
This is a corrected version of the article that appeared in print.
Am Fam Physician. 2019;99(6):362-369
Author disclosure: No relevant financial affiliations.
Lower extremity peripheral artery disease (PAD) affects 12% to 20% of Americans 60 years and older. The most significant risk factors for PAD are hyperlipidemia, hypertension, diabetes mellitus, chronic kidney disease, and smoking; the presence of three or more factors confers a 10-fold increase in PAD risk. Intermittent claudication is the hallmark of atherosclerotic lower extremity PAD, but only about 10% of patients with PAD experience intermittent claudication. A variety of leg symptoms that differ from classic claudication affects 50% of patients, and 40% have no leg symptoms at all. Current guidelines recommend resting ankle-brachial index (ABI) testing for patients with history or examination findings suggesting PAD. Patients with symptoms of PAD but a normal resting ABI can be further evaluated with exercise ABI testing. Routine ABI screening for those not at increased risk of PAD is not recommended. Treatment of PAD includes lifestyle modifications—including smoking cessation and supervised exercise therapy—plus secondary prevention medications, including antiplatelet therapy, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, and statins. Surgical revascularization should be considered for patients with lifestyle-limiting claudication who have an inadequate response to the aforementioned therapies. Patients with acute or limb-threatening limb ischemia should be referred immediately to a vascular surgeon.
Atherosclerotic lower extremity artery occlusive disease—commonly referred to as peripheral artery disease (PAD)—affects 12% to 20% of Americans 60 years and older, increasing to nearly 50% in those 85 years and older.1 Prevalence increases dramatically with age, and PAD disproportionately affects black persons. The global disease burden exceeds 200 million persons worldwide, and PAD increased in prevalence by 23.5% between 2000 and 2010.2
| Clinical recommendation | Evidence rating | References | Comments |
|---|---|---|---|
| Patients at increased risk of PAD should be assessed for exertional leg symptoms, ischemic rest pain, and nonhealing wounds. Vascular examination should include palpation of lower extremity pulses and auscultation for femoral bruits. | C | 9 | Based on multiple well-designed, well-executed observational studies |
| Resting ABI testing should be performed for patients with history or examination findings suggestive of PAD. Exercise ABI testing should be considered for those with a normal resting ABI despite symptoms of exertional claudication. | C | 9, 12 | Based on a retrospective review of a vascular diagnostic laboratory database |
