptomatic vascular disease.
Tobacco is directly toxic to the vascular endothelium and is implicated in initiating and perpetuating atherosclerosis.16 All patients must be strongly encouraged to abstain from tobacco use.
ANTIPLATELET AGENTS
Three antiplatelet agents are available for use in patients with vascular disease. Aspirin should be considered for use in any patient with coronary artery disease, cerebrovascular disease or PAOD. In the Physicians' Health Study,17 patients who were randomized to receive aspirin therapy had a relative risk of 0.54 for peripheral arterial surgery when compared with patients who received placebo.17 The Antiplatelet Trialists' Collaboration Study18 demonstrated that patients with intermittent claudication who were treated with antiplatelet therapy had a 17.8 percent relative reduction in the incidence of myocardial infarction, stroke and vascular death.
Treatment with ticlopidine (Ticlid) or clopidogrel (Plavix) should be considered in patients who are intolerant of aspirin therapy. In the Swedish Ticlopidine Multicentre Study,19 the group treated with ticlopidine had an incidence of myocardial infarction, stroke and transient ischemic attack of 13.8 percent versus an incidence of 22.4 percent in the group taking placebo. A lower rate of mortality from all causes was also demonstrated—18.7 percent of the ticlopidine group compared with 26.1 percent of the placebo group.
The mechanism of action of clopidogrel is similar to that of ticlopidine, with fewer hematologic side effects. In the Clopidogrel versus Aspirin in Patients at Risk of Ischemic Events (CAPRIE) trial,20 patients with recent ischemic stroke, recent myocardial infarction or symptomatic PAOD were evaluated. Patients who were treated with clopidogrel for the combined end points of ischemic stroke, myocardial infarction and vascular death demonstrated an overall relative risk reduction of 8.7 percent compared with patients who were treated with aspirin without a significant reduction in overall mortality. In the subgroup analysis, patients with PAOD had a relative risk reduction of 23.8 percent for the combined end points.
EXERCISE
Walking improves the symptoms of claudication in several ways. The muscle can better adapt to anaerobic metabolism with repeated exposure to an ischemic environment. Oxidative metabolism and the overall number of available mitochondria increase. A meta-analysis21 showed an increase of 179 percent in the initial claudication distance and 122 percent in the absolute claudication distance in patients who followed a walking program. Five components of a successful program were also identified. Walking is the preferred mode of exercise. Patients should walk at least three times per week for at least 30 minutes at each session. Near-maximal claudication pain (absolute claudication distance) should be the resting point, and the patients should follow the program for at least six months.21 A supervised program is superior to a home-based exercise program.22 A walking program can increase the objective distance that the patient with claudication can ambulate. This may result in subjective improvement and lead to an enhanced quality of life.
MEDICATION
Pentoxifylline (Trental) is approved for the treatment of intermittent claudication. While the overall efficacy of pentoxifylline has been questioned,23 a recent meta-analysis24 of patients treated with pentoxifylline demonstrated small improvements in the initial claudication distance and absolute claudication distance. Sometimes, small gains obtained by patients on a treadmill protocol equal much larger gains in walking associated with daily activities. Because of this, a trial of pentoxifylline therapy can be considered. If no improvement occurs after three months, therapy may be discontinued.
The newest agent for treating intermittent claudication is cilostazol (Pletal). Cilostazol is a phosphodiesterase inhibitor that suppresses platelet aggregation and acts as a direct arterial vasodilator.25–27 In one study,27 the patients who received cilostazol had a 35 percent increase in the distance they could walk before claudication and a 41 percent increase in absolute claudication distance when compared with the subjects who received placebo. One half of the patients treated with cilostazol judged their walking to be “better” or “much better”; 84 percent of patients taking placebo felt that their symptoms were unchanged or worse.27 Other patients taking cilostazol documented improvement in the absolute claudication distance and ankle-brachial index, along with similar subjective improvements in quality of life and walking ability.28
Final Comment
Identifying the patient with intermittent claudication is highly important. Successful management of the disease involves aggressive risk factor modification, antiplatelet therapy and an exercise program. Overall, the prognosis for the diseased extremity is favorable. However, the excessive five- and 10-year mortality rate is heavily influenced by underlying cardiovascular disease.