
Am Fam Physician. 2000;62(1):95-102
A more recent article on interventional radiology is available.
Procedures performed by an interventional radiology specialist are becoming increasingly important in the management of patients with cancer. Although general interventional radiology procedures such as angiography and angioplasty are used in patients with and without cancer, certain procedures are reserved for the diagnosis and treatment of cancer or cancer-related complications. Interventional radiology procedures include imaging-guided biopsies to obtain samples for cytologic or pathologic testing without affecting adjacent structures. Transjugular liver biopsy is used to diagnose hepatic parenchymal abnormalities without traversing Glisson's capsule. This biopsy procedure is particularly useful in patients with coagulopathies. Because the transjugular liver biopsy obtains random samples, it is not recommended for biopsy of discrete hepatic masses. Fluid collections can also be sampled or drained using interventional radiology techniques. Transcatheter chemoembolization is a procedure that delivers a chemotherapeutic agent to a tumor along with sponge particles that have an ischemic effect on the mass. Tumor ablation, gene therapy and access of central veins for treatment are performed effectively under radiographic guidance. Cancer complications can also be treated with interventional radiology techniques. Examples include pain control procedures, vertebroplasty and drainage of obstructed organs. Interventional radiology techniques typically represent the least invasive definitive diagnostic or therapeutic options available for patients with cancer. They can often be performed at a lower cost and with less associated morbidity than other interventions.
Interventional radiology offers minimally invasive diagnostic and therapeutic procedures for cancer and cancer-related processes. Surgery, chemotherapy and radiation therapy remain the mainstays of cancer treatment, but interventional radiology continues to grow in importance as an alternative mode of therapy or an altogether new form of treatment for patients with cancer.
Many interventional radiology procedures can be performed on an outpatient basis or during a short hospital stay. Consequently, these procedures tend to be less expensive than other forms of therapy and frequently are associated with less morbidity.1,2 In one study,1 for example, radiologic placement of long-term hemodialysis catheters resulted in a 50 percent savings in total hospital costs compared with surgical placement of the same type of catheter.
The primary goals of the procedures performed in the interventional radiology suite can be categorized based on the purpose of each procedure. These goals include the diagnosis of cancer or cancer-related diseases, the treatment of cancer and the treatment of complications arising from cancer (Table 1).
| Diagnosis |
| Imaging-guided biopsy |
| Transjugular liver biopsy |
| Aspiration or drainage of fluid collections |
| Primary treatment of cancer |
| Transcatheter chemoembolization |
| Tumor ablation (sclerotherapy, radiofrequency ablation) |
| Transcatheter gene therapy (investigational) |
| Central venous access |
| Treatment of cancer-related complications |
| Pain control (celiac plexus ablation, transcatheter embolization) |
| Vertebroplasty |
| Drainage of obstructed organs |
| Drainage and sclerosis of pericardial fluid |
Diagnosis of Cancer or Cancer-Related Diseases
IMAGING-GUIDED BIOPSY
Many disease processes have a similar appearance on imaging studies. For example, a cavitary lung infection caused by tuberculosis or fungal disease may look identical to a cavitary neoplasm on a computed tomographic (CT) scan. Clearly, the need to differentiate these processes is vital to determining the most appropriate mode of therapy.
One of the most common interventional radiology procedures performed to diagnose or exclude cancer is imaging-guided biopsy. Under fluoroscopic, CT or ultrasound guidance, small needles can be placed in areas of abnormality, and samples can be taken for cytologic or pathologic testing. With imaging guidance, biopsies of an abnormality can be obtained while important adjacent structures, such as blood vessels or bowel, may be avoided. Often, imaging-guided biopsy is performed, instead of a surgical or open biopsy, to spare the patient a much more invasive procedure with its associated inherent risks.3
CT fluoroscopy, or real-time CT, is a new technologic development that aids in imaging-guided biopsies. Because this technique allows direct visualization of the needle during a procedure, it results in significant time savings.4
TRANSJUGULAR LIVER BIOPSY
Transjugular liver biopsy is a specialized procedure designed to decrease the risk of postbiopsy bleeding from the liver. This technique is particularly useful in patients with coagulopathies.
The procedure consists of the insertion of a long, thin (19- to 20-gauge) biopsy device into the right internal jugular vein and moving it into the right or middle hepatic vein. Random biopsy samples can then be obtained from the liver. Because the samples are essentially taken from the “inside-out,” without traversing the liver capsule, the risk of extracapsular hemorrhage is decreased.3,5
Transjugular liver biopsy is especially useful in the diagnosis of diffuse hepatic parenchymal abnormalities, such as graft-versus-host disease in patients who have undergone bone marrow transplantation. Because of the random nature of the samples obtained with this procedure, biopsy samples of specific hepatic masses are best obtained with other percutaneous imaging-guided biopsy procedures.
IMAGING-GUIDED FLUID ASPIRATION
Imaging-guided aspiration of fluid collections is another diagnostic aid. Either CT or ultrasonography can be used to place a small (18- to 22-gauge) needle into a fluid collection to determine whether the collection is benign or malignant.