| ABI screening should not be performed in asymptomatic patients who are not at increased risk of PAD. | C | 9 | Based on data from population-based cohort studies demonstrating low prevalence of abnormal resting ABI in younger, asymptomatic individuals |
| The primary treatment strategies for lower extremity PAD include the following: | |||
| Lifestyle modifications | C | 9, 24–31 | Based on expert opinion and consensus guidelines in the absence of clinical trials |
| Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers | A | 9, 30–33 | Based on consistent evidence from RCTs showing reduced morbidity and mortality |
| Statins | A | 9, 28, 29 | Based on consistent evidence from RCTs showing reduced morbidity and mortality |
| Antiplatelet therapy | A | 9, 24–27 | Based on consistent evidence from RCTs showing reduced morbidity and mortality. |
| Recommendation | Sponsoring organization |
|---|---|
| Interventions, including surgical bypass, angiogram, angioplasty, or stent, should not be used as a first line of treatment for most patients with intermittent claudication. | Society for Vascular Surgery |
Risk Factors and Mortality Rates
Analysis of data from the National Health and Nutrition Examination Survey demonstrated that the most significant PAD risk factors are hypertension, diabetes mellitus, chronic kidney disease, hyperlipidemia, and smoking. The odds of having PAD increase with each additional risk factor, from a 1.5-fold increase with one risk factor to a 10-fold increased risk with three or more risk factors.3 In one large study, more than 80% of patients with PAD were current or former smokers.4 Cardiovascular mortality rates of current smokers with PAD are more than double that of those with PAD who have never smoked.5 High-density lipoprotein cholesterol that is low (less than 40 mg per dL [1.04 mmol per L] in men and less than 50 mg per dL [1.29 mmol per L] in women) is also associated with increased risk of death in PAD.6
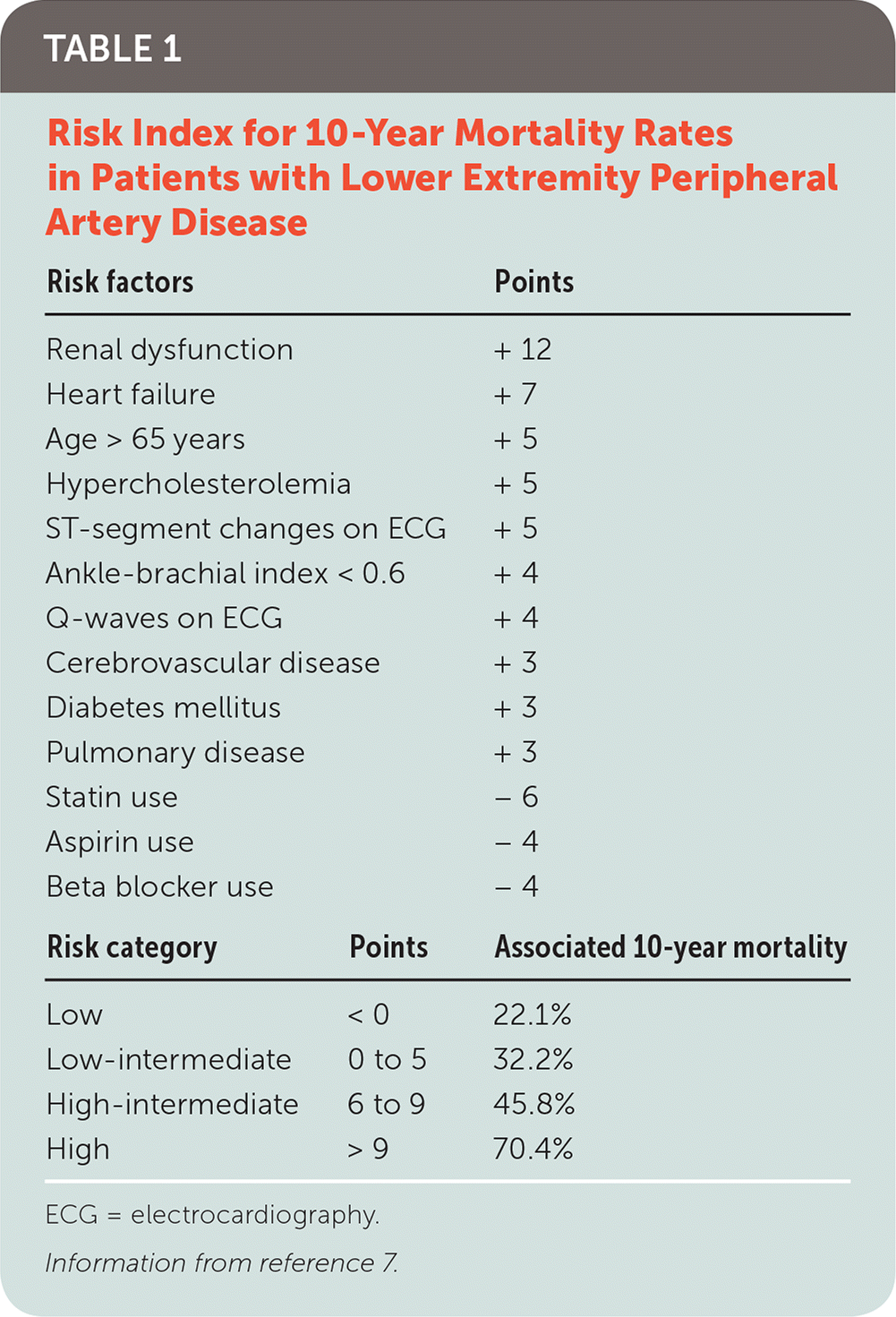
| Risk factors | Points | |
| Renal dysfunction | + 12 | |
| Heart failure | + 7 | |
| Age > 65 years | + 5 | |
| Hypercholesterolemia | + 5 | |
| ST-segment changes on ECG | + 5 | |
| Ankle-brachial index < 0.6 | + 4 | |
| Q-waves on ECG | + 4 | |
| Cerebrovascular disease | + 3 | |
| Diabetes mellitus | + 3 | |
| Pulmonary disease | + 3 | |
| Statin use | – 6 | |
| Aspirin use | – 4 | |
| Beta blocker use | – 4 | |
| Risk category | Points | Associated 10-year mortality |
| Low | < 0 | 22.1% |
| Low-intermediate | 0 to 5 | 32.2% |
| High-intermediate | 6 to 9 | 45.8% |
| High | > 9 | 70.4% |
Clinical Presentation
Intermittent claudication is the hallmark of PAD and is defined as fatigue, discomfort, cramping, or pain of vascular origin in the calf muscles of the lower extremities that is consistently induced by exercise and consistently relieved within 10 minutes by rest.
