
Am Fam Physician. 2001;64(7):1183-1191
See patient information handout on refractive surgery, written by the authors of this article.
Patients with myopia, hyperopia and astigmatism can now reduce or eliminate their dependence on contact lenses and eyeglasses through refractive surgery that includes radial keratotomy (RK), photorefractive keratectomy (PRK), laser-assisted in situ keratomileusis (LASIK), laser thermal keratoplasty (LTK) and intrastromal corneal rings (ICR). Since the approval of the excimer laser in 1995, the popularity of RK has declined because of the superior outcomes from PRK and LASIK. In patients with low-to-moderate myopia, PRK produces stable and predictable results with an excellent safety profile. LASIK is also efficacious, predictable and safe, with the additional advantages of rapid vision recovery and minimal pain. LASIK has rapidly become the most widely performed refractive surgery, with high patient and surgeon satisfaction. Noncontact Holium: YAG LTK provides satisfactory correction in patients with low hyperopia. ICR offers patients with low myopia the potential advantage of removal if the vision outcome is unsatisfactory. Despite the current widespread advertising and media attention about laser refractive surgery, not all patients are good candidates for this surgery. Family physicians should be familiar with the different refractive surgeries and their potential complications.
Extensive television, radio and newspaper advertising have promoted laser vision correction, and the public has a variety of refractive procedures from which to choose. Because of this media attention, many patients will be asking their family physician about these procedures. Family physicians should be familiar with the indications and contraindications of the refractive procedures, as well as the results, follow-up courses and potential complications.
Anatomy of the Cornea
The transparent cornea is about 0.5 mm thick, with five distinct layers: the epithelium, Bowman's membrane, stroma, endothelium and Descemet's membrane. The epithelium is the most exterior layer, providing the smooth refractive surface and serving as a barrier against infection. The function of Bowman's membrane, which lies beneath the epithelium and its basement membrane, is unclear. The stroma, made up of intertwining lamellae of collagen fibrils, provides structure to the cornea and accounts for 90 percent of the corneal thickness. The endothelium and its basement membrane (Descemet's membrane) form the innermost layers. Endothelial cells, via an active sodium potassium–adenosine triphosphatase pump, are responsible for the relative corneal dehydration necessary for corneal clarity.
Optics, Refraction and Refractive Error
Refraction is the bending of light rays as they pass from one transparent medium to another medium of a different density; it is measured in diopters. The refractive power of a lens is the reciprocal of its focal length measured in meters (e.g., a one-diopter lens has a focal point of 1 m; a two-diopter lens has a focal length of 0.5 m). The cornea and the crystalline lens refract light that enters the eye. The cornea is responsible for two thirds of the eye's total focusing power, while the crystalline lens accounts for the remaining one third. The focusing power of the cornea is fixed, whereas the focusing power of the crystalline lens is not. Through a process called accommodation, the lens changes its shape to bring objects into focus.
In emmetropia (an eye with normal vision), the focusing powers of the cornea and the lens are perfectly matched to the length of the globe. When a person with normal vision acuity views an object, the cornea and the lens focus the parallel light rays emitted from the object precisely on the retina (Figure 1a), and a clear image is perceived. The eye's focal point is at infinity.
Refractive errors occur when the cornea and the lens do not properly focus the light rays on the retina. In myopia (nearsightedness), the most common type of refractive error, the cornea is too curved or the lens too powerful for the length of the globe. Distant objects cannot be seen clearly because light rays are focused in front of the retina (Figure 1b); however, near objects appear clear. Concave lenses with minus or divergent power correct this refractive error and refocus the light rays on the correct point on the retina.
In hyperopia (farsightedness), the cornea is too flat or the lens too weak for the length of the globe. As a result, the cornea and lens focus the light rays behind the retina (Figure 1c). The process of accommodation may bring a distant object into focus; however, near vision is unclear. Convex lenses with plus or convergent power correct this refractive error and refocus the light rays to the correct point on the retina.