If indicated, a drainage catheter may be placed at the same time as the needle aspiration. Catheter drainage may be especially helpful in patients with infected fluid collections (Figure 1) or fluid collections that otherwise might require surgical drainage (e.g., pericardial effusions in pericardial tamponade or loculated fluid collections).
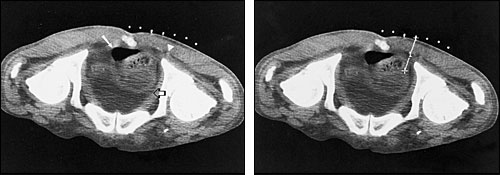
Sclerosing agents may also be injected into the indwelling catheter. This measure can decrease the likelihood of fluid reaccumulation in patients with conditions such as recurrent malignant pleural effusions.
Treatment of Cancer
In the past, cancer treatment in the radiology department typically consisted of external-beam radiotherapy delivered by a radiation oncologist. Recent advances in interventional radiology, such as transcatheter chemoembolization and tumor ablation, have expanded the role of the radiologist to include the direct delivery of chemotherapeutic and radiotherapy agents to malignancies. New treatment modalities have also been introduced.
TRANSCATHETER CHEMOEMBOLIZATION
For transcatheter chemoembolization, a chemotherapeutic agent is mixed with small sponge particles and injected into an artery that supplies a tumor. With this direct delivery technique, far lower dosages of the chemotherapeutic agent are needed than when the agent is delivered systemically. Concurrent injection of sponge particles causes vascular stasis and has an ischemic effect on the tumor itself. The sponge particles also decrease blood flow through the tumor and prolong the time that the chemotherapy agent is in contact with tumor cells.
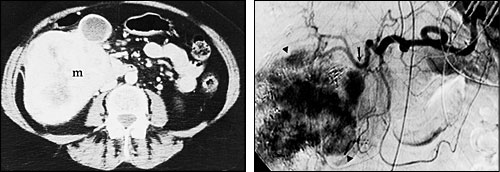
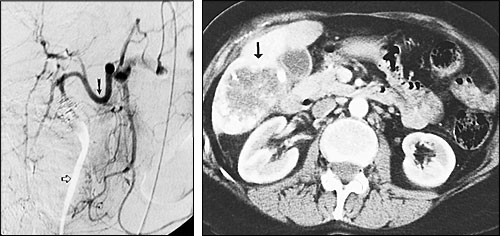
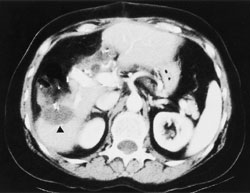
Hepatic transcatheter chemoembolization is especially useful in patients with primary hepatocellular carcinoma and in patients with hypervascular metastases that are confined to the liver.6–9 Two-year survival rates for patients with hepatocellular carcinoma who are treated with this procedure are typically 30 to 40 percent, compared with a survival rate of less than 10 percent in untreated patients.8 The procedure has been found to provide symptomatic relief in 80 to 90 percent of patients with metastatic neuroendocrine tumors.9
Patients who undergo transcatheter chemoembolization are typically hospitalized for only one or two days, and the procedure may be repeated multiple times in the same patient. The most common side effects are low-grade fever and pain. These side effects, which occur in fewer than 5 percent of treated patients, generally are well controlled with conservative therapy.6–9
TUMOR ABLATION
Until a few years ago, tumor ablation therapy consisted of the injection of sclerosing agents (e.g., absolute alcohol) into metastatic or primary tumors of the liver.10,11 In one large series,10 patients with a single hepatocellular carcinoma who underwent alcohol ablation had a five-year survival rate of 48 percent, which was comparable to the five-year survival rate (49 percent) in a cohort of patients who underwent liver resection. Although alcohol ablation therapy has been a successful mode of therapy, its use has generally been confined to patients with cirrhosis whose tumors are anatomically amenable to a percutaneous approach.
Radiofrequency ablation is a relatively new treatment modality. For this procedure, a small (15- to 18-gauge) needle, attached to a radiofrequency device that heats the end of the needle, is inserted into a tumor, causing coagulation necrosis of the surrounding tumor. Early reports on the applications of this procedure in liver tumors have been quite promising,12–16 with one-year disease-free survival rates often exceeding 50 percent.12,15,16
Studies comparing radiofrequency ablation and other modes of therapy are currently being conducted. In the near future, the clinical utility of and indications for radio frequency ablation are likely to expand rapidly to include the treatment of other types of tumors.
GENE THERAPY
Currently undergoing clinical investigation, interventional radiology is likely to play a highly significant role in gene therapy for the treatment of cancer. It will probably be used to deliver the gene-carrying vector to the tumor. At present, this delivery is confined to systemic intravascular administration or to percutaneous placement of vectors directly into a tumor through a needle.17,18 Significant advances in the delivery of gene therapy have been achieved in patients without cancer, and transvascular delivery of gene-carrying vectors through an angiographic catheter is soon to be explored.19
VENOUS ACCESS
Although not a direct form of cancer therapy, the placement of central venous access devices is increasingly coming under the realm of interventional radiology. With imaging guidance and under sterile conditions, the placement of ports, tunneled catheters and nontunneled devices (e.g., peripherally inserted central catheter lines) can usually be performed in a more cost-effective and time-effective manner in the interventional radiology suite than in the operating room.2,20–22
Technical success rates for the imaging-guided placement of central venous access devices approach 100 percent, and complication rates are typically more favorable than those for surgical placement.2,20–22 As a result, many interventional radiology departments currently devote more than one half of their time to the placement of these devices.