In the general population, only about 10% of persons with known PAD have the classic symptom of intermittent claudication. Approximately 40% do not complain of leg symptoms at all, and 50% have a variety of leg symptoms different from classic claudication, such as exertional pain that does not stop the individual from walking, does not involve the calves, or does not resolve within 10 minutes of rest.8
The 2016 American Heart Association/American College of Cardiology (AHA/ACC) guideline on the management of patients with lower extremity PAD recommends patients at increased risk of PAD should be assessed for exertional leg symptoms, ischemic rest pain, and nonhealing wounds.9 Table 2 outlines characteristics of patients at increased risk of PAD.9
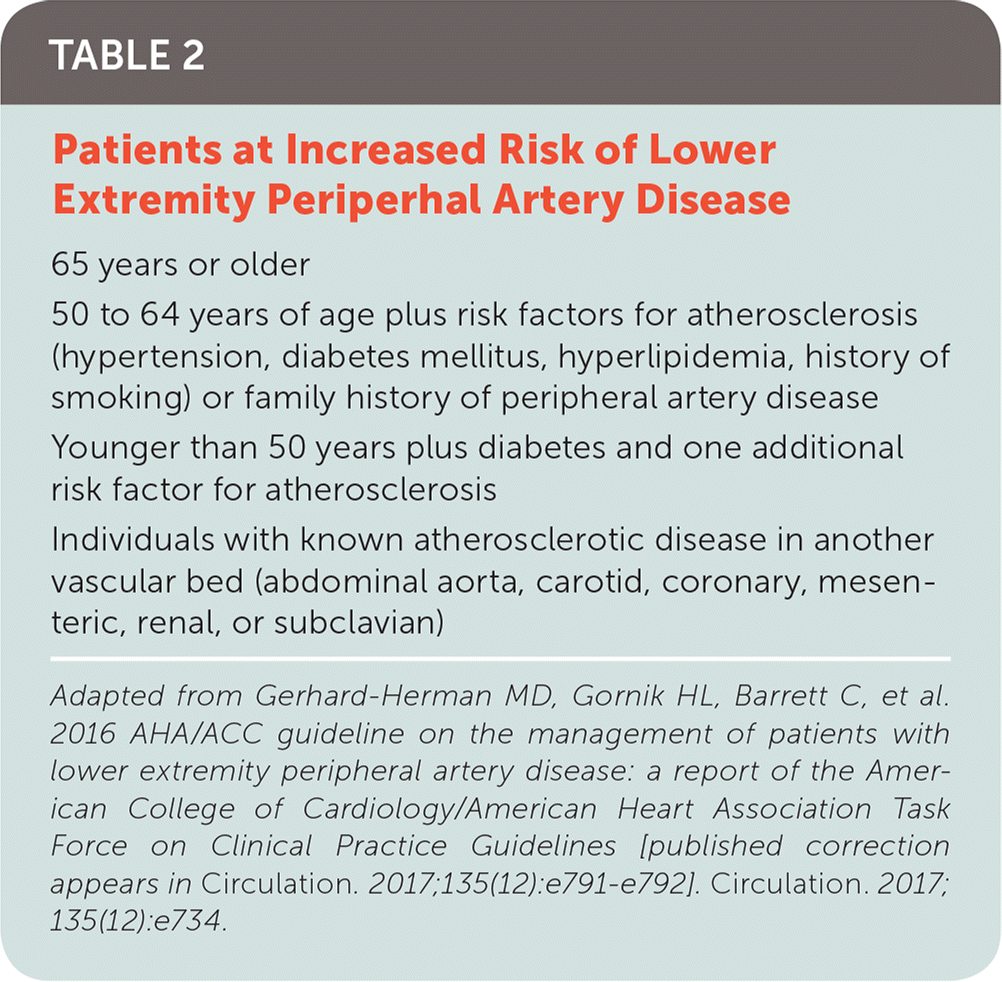
| 65 years or older |
| 50 to 64 years of age plus risk factors for atherosclerosis (hypertension, diabetes mellitus, hyperlipidemia, history of smoking) or family history of peripheral artery disease |
| Younger than 50 years plus diabetes and one additional risk factor for atherosclerosis |
| Individuals with known atherosclerotic disease in another vascular bed (abdominal aorta, carotid, coronary, mesenteric, renal, or subclavian) |
Physical Examination
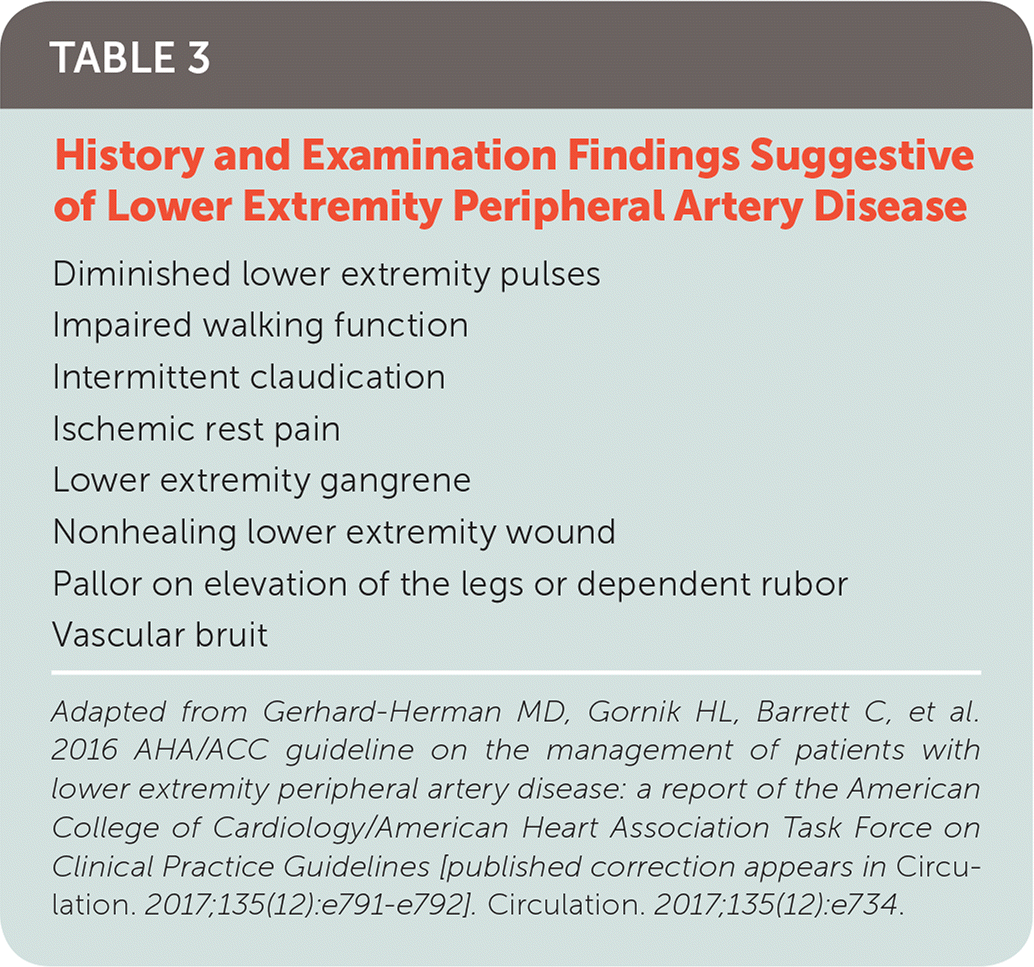
| Diminished lower extremity pulses |
| Impaired walking function |
| Intermittent claudication |
| Ischemic rest pain |