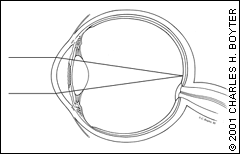
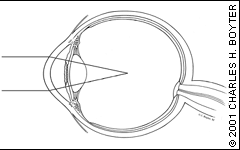
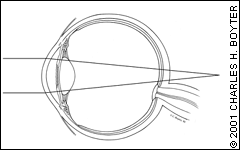
In astigmatism, the refractive power of the eye is different in different meridians. The cornea and the lens cannot bring the light rays to the precise point on the retina to provide clear vision; thus, objects will appear blurry at any distance. Astigmatism may occur with myopia or hyperopia.
Refractive Surgery
Radial keratotomy (RK), photorefractive keratectomy (PRK), laser-assisted in situ keratomileusis (LASIK), laser thermal keratoplasty (LTK) and intrastromal corneal rings (ICR) are the most common refractive surgeries presently performed in the United States in the treatment of patients with myopia, hyperopia and astigmatism. The object of these procedures is to change the refractive state of the eye by changing the shape of the cornea.
RADIAL KERATOTOMY
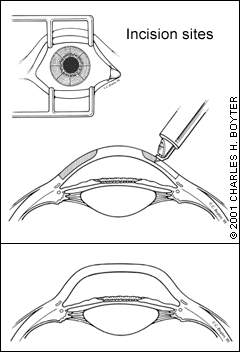
In the past, RK was performed to treat patients with myopia. The surgeon makes a number of microscopic corneal incisions in a radial or spoke-like pattern. This allows the outer cornea to relax so that the central cornea flattens (Figure 2). The new shape of the cornea is permanently retained as the cornea heals.
Potential serious complications include loss of best-corrected vision acuity, perforation of the cornea, infection and rupture of the globe. Some of the major concerns with this procedure relate to the significant corneal instability induced by the surgery, including diurnal fluctuation of refractive error, overcorrection, hyperopic shift and potential rupture of the globe with blunt trauma.1 This procedure has declined in popularity since 1995, when the U.S. Food and Drug Administration (FDA) approved the use of the excimer laser, and because of the superior results of the other commonly performed refractive surgeries.
THE EXCIMER LASER
The excimer laser is used to perform PRK and LASIK procedures and works by changing the shape of the cornea. The excimer laser emits an ultraviolet beam that has sufficient energy to break intermolecular bonds within the cornea (photoablation). Because little or no thermal damage occurs to adjacent tissue, this is often referred to as a “cool” laser beam. A computer, programmed with the patient's refraction and corneal topography, controls the laser beam to precisely remove corneal tissue.2 With improving technology, the width of the laser beam has continued to decrease to less than 100μ. In addition, laser eye-tracking systems are now available that allow precision corneal ablation during eye movements.3
In myopia, the laser flattens the central cornea to decrease its focusing power. In hyperopia, the laser indirectly steepens the central cornea by removing tissue from the periphery, thus increasing the cornea's focusing power. Astigmatism is treated with an elliptic or cylindrical beam that flattens the steepest corneal meridian (Figure 3).
Not every patient is a candidate for treatment using the excimer laser. Age, high refractive error, and ocular and medical disease may prevent a patient from obtaining a predictable refractive outcome. Table 1 reviews patient selection criteria for PRK and LASIK procedures.
PHOTOREFRACTIVE KERATECTOMY
PRK effectively treats patients with low-to-moderate myopia, myopia with astigmatism and low-to-moderate hyperopia without astigmatism.3–10 It is performed on an outpatient basis using topical anesthetic. The corneal epithelium in the ablation zone is first removed or pushed to the side to allow a more accurate ablation of corneal tissue. The laser treatment is then applied to the exposed corneal stroma.4 Immediately following the laser treatment, the ophthalmologist applies topical antibiotic, steroid and nonsteroidal anti-inflammatory drugs (NSAIDs). A disposable bandage contact lens is then placed over the cornea.