Treatment of Cancer-Related Complications
Complications arising from cancer include primary or metastatic disease causing pain or bleeding, obstruction of vital organs such as ureters and biliary ducts, infectious complications in immunocompromised patients, and thromboembolic disease of the lower extremities. Some treatments performed by interventional radiology for cancer complications (e.g., abscess drainage catheter placement or inferior vena cava filter placement) are identical to those performed in patients without cancer; these procedures are not discussed in detail in this article.
PAIN CONTROL
Pain control is essential in patients with cancer. Pain often arises from local spread of a tumor, such as invasion of the celiac plexus in a patient with upper gastrointestinal or pancreatic cancer. Ablation of the celiac plexus with a sclerosing agent can be easily and safely performed under imaging guidance in the interventional radiology suite, and this procedure often greatly relieves the patient's pain.23,24
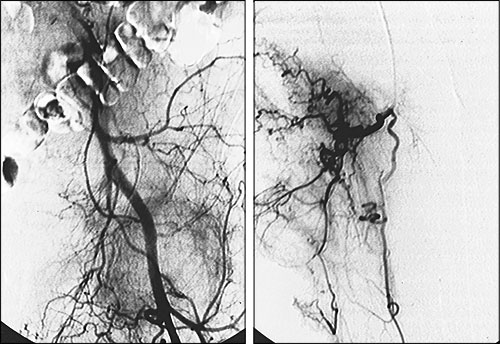
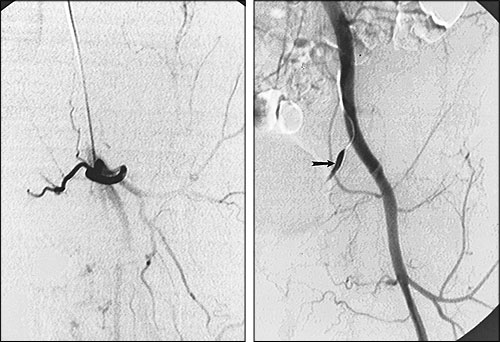
If a patient's pain arises from a hypervascular process, such as renal carcinoma that metastasizes to bone, transcatheter embolization (injecting small sponge particles through a catheter placed into the artery supplying the tumor) may significantly ease the patient's pain and decrease the likelihood of pathologic fracture (Figures 5 and 6). In one series,25 palliative transcatheter embolization totally relieved bone pain in 63 percent of patients; in 100 percent of treated patients, opiates were no longer required for pain control.
VERTEBROPLASTY
Vertebroplasty is a relatively new technique in which a strong glue is injected through a needle into a collapsed or weakened vertebra to keep the vertebra from collapsing further and causing symptoms of cord compression.26–28 Vertebroplasty is still under investigation, particularly in patients with cancer, but early results are quite promising. In one study,29 pain control was noted in 94 percent of patients immediately after vertebroplasty, and stable pain control was present in 73 percent of patients six months after the procedure.
DRAINAGE
Percutaneous placement of drainage catheters, such as percutaneous nephrostomy tubes and biliary drainage catheters, is performed under imaging guidance to allow external drainage of obstructed urine or bile for the purpose of preventing or treating organ failure and infection.
In addition to placing external drains, it is often possible to place a stent in an obstructed organ to bypass the obstruction and facilitate internal drainage. The placement of metal and plastic stents in the interventional radiology suite is performed from an entirely percutaneous approach, precluding the need for surgery or endoscopy (Figure 7).
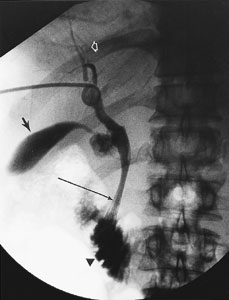
A relatively new interventional radiology procedure involves the placement of drainage catheters in pericardial fluid collections to allow drainage of the fluid and provide access to the pericardial space for the injection of sclerosing agents.30–33 Pericardial drain placement decreases the need for a surgical pericardial window procedure (an involved procedure performed in an already hemodynamically compromised patient). The role of pericardial catheter placement and subsequent sclerosis is still being investigated.30–32
Final Comment
The role of interventional radiology in the care of patients with cancer continues to expand. Radiologists, who have traditionally been relegated to the role of diagnosis in cancer patients, are becoming more involved in the treatment of cancer and its complications.
Family physicians are encouraged to communicate directly with an interventional radiology specialist to determine the utility of interventional radiology procedures in the individual patient. Resources for physician referrals or patient communications can be obtained from the Society of Cardiovascular and Interventional Radiology (10201 Lee Highway, Suite 500, Fairfax, VA 22030; telephone: 703–691–1805; Web site: www.scvir.org).