| Lower extremity gangrene |
| Nonhealing lower extremity wound |
| Pallor on elevation of the legs or dependent rubor |
| Vascular bruit |
Other common lower extremity findings include hair loss, shiny skin, and muscle atrophy. Arterial ulcerations are characterized by well-demarcated, “punched-out” lesions. Dependent rubor and elevation pallor may be present in advanced disease and result from impaired autoregulation in the dermal arterioles and capillaries10 (Figure 1).
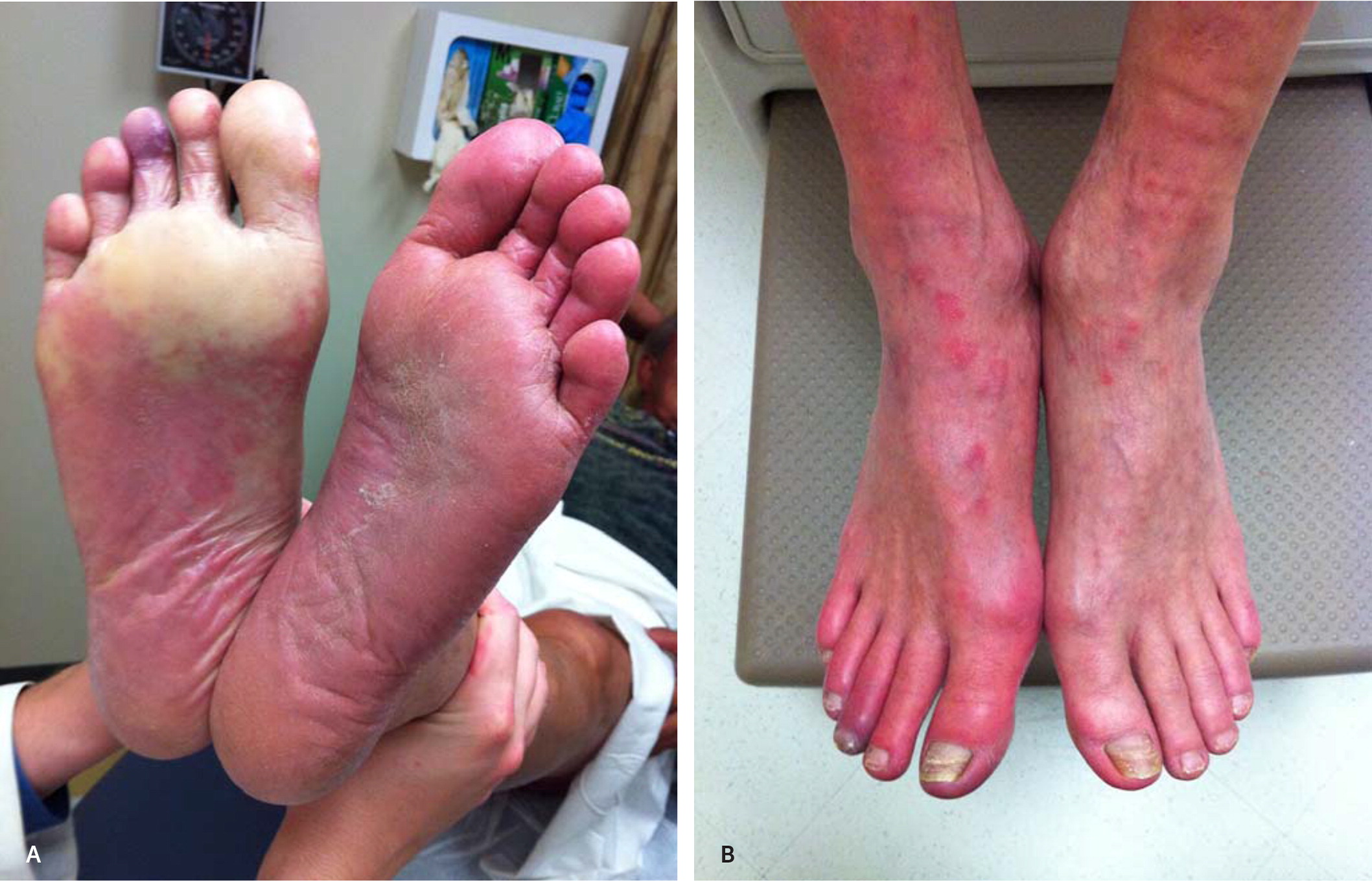
Diagnostic Testing
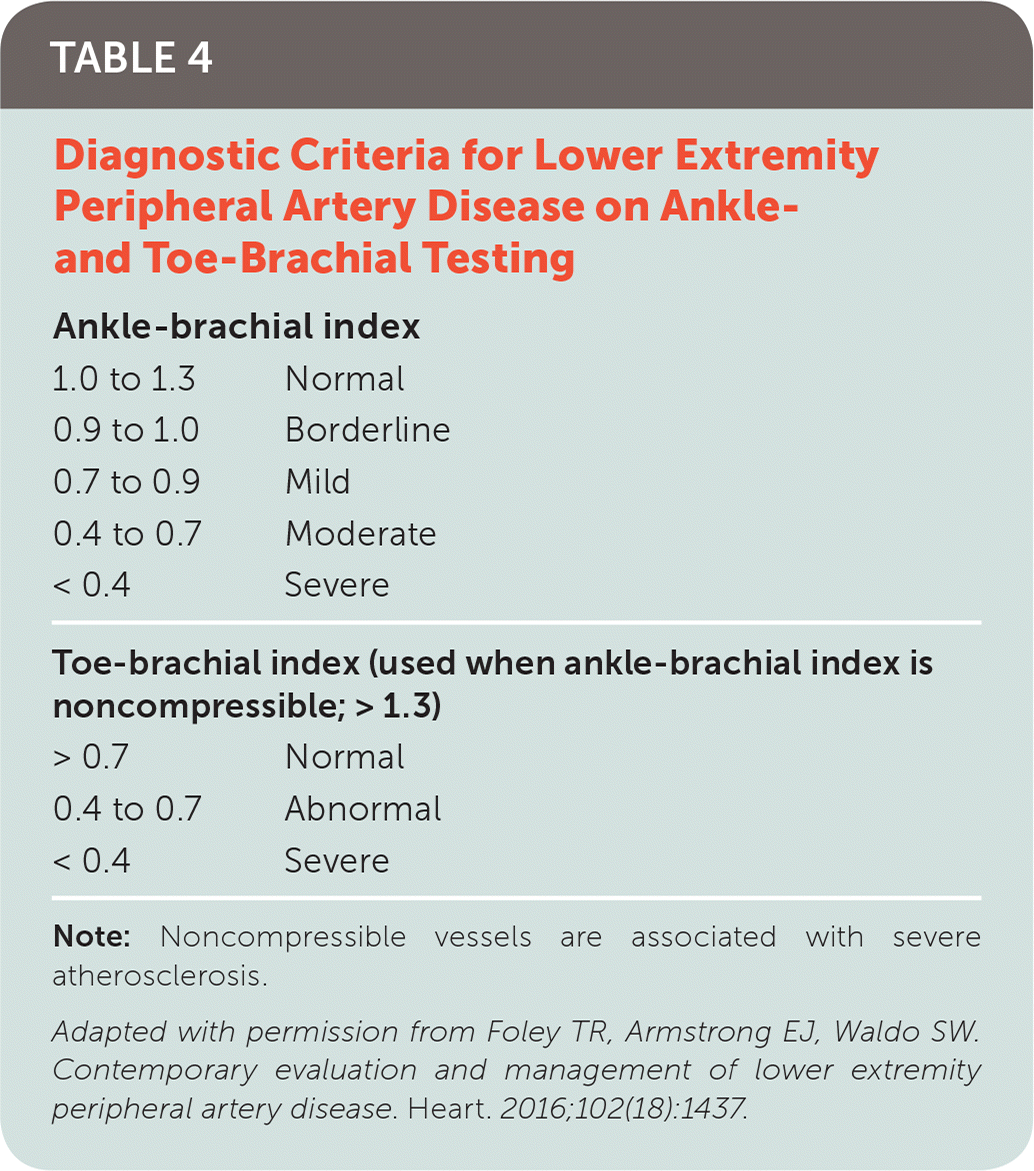
The ankle-brachial index (ABI) is an inexpensive and reproducible method for assessing lower extremity hemodynamics. The ABI is the ratio of the highest systolic pressure in each leg, obtained at the dorsalis pedis and posterior tibial recurrent arteries using a Doppler probe, to the higher of the right or left arm brachial artery pressures. Interpretation of ABI results is outlined in Table 4.10
| Ankle-brachial index | |
| 1.0 to 1.3 | Normal |
| 0.9 to 1.0 | Borderline |
| 0.7 to 0.9 | Mild |
| 0.4 to 0.7 | Moderate |
| < 0.4 | Severe |
| Toe-brachial index (used when ankle-brachial index is noncompressible; > 1.3) | |
| > 0.7 | Normal |
| 0.4 to 0.7 | Abnormal |
| < 0.4 | Severe |
