This is an updated and corrected version of the article that appeared in print.
Am Fam Physician. 2013;88(5):306-310
A more recent article on peripheral artery disease is available.
Patient information: A handout on this peripheral arterial disease is available at https://familydoctor.org/familydoctor/en/diseases-conditions/peripheral-arterial-disease-and-claudication.html.
Author disclosure: No relevant financial affiliations.
Peripheral arterial disease (PAD) is atherosclerosis leading to narrowing of the major arteries distal to the aortic arch. The most common presenting symptom is claudication; however, only 10% of patients have classic claudication. The ankle-brachial index (ABI) can be used to screen for and diagnose PAD in the primary care setting. An ABI of less than 0.9 is associated with a two- to fourfold increase in relative risk for cardiovascular events and all-cause mortality. To improve cardiovascular risk stratification and risk factor modification, the American Diabetes Association recommends ABI screening for patients older than 50 years who have diabetes mellitus, and the American Heart Association recommends screening all patients 65 years and older and those 50 years and older who have a history of diabetes or smoking. Because there is no evidence that screening leads to fewer cardiovascular events or lower all-cause mortality, the U.S. Preventive Services Task Force concluded that the current evidence is insufficient to assess the balance of benefits and harms of screening for PAD and of cardiovascular risk assessement with ABI in adults. Management of claudication includes exercise, smoking cessation, statin therapy, and antiplatelet therapy with aspirin or clopidogrel, and possibly cilostazol in patients with no history of heart failure. Surgical revascularization may be considered in patients with lifestyle-limiting claudication symptoms that do not respond to medical therapy.
Peripheral arterial disease (PAD) is atherosclerosis leading to narrowing of the major arteries distal to the aortic arch. It can involve both the upper and lower extremities. Progressive occlusion results in arterial stenosis, reduced blood flow, and claudication, the most common presenting symptom. Classic claudication is defined as muscle discomfort in the lower limbs that is reproduced by exercise and relieved by rest within 10 minutes.1 Only 10% of patients with PAD have classic claudication; others have atypical leg pain (50%) or are asymptomatic (40%).2 The American Heart Association estimates that approximately 8 to 12 million Americans have PAD, with an overall prevalence of 3% to 10% (15% to 20% in those older than 70 years).1,3
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Screening for PAD with the ankle-brachial index may be considered in patients 65 years and older and in those 50 years and older who have a history of diabetes mellitus, smoking, exertional leg pain, or a nonhealing extremity wound. However, there is no prospective evidence that screening improves health outcomes in these patients. | C | 1, 4, 7, 9, 11, 13 |
| Patients with PAD and lifestyle-limiting claudication should be prescribed a supervised exercise program and trial of cilostazol (Pletal; 100 mg twice per day) in the absence of heart failure. | A | 14, 15, 22–24 |
| Patients with PAD who smoke should be offered smoking-cessation counseling and interventions. | A | 4, 16 |
| Treatment of PAD should include statin therapy to achieve a low-density lipoprotein level of 100 mg per dL (2.59 mmol per L) or less. | C | 4, 17 |
| Patients with symptomatic PAD should be treated with aspirin (81 mg per day) or clopidogrel (Plavix; 75 mg per day) to prevent cardiovascular events. | B | 7, 20, 21 |
Diagnosis
Risk factors for PAD include older age, smoking, diabetes mellitus, hypertension, hyperlipidemia, renal insufficiency (glomerular filtration rate less than 60 mL per minute per 1.73 m2), and non-Hispanic black race.1,3,4 Smoking and diabetes are associated with the highest relative risk for developing lower-extremity PAD.