| Age 18 years or older | |
| Stable refraction of at least one year's duration | |
| Myopia between −0.50 and −12.00 diopters | |
| Astigmatism 5.00 diopters | |
| Hyperopia < +6.00 diopters | |
| Absence of ocular contraindications: | |
| Keratoconus | |
| Herpetic keratitis | |
| Progressive myopia | |
| Corneal disease† | |
| Glaucoma† | |
| Cataract† | |
| Any other preexisting pathology of the cornea or anterior segment, including scarring, agophthalmos, dry eye and blepharitis† | |
| Absence of medical contraindications: | |
| Uncontrolled vascular disease | |
| Autoimmune disease | |
| Immunosuppressed/immunocompromised | |
| Pregnant or nursing | |
| History of keloids | |
| Diabetes mellitus† | |
During the early postoperative period, patients may experience significant tearing, photophobia, blurred vision and discomfort because of the central corneal abrasion. With the use of the bandage contact lens and NSAID eyedrops, postoperative pain is usually mild to moderate; however, patients occasionally require systemic analgesia for more severe pain. The contact lens remains on the eye until the epithelial defect is healed (an average of three to four days). Antibiotic therapy is usually continued for two to three days after the defect has healed, and topical steroid eye drops may be continued for up to three months postoperatively.5
Vision acuity improves once the epithelial defect heals—usually within one week postoperatively. Vision acuity typically fluctuates following surgery before stabilizing at around three months postoperatively. Glare, halos and dry-eye symptoms are common during the first month following surgery but usually diminish or disappear entirely by three to six months postoperatively.
LASER-ASSISTED IN SITU KERATOMILEUSIS
A microkeratome, which works like a carpenter's plane, is used to raise a corneal flap about the size of a contact lens (Figure 4). This flap, which averages 160μ in thickness, is folded back to expose the underlying stroma.14 The excimer laser is used to ablate a precise amount of corneal stroma, and the flap is irrigated and placed back in its original position. The corneal flap is stabilized without sutures by the relative corneal dehydration created by the endothelial pump. The stability of the corneal flap and adherence to the corneal stroma is checked following surgery, and patients are usually sent home with topical steroid, topical antibiotic and topical NSAID eye drops. In addition, the patient is instructed to use an eye shield overnight, with follow-up typically scheduled on postoperative day 1 and again at one week.18 The patient is usually checked again at one, three and six months.
The LASIK procedure has significant attractions for patients. It causes little pain, provides quick recovery of vision and has the potential for treating higher levels of myopia.15 LASIK has also been found to be safe and effective in treating both eyes on the same day; whereas, PRK is usually performed on two separate days.19
LASIK enhancements are more easily performed (at least within the first six to 12 months following the initial surgery) by lifting the original corneal flap and re-treating the stromal bed to correct any residual refractive error. Unlike PRK, LASIK does not produce stromal haze, and patients who have undergone this procedure do not usually require topical steroid eye drops beyond the first postoperative week. At one year postoperatively, patients who have undergone the LASIK procedure are more satisfied than patients who have undergone the PRK procedure (90 versus 52 percent, respectively).11 Despite the different surgical techniques of PRK and LASIK, the refractive outcomes are similar.6,7,11 Table 23,7,8,11 compares outcomes of PRK and LASIK.
LASER THERMAL KERATOPLASTY
LTK is performed using the noncontact Holmium: YAG laser. The ophthalmologist uses the laser to symmetrically place radial spots that are outside the visual axis. This heats the cornea, resulting in stromal collagen shrinkage, thus modifying the anterior corneal curvature. This procedure is performed under topical anesthetic with a variable follow-up schedule.