The sensitivity of ABI in detecting angiographically significant stenoses has been reported to be as high as 94% to 97%.10 The sensitivity of ABI is diminished, however, in patients with small vessel disease resulting from hypertension, diabetes, or chronic kidney disease. When added to the Framingham Risk Score, a measured ABI less than or equal to 0.9 nearly doubles the risk of overall mortality, cardiovascular mortality, and major coronary events in each Framingham Risk Score category.2
The 2016 AHA/ACC guideline recommends resting ABI testing for patients with history or examination findings suggestive of PAD (Table 3).9 For patients at increased risk of PAD, but without suggestive history or examination findings, resting ABI testing is considered reasonable. The guideline does not recommend ABI screening in patients who are not at increased risk of PAD.9 The U.S. Preventive Services Task Force found insufficient evidence to assess the balance of benefits and harms of using ABI to screen asymptomatic adults for PAD.11
In patients with noncompressible lower extremity vessels (ABI greater than 1.3), the toe-brachial index can be used.10 If the physical examination and resting ABI or toe-brachial index do not definitively diagnose lower extremity PAD despite a history of exertional claudication, then exercise ABI testing may be performed. As many as 30% of symptomatic patients with normal resting studies may have an abnormal ABI after exercise.12 Although there is no formally established exercise ABI protocol, participants in one study