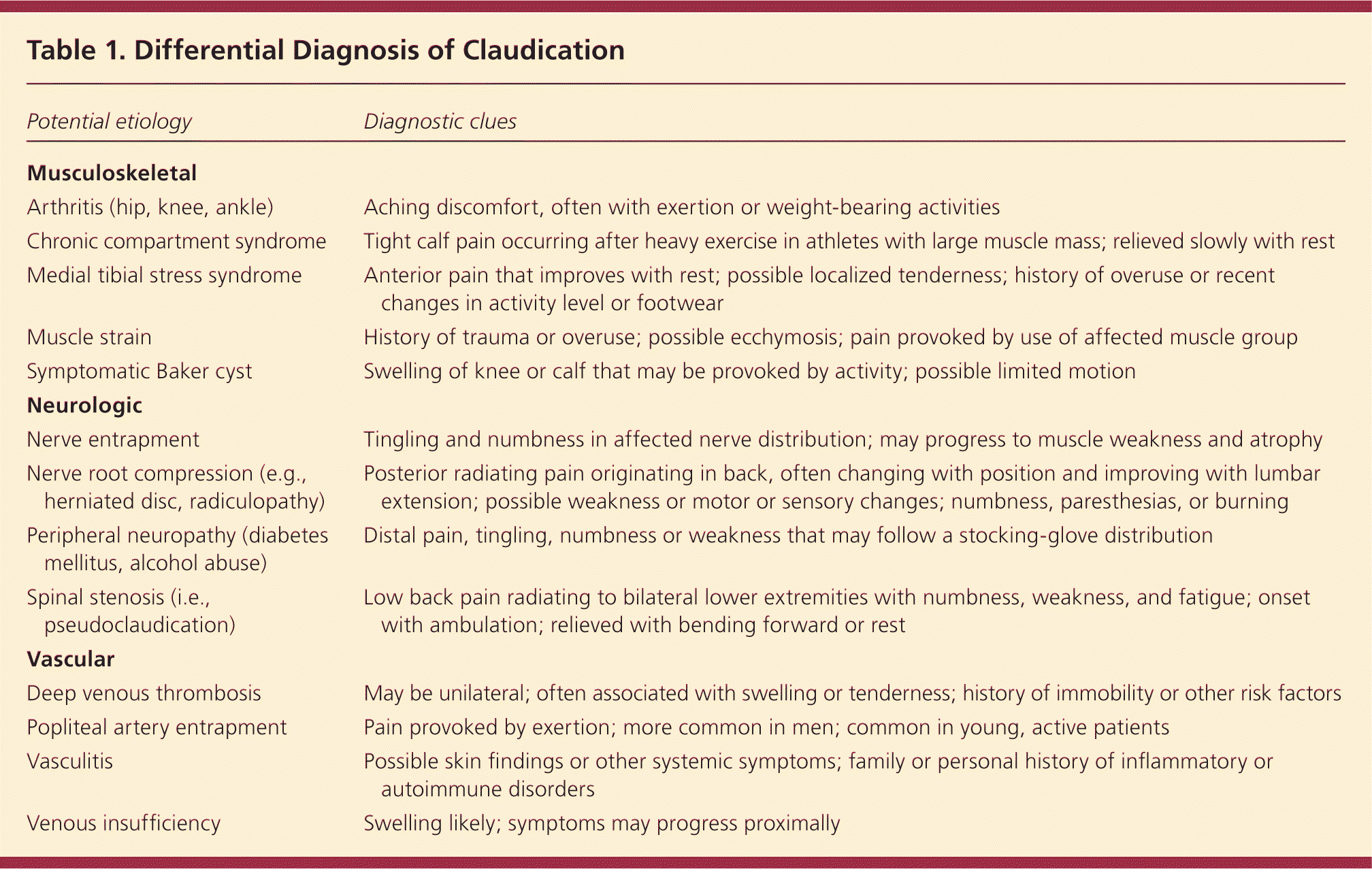
The clinical history and physical examination findings may suggest a diagnosis of PAD, especially in patients with multiple risk factors or classic claudication. Claudication must be distinguished from spinal stenosis (i.e., pseudoclaudication), peripheral neuropathies, musculoskeletal disorders and injuries, and deep venous thrombosis (Table 1) [ corrected]. Physical examination findings may include cool skin; nonpalpable distal pulses; auscultation of bruits over the iliac, femoral, or popliteal arteries; abnormal capillary refill time; nonhealing wounds; shiny skin; absence of hair in the affected area; and distal extremity pallor on elevation (Figure 1). An estimated 1% of patients with PAD present with evidence of tissue loss, gangrene, or chronic pain at rest; such symptoms suggest critical limb ischemia, and these patients should be referred for surgical consultation.4
| Potential etiology | Diagnostic clues |
|---|---|
| Musculoskeletal | |
| Arthritis (hip, knee, ankle) | Aching discomfort, often with exertion or weight-bearing activities |
| Chronic compartment syndrome | Tight calf pain occurring after heavy exercise in athletes with large muscle mass; relieved slowly with rest |
| Medial tibial stress syndrome | Anterior pain that improves with rest; possible localized tenderness; history of overuse or recent changes in activity level or footwear |
| Muscle strain | History of trauma or overuse; possible ecchymosis; pain provoked by use of affected muscle group |
| Symptomatic Baker cyst | Swelling of knee or calf that may be provoked by activity; possible limited motion |
| Neurologic | |