| Procedure | UCVA efficacy of 20/20 (%) | UCVA efficacy of 20/40 (%) | Predictability within one diopter (%) | Satisfaction (%) |
|---|---|---|---|---|
| PRK | 53 to 86 | 94.4 to 98.1 | 87 to 94.4 | 52 |
| LASIK | 67 to 83 | 97 to 100 | 90 to 98.7 | 90 |
LTK is used to treat patients with a hyperopic refractive error of up to +4.00 diopters who have become presbyopic and have no ocular pathology. The refractive outcomes vary with the amount of laser treatment.20 The most common complications include irregular, induced astigmatism and hyperopic regression with a need for retreatment using LTK or the excimer laser. LTK is safe, effective and provides satisfactory correction of low hyperopia with minimal complications.21
INTRASTROMAL CORNEAL RING
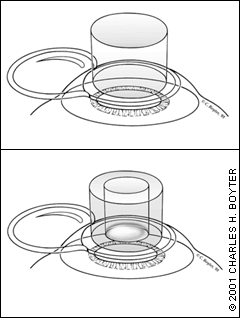
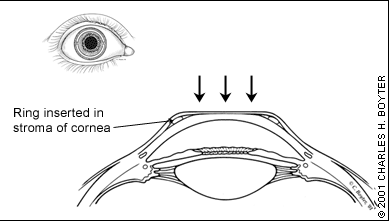
Results of preliminary studies indicate that ICR is a promising option in correcting myopic refractive errors of less than −3.00 diopters.22 During this procedure, the ophthalmologist places a polymethylmethacrylate ring into the periphery of the cornea at about two thirds of its depth. The ring causes the cornea to flatten, thus correcting the refractive error (Figure 5). The benefits of the ICR include rapid vision recovery following placement (because surgical manipulation does not occur over the central cornea/visual axis). An added benefit is that the ophthalmologist can remove the device at any time. Risks include infection, abnormal wound healing and irregular astigmatism.23
POSTOPERATIVE COMPLICATIONS
Complications of refractive surgery may arise intraoperatively or during the postoperative period (Tables 31,9,24–31 and 4). With the LASIK procedure, intraoperative flap complications that occur with the use of the microkeratome include incomplete flaps, irregular flaps, small flaps, flap decentration, buttonhole flaps or free cap flaps.18,24 Early postoperative complications include dislodging of the flap from the corneal stroma, flap striae, interface debris, epithelial downgrowth into the flap, corneal stroma interface or a sterile inflammatory response termed diffuse lamellar keratitis, or “Sands of Sahara” syndrome.18,25,26
| PRK and LASIK | More common with PRK | More common with LASIK |
|---|---|---|
| Overcorrection/undercorrection | Postoperative pain | Flap complications |
| Astigmatism | Delayed epithelial healing | Epithelial ingrowth |
| Regression | Infection | Diffuse lamellar keratitis, or “Sands of Sahara” syndrome |
| Glare, halo, monocular diplopia | Scarring/corneal haze | — |
| Dry-eye symptoms | — | — |
| Reduced contrast sensitivity | — | — |
Intraoperative PRK complications most commonly include decentration of the laser ablation and central islands of higher refractive power.4,27,28 Postoperative complications include pain secondary to an epithelial defect and/or delayed epithelial healing, which may increase the risk of infection, as well as late haze formation and corneal scarring.27
With any procedure using the excimer laser, the refractive outcome may not always result in an uncorrected vision acuity or best-spectacle corrected vision acuity of 20/20 or better. Some patients may develop a worsening of vision clarity and acuity secondary to scarring, glare, halos, monocular diplopia and reduced contrast sensitivity.4,5,7,29,30 Patients may have a postoperative overcorrection, undercorrection and astigmatism that may need an enhancement to correct the residual refractive error.6,9,10,17,32 Furthermore, following excimer laser refractive surgery, most patients will complain of dry-eye symptoms secondary to disruption of the corneal nerve innervation. These patients are most effectively treated with nonpreserved artificial tears or punctual plugs, as needed; the dry-eye symptoms usually resolve within three months.
| Decreased vision acuity |
| Pain secondary to epithelial abrasion or LASIK flap complication |
| Redness, with or without drainage, secondary to infection |
Finally, patients should understand that there is a possibility they may still require correction with eyeglasses or contact lenses in order to obtain the best-vision acuity and that over time, postoperative refractive error regression may require additional laser treatment.31