received ABI testing immediately after walking for five minutes on a treadmill at 12% grade and 2.0 miles per hour (3.2 km per hour) or until symptoms forced the patient to stop.12 Alternatively, the six-minute walk test (how far and how fast the patient can walk in six minutes) correlates well with the hemodynamic severity of PAD and may be more practical than treadmill testing.13
It is important to recognize that PAD typically does not occur in isolation. The AHA/ACC considers the use of ultrasonography for abdominal aortic aneurysm to be reasonable in patients with symptomatic PAD.9 In a series of 210 patients with PAD and an average age of 65 years, the prevalence of abdominal aortic aneurysm was 9.0%, rising to 15.8% in patients older than 75 years.14 The prevalence of abdominal aortic aneurysm in the general population is only 4% to 7.6%.15
Critical and Acute Limb Ischemia
CRITICAL LIMB ISCHEMIA
Limb-threatening or critical limb ischemia (CLI) is manifested by chronic (more than two weeks) ischemic rest pain, ischemic wounds or tissue loss, or gangrene in one or both legs. Ischemic rest pain typically occurs soon after falling asleep; the patient is awakened by burning pain or numbness in the forefoot. Symptoms are often relieved by hanging the limb over the side of the bed, triggering dependent rubor of the foot. Patients with any of these findings require urgent referral to a vascular surgeon.