| Nerve entrapment | Tingling and numbness in affected nerve distribution; may progress to muscle weakness and atrophy |
| Nerve root compression (e.g., herniated disc, radiculopathy) | Posterior radiating pain originating in back, often changing with position and improving with lumbar extension; possible weakness or motor or sensory changes; numbness, paresthesias, or burning |
| Peripheral neuropathy (diabetes mellitus, alcohol abuse) | Distal pain, tingling, numbness or weakness that may follow a stocking-glove distribution |
| Spinal stenosis (i.e., pseudoclaudication) | Low back pain radiating to bilateral lower extremities with numbness, weakness, and fatigue; onset with ambulation; relieved with bending forward or rest |
| Vascular | |
| Deep venous thrombosis | May be unilateral; often associated with swelling or tenderness; history of immobility or other risk factors |
| Popliteal artery entrapment | Pain provoked by exertion; more common in men; common in young, active patients |
| Vasculitis | Possible skin findings or other systemic symptoms; family or personal history of inflammatory or autoimmune disorders |
| Venous insufficiency | Swelling likely; symptoms may progress proximally |
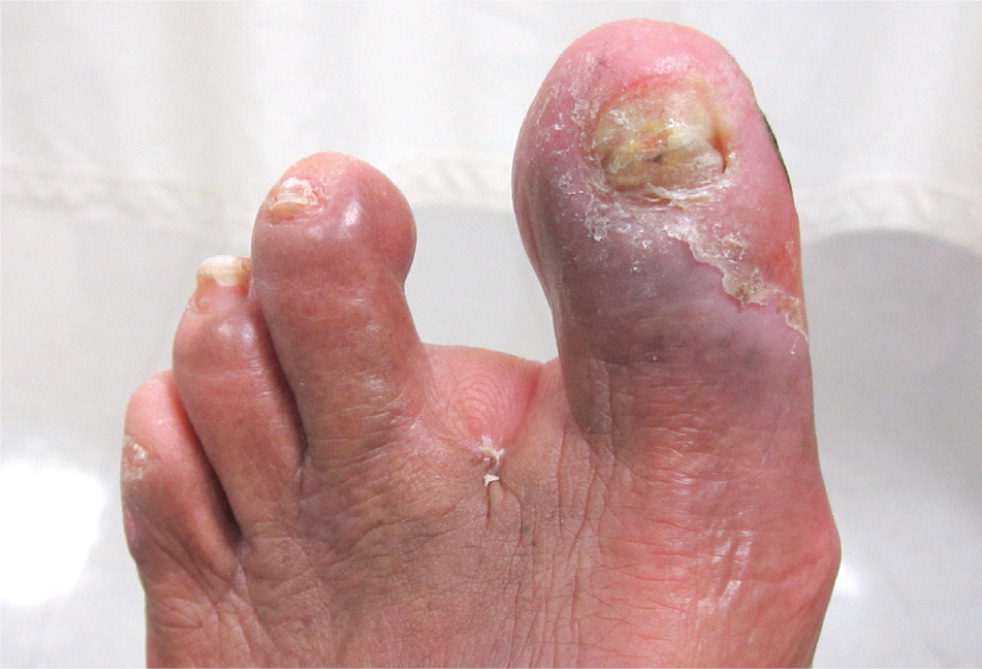
Computed tomographic angiography, magnetic resonance angiography, and contrast-enhanced angiography may be used for the diagnosis of PAD; however, these modalities are typically reserved for patients who are being considered for surgery to localize and quantify arterial stenosis.5 The ankle-brachial index (ABI), the ratio of the ankle blood pressure to the highest brachial systolic pressure, is an inexpensive and efficient method to diagnose PAD in the primary care setting. It