
A more recent article on common types of supraventricular tachycardia is available.
Am Fam Physician. 2002;65(12):2479-2487
This is part I of a two-part article on common arrhythmias. Part II, “Ventricular Arrhythmias and Arrhythmias in Special Populations,” appears on page 2491 of this issue.
Family physicians frequently encounter patients with symptoms that could be related to cardiac arrhythmias, most commonly atrial fibrillation or supraventricular tachycardias. The initial management of atrial fibrillation includes ventricular rate control to provide adequate cardiac output. In patients with severely depressed cardiac output and recent-onset atrial fibrillation, immediate electrical cardioversion is the treatment of choice. Hemodynamically stable patients with atrial fibrillation for more than two days or for an unknown period should be assessed for the presence of atrial thrombi. If thrombi are detected on transesophageal echocardiography, anticoagulation with warfarin for a minimum of 21 days is recommended before electrical cardioversion is attempted. Patients with other supraventricular arrhythmias may be treated with adenosine, a calcium channel blocker, or a short-acting beta blocker to disrupt reentrant pathways. When initial medications are ineffective, radiofrequency ablation of ectopic sites is an increasingly popular treatment option.
Heart palpitations and cardiac arrhythmias are common problems encountered by family physicians. Patients may present with acute cardiac rhythm abnormalities. Although these arrhythmias are usually benign, they can indicate significant underlying heart disease. More often, patients have chronic arrhythmias, such as atrial fibrillation, that may require treatment to reduce the risk of future complications. The challenges for the family physician are to determine which arrhythmias are benign and which indicate probable cardiac malfunction, and to manage recurrent or chronic rhythm abnormalities.
This two-part article reviews common atrial and ventricular arrhythmias, with a focus on initial management decisions. Part I discusses supraventricular arrhythmias. Part II discusses ventricular arrhythmias and the management of rhythm abnormalities in special populations, including pregnant women, athletes, and children.
Atrial Fibrillation
Atrial fibrillation is the most common cardiac arrhythmia family physicians are likely to encounter. This rhythm abnormality affects 3 to 5 percent of patients more than 60 years of age1 and becomes increasingly common with advancing age. The median age of patients with atrial fibrillation is 75 years, and the prevalence of the arrhythmia doubles every 10 years after the age of 55.2,3 In the United States, atrial fibrillation is estimated to affect almost 9 percent of patients more than 75 years of age.2
Most risk factors for atrial fibrillation are associated with structural or ischemic heart disease. Risk factors include hypertension, left ventricular hypertrophy, dilated and restrictive cardiomyopathies, coronary artery disease, chronic obstructive pulmonary disease, and diabetes in women.1
The annual risk of stroke in patients with atrial fibrillation and normal valve function has been reported to be 4.5 percent per year.4 Anticoagulation with warfarin (Coumadin) reduces the risk by about two thirds.4 The mortality rate for stroke in patients with atrial fibrillation is approximately twice as high as the rate in patients without this rhythm abnormality.5 Although anticoagulation is contraindicated in some elderly patients, a study in Great Britain6 found that about 60 percent of patients identified in community screenings as having atrial fibrillation were eligible for, and would benefit from, this treatment.
MANAGEMENT
The first step in managing a patient with atrial fibrillation is to decide whether there is a high likelihood of safe conversion to sinus rhythm or whether the patient should be allowed to remain in atrial fibrillation. A patient with recent onset of atrial fibrillation (within the previous 12 months) and no evidence of enlargement of the left atrium has a greater chance of achieving and maintaining sinus rhythm. If the arrhythmia is long-standing and the patient is not a suitable candidate for rate cardioversion, initial treatment should focus on ventricular rate control, with consideration given to long-term stroke prophylaxis.
Restoration of Sinus Rhythm
Patients who present within 48 hours of the onset of new atrial fibrillation are candidates for cardioversion with a low risk of embolism. Conversion to sinus rhythm can be attempted by electrical shock or with antiarrhythmic drugs. Patients who have been in atrial fibrillation for more than 48 hours or for an undetermined period are more likely to have atrial thrombi and may develop emboli with immediate electrical or medical (pharmacologic) cardioversion.
Atrial thrombi are not evident on transthoracic echocardiograms, but they can been seen on transesophageal echocardiograms.7 If the transesophageal echocardiogram reveals thrombi, anticoagulation is recommended before cardioversion is attempted. Anticoagulation can be accomplished using warfarin, with the dosage adjusted to achieve an International Normalized Ratio (INR) between 2.0 and 3.0 for a minimum of 21 days.8
If the transesophageal echocardiogram does not show thrombi on multiplane views, cardioversion can be attempted. Short-term anticoagulation with heparin should be started before the procedure, and warfarin therapy should be initiated after cardioversion.8
When rhythm conversion is indicated, it can be accomplished using direct-current cardioversion or pharmacologic therapy. Synchronized cardioversion is currently considered the treatment of choice for the restoration of sinus rhythm and, in appropriately selected patients, has a success rate of at least 