The reported incidence of PAD and CLI varies depending on the population studied. Up to 21% of patients with intermittent claudication may progress to CLI.16 One large cohort study found the most significant risk factors for development of CLI were diabetes, renal failure, heart failure, and prior stroke.17 Annual mortality rates of patients with CLI are approximately 25%.18
ACUTE LIMB ISCHEMIA
Acute limb ischemia is a medical emergency and describes the abrupt interruption of arterial blood flow to an extremity. Acute limb ischemia presents as a cold, painful, and pale extremity with diminished or absent pulses, motor weakness, and sensory impairment. In contrast to CLI, in which gradual ischemic conditioning promotes the development of collateral vessels that maintain limb perfusion, the acute disruption of arterial flow with acute limb ischemia threatens limb integrity unless prompt revascularization is undertaken.10 The urgency of acute limb ischemia is attributable to the period that skeletal muscle will tolerate ischemia: approximately four to six hours.19 Patients with acute limb ischemia should be evaluated by a vascular subspecialist on an emergency basis.
Treatment
PAD is considered a coronary artery disease risk equivalent, and patients with PAD are at increased risk for major adverse cardiac events (MACE), including myocardial infarction (MI), ischemic stroke, and cardiovascular death. They are also at risk for major adverse limb events, which include major amputations and acute limb ischemia. Among patients with symptomatic PAD, annual rates of MACE are 4% to 5%, and rates of major adverse limb events are 1% to 2%.20
Patients with PAD should receive a comprehensive program of guideline-directed medical therapy, including structured exercise and lifestyle modification, to reduce MACE and major adverse limb events and to improve functional status. Smoking cessation is a vital component of care for patients with PAD who smoke.9
EXERCISE
Current guidelines endorse supervised exercise therapy as a first-line treatment for all patients with PAD.10 Exercise therapy for patients with PAD has typically involved walking to the point of significant claudication pain, then briefly resting until pain subsides. For patients unwilling to endure repeated bouts of pain, modalities of exercise that avoid claudication or walking performed at intensities that are pain free or that produce only mild levels of claudication can achieve health benefits comparable with walking at moderate or higher levels of claudication pain.21,22 A structured community- or home-based exercise program with behavioral change techniques, such as health coaching and activity tracking, can also improve walking ability and functional status.10 Unstructured programs or general recommendations to simply walk more are not usually effective.23
Drug Therapy
ANTIPLATELET THERAPY
Current AHA/ACC guidelines recommend antiplatelet therapy with aspirin alone (75 to 325 mg per day) or clopidogrel (Plavix) alone (75 mg per day) to reduce the risk of MI, stroke, and vascular death in patients with symptomatic PAD.9 Meta-analysis of the Antithrombotic Trialists' Collaboration found a 23% relative reduction and 1.3% absolute reduction in serious vascular events among patients with PAD using antiplatelet therapy (primarily aspirin) compared with those using no antiplatelet therapy.24
In asymptomatic patients with PAD (ABI less than or equal to 0.90), antiplatelet therapy is reasonable to reduce the risk of MI, stroke, or vascular death.9 However, one large randomized controlled trial evaluating the use of aspirin in this population showed no significant reduction in vascular events.25 In asymptomatic patients with borderline ABI (0.91 to 0.99), the usefulness of antiplatelet therapy to reduce the risk of MI, stroke, or vascular death is uncertain.9
For patients with PAD-associated limb symptoms in addition to stable coronary artery disease or cerebrovascular disease, the benefit of aspirin (or clopidogrel if the patient is aspirin intolerant) is well established, but dual antiplatelet therapy in this setting (combining aspirin with clopidogrel) is no more effective and is associated with greater risk of major bleeding.9 In those with prior MI, however, ticagrelor (Brilinta) may be added to aspirin to further reduce MACE, although the overall benefit is small (absolute risk reduction = 1.2% over three years; number needed to treat = 83).26