is highly sensitive (90%) and specific (98%).6 Ankle blood pressure is obtained by inflating a blood pressure cuff above the ankle and detecting the return of the dorsalis pedis or posterior tibial artery pulse by Doppler ultrasonography as the cuff is slowly deflated (Figure 2). Diagnostic parameters for PAD at specific ABI values are listed in Table 2.7,8
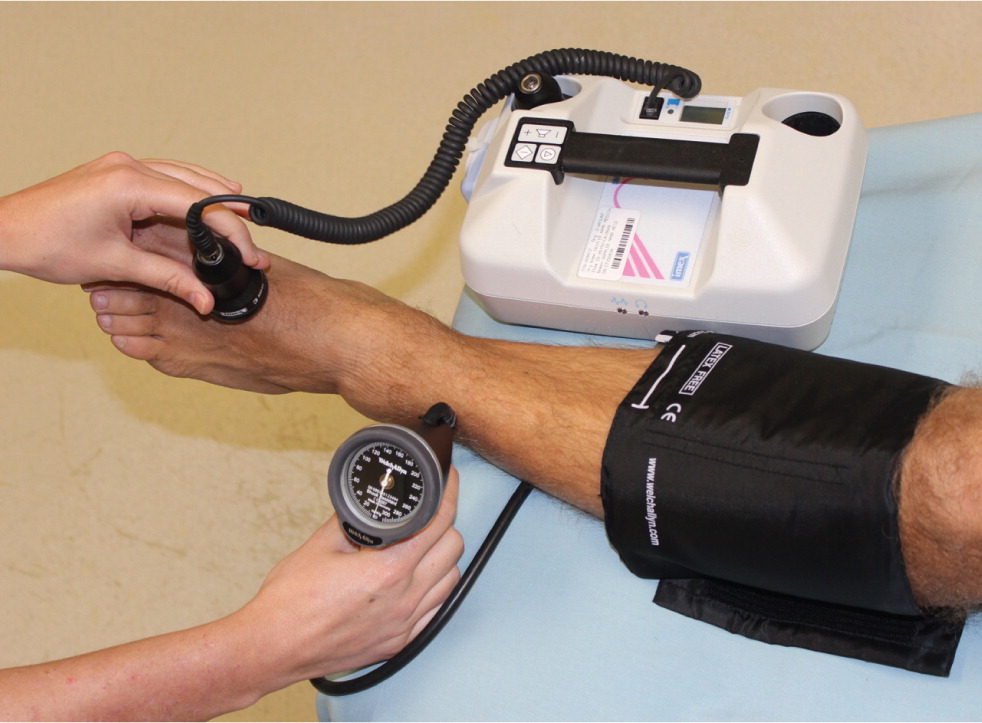
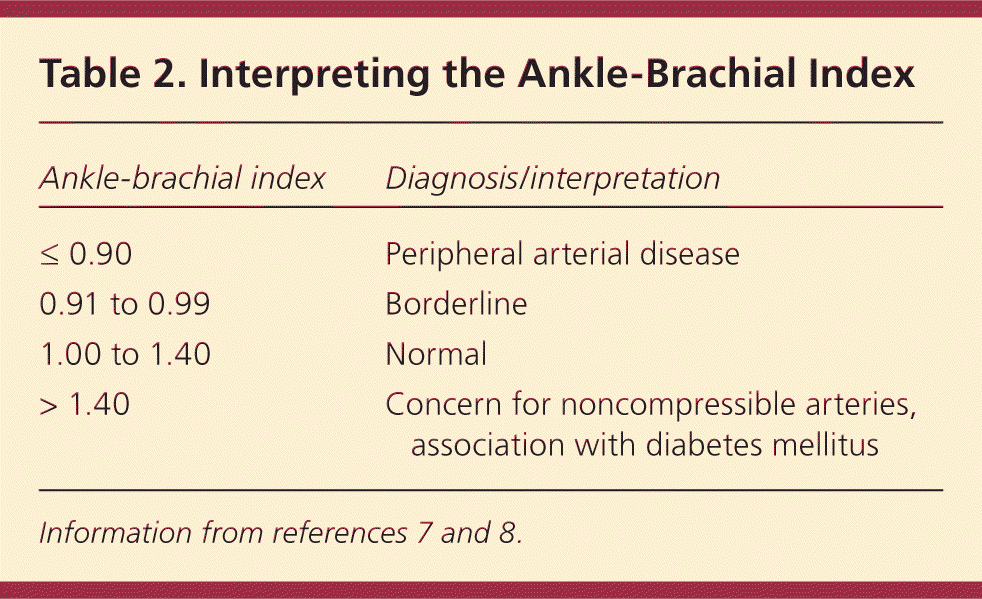
| Ankle-brachial index | Diagnosis/interpretation |
|---|---|
| ≤ 0.90 | Peripheral arterial disease |
| 0.91 to 0.99 | Borderline |
| 1.00 to 1.40 | Normal |
| > 1.40 | Concern for noncompressible arteries, association with diabetes mellitus |
Screening
The U.S. Preventive Services Task Force (USPSTF) found insufficient evidence to recommend routine screening for PAD based on a lack of evidence that screening improves outcomes such as cardiovascular morbidity or mortality.9 However, there may be a role for screening in certain high-risk populations. The American Diabetes Association recommends ABI screening in all patients with diabetes who are older than 50 years; if results are normal, screening should be repeated every five years.10 Patients with diabetes who are younger than 50 years should be screened if they have risk factors (e.g., smoking, hypertension, hyperlipidemia, duration of diabetes more than 10 years).
Guidelines from the American College of Cardiology and American Heart Association recommend ABI screening and a directed review of symptoms in high-risk populations (e.g., persons 65 years and older; persons 50 years and older with a history of diabetes or smoking, exertional leg pain symptoms, or a nonhealing extremity wound).4,7 The Trans-Atlantic Inter-Society Consensus guidelines recommend screening in patients with a 10-year Framingham cardiovascular risk score of 10% to 20%.1 There are no randomized trials on the use, effectiveness, or long-term clinical outcomes of ABI screening.11
Combining the results of ABI screening with Framingham risk scores can improve 10-year cardiovascular risk prediction and alter risk factor modification goals in some patients.8 A meta-analysis demonstrated that inclusion of the ABI in the Framingham risk calculation resulted in risk category reclassification in approximately 19% of men and 36% of women.8 Specifically, 7% of low-risk and 10% of intermediate-risk women classified by Framingham risk alone were changed to the high-risk category when an ABI of 0.9 or less was also considered.12 The USPSTF's evidence review concluded that the ABI has limited value for cardiovascular risk prediction when added to the Framingham risk score; in one randomized trial, treatment of persons with a screen-detected low ABI did not reduce cardiovascular events, but likely increased the risk of major bleeding.13
Treatment
PAD represents a significant systemic atherosclerotic burden and should be considered equivalent to coronary heart disease when assessing overall cardiovascular risk. Management of PAD includes smoking cessation, exercise, statin therapy to reach a goal low-density lipoprotein level of 100 mg per dL (2.59 mmol per L) or less, and antiplatelet therapy with either 75 to 325 mg of aspirin or 75 mg of clopidogrel (Plavix) daily. Patients with lifestyle-limiting claudication should be considered for a trial of cilostazol (Pletal) in the absence of heart failure.