ANTICOAGULANT THERAPY
In the Cardiovascular Outcomes for People Using Anticoagulation Strategies trial, the addition of low-dose rivaroxaban (Xarelto; 2.5 mg twice daily) to aspirin in patients with coronary artery disease and symptomatic PAD reduced MACE and major adverse limb events (absolute risk reduction = 1.0% for each; number needed to treat = 100), although it also increased the absolute risk of major bleeding by 1%. [corrected]
STATIN THERAPY
Substantial evidence supports the use of statin therapy in all patients with symptomatic PAD.9 One trial evaluating patients with symptomatic PAD found that atorvastatin (Lipitor) reduced the risk of long-term adverse limb outcomes (revascularization procedures and need for ischemic amputation) by 18% compared with those not receiving statins.28 High-intensity atorvastatin also improves pain-free walking distance and community-based physical activity in those with intermittent claudication.29
ANTIHYPERTENSIVE THERAPY
Antihypertensive therapy should be administered to patients with hypertension and PAD to reduce the risk of MI, stroke, heart failure, and cardiovascular death.9 Angiotensin-converting enzyme inhibitors directly inhibit the atherosclerotic process and improve vascular endothelial function, independent of their antihypertensive effects.30,31 The Heart Outcomes Prevention Evaluation study showed that the use of ramipril (Altace) significantly reduced the rates of mortality, MI, and stroke in high-risk patients with evidence of vascular disease or diabetes but without a low ejection fraction or heart failure.32 Telmisartan (Micardis) has demonstrated reductions in adverse outcomes similar to ramipril.33 Consequently, AHA/ACC guidelines recommend the use of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers in patients with PAD.9
MEDICATIONS TO IMPROVE CIRCULATORY FLOW
Cilostazol (Pletal), a vasodilator with antiplatelet activity, is an effective therapy to improve symptoms and increase walking distance in patients with claudication. The usefulness of the drug is limited, however, by its adverse effect profile (e.g., dizziness, gastrointestinal symptoms) and its contraindication for use in patients with heart failure. Pentoxifylline (Trental), a drug that increases red blood cell deformability to improve circulatory flow, does not improve maximal walking distance in patients with PAD, and guidelines do not recommend using it for treatment of claudication.9
Surgery
Revascularization is a reasonable treatment option for patients with lifestyle-limiting claudication who have an inadequate response to other guideline-directed therapies. Preoperative evaluation includes angiography to define the location and severity of vascular occlusion and to guide selection of the appropriate surgical intervention (Figure 2). Commonly used interventions include bypass grafting, endarterectomy, and angioplasty with stenting.34
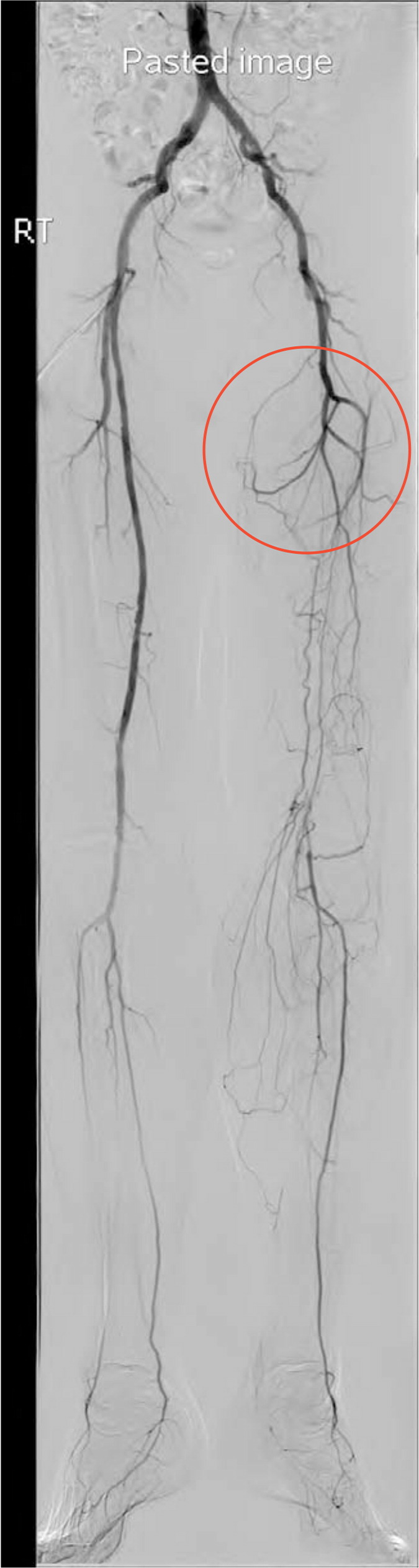
No data support performing endovascular procedures on patients with PAD for the purpose of preventing progression of claudication symptoms to CLI. Similarly, no data support revascularization in patients with asymptomatic PAD.9 Patients with claudication in whom maximal risk factor treatment and supervised exercise therapy have failed and who are truly limited in daily activities should be referred to a vascular surgeon. Patients in whom the diagnosis of claudication is unclear from history, physical examination, and ABI determination should also be referred to a vascular surgeon.
This article updates previous articles by Hennion and Siano35 ; Sontheimer36 ; Gey, et al.37 ; and Carman and Fernandez.38
Data Sources: An online search via PubMed was completed using the keywords peripheral vascular disease. The search included systematic reviews, meta-analyses, randomized controlled trials, and review articles. Additionally, the search included the Essential Evidence Plus, Cochrane Library, and U.S. Preventive Services Task Force. Finally, references within these resources were searched. Search dates: July 2017; February and December 2018.