COUNSELING AND LIFESTYLE MODIFICATION
Supervised exercise programs may increase walking time and distance. A 2008 Cochrane review showed that exercise increased the length of time patients could walk without claudication, and that it improved overall walking time and distance by an average of five minutes and 113.2 m; these results were sustained for more than two years.14 In most studies, supervised exercise programs consisted of lower-extremity exercises or walking on a treadmill for 30 minutes two or three times per week. Prescribed home exercise programs also improve maximal and pain-free walking distance and may be more convenient for patients. However, the same Cochrane review found that supervised exercise programs were more beneficial than unsupervised exercise programs.14 A systematic review found that supervised exercise programs are equivalent to percutaneous angioplasty in improving walking distance and quality of life in patients with intermittent claudication.15 There is no evidence that exercise programs improve ABI values or overall mortality.14
Smoking-cessation interventions and counseling should be offered to all patients with PAD who use tobacco.4 Although no randomized studies have been done to determine the benefit of smoking cessation in this population, observational data indicate that smoking cessation is associated with overall increased walking time in patients with PAD and claudication.16
MEDICATIONS
Statin therapy modestly improves claudication symptoms in addition to lowering lipid levels.17 A randomized, double-blind study compared 10 mg and 80 mg of daily atorvastatin (Lipitor) with placebo.18 Persons in the 80-mg group had the most improvement in pain-free walking time after 12 months, increasing by 81 seconds vs. 39 seconds for those in the placebo group; however, these results were not statistically significant. In a retrospective study, patients with PAD who were receiving statins had significant improvements in usual- and rapid-pace 4-m walking velocity and in six-minute walking distance compared with patients who were not receiving statins.19 PAD should be managed as a coronary heart disease risk equivalent even in asymptomatic patients, and patients should be treated with lipid-lowering therapy to a target low-density lipoprotein level of 100 mg per dL or less.4,17
Although neither aspirin nor clopidogrel improves claudication symptoms, antiplatelet therapy is recommended to reduce the risk of myocardial infarction, stroke, or vascular death in patients with symptomatic PAD. Aspirin (75 to 325 mg daily) and clopidogrel (75 mg daily) are considered safe and effective.7 In a randomized trial of patients with atherosclerosis, about one-third of whom had symptomatic PAD, clopidogrel demonstrated a relative risk reduction of 8.7% compared with 325 mg of aspirin, with a number needed to treat of 196 to prevent one additional cardiovascular event over one year.20 However, because this trial was not specifically designed to compare clopidogrel with aspirin in patients with PAD, aspirin at a dosage of 81 mg per day is considered first-line therapy. Clopidogrel may be substituted in patients with contraindications and in those who experience cardiovascular events while receiving aspirin therapy.
Antiplatelet therapy may also reduce the risk of myocardial infarction, stroke, or vascular death in asymptomatic patients with moderate to severe PAD. However, the evidence is inadequate to determine whether antiplatelet therapy improves cardiovascular outcomes in asymptomatic patients with mildly decreased ABI values (0.91 to 0.99).7,21
The phosphodiesterase inhibitor cilostazol suppresses platelet aggregation and is a direct arterial vasodilator. It has been shown to improve claudication symptoms and increase maximal and pain-free walking distances in patients with PAD by at least 50% compared with placebo and pentoxifylline (Trental). Cilostazol does not affect overall mortality in patients with PAD, and it is contraindicated in patients with a history of heart failure.22,23 Cilostazol can be used safely with aspirin or clopidogrel. Pentoxifylline is another antiplatelet agent commonly used for claudication. Because head-to-head studies have shown it to be less effective than cilostazol,24 it should be considered a second-line alternative.
The angiotensin-converting enzyme inhibitor ramipril (Altace) has been evaluated for treating functional limitations in patients with PAD. A randomized controlled trial comparing 10 mg of ramipril with placebo in patients with intermittent claudication reported a 77% increase in pain-free walking time and a 123% increase in maximum walking time in the treatment group at six months.25 However, the trial's publication was subsequently retracted when one of the authors admitted to fabricating data collection at one of the study sites (see Editor's Note). [corrected]
SURGICAL REFERRAL
Prognosis
A low ABI is an independent predictor of future cardiovascular events.27 Specifically, an ABI less than 0.9 is associated with a two- to fourfold relative risk increase for cardiovascular events and all-cause mortality.11 A systematic review included studies that measured ABI at baseline and tracked coronary heart disease, stroke, and all-cause mortality in patients who had not had a previous myocardial infarction or stroke.6 The results showed that a low ABI was 92.7% specific for predicting incident coronary heart disease. At five years, 20% of patients with PAD had had a nonfatal myocardial infarction, and 15% to 30% died; three-fourths of these were cardiovascular deaths.1,3,4,26
Data Sources: Essential Evidence Plus, PubMed, the Cochrane database, the Agency for Healthcare Research and Quality Evidence Reports, and UpToDate were searched using the key terms claudication, peripheral arterial disease, and peripheral vascular disease. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. Search dates: March 2012 to May 2012.
EDITOR'S NOTE: On September 14, 2015, JAMA published a Notice of Retraction regarding Reference #25 due to "an admission of fabricated results by Anna A. Ahimastos, PhD, who is both the first and corresponding author and was responsible for data collection and integrity for the article". The relevant text of the article has been modified in order to avoid misleading readers about the effectiveness of ramipril in treating functional limitations in patients with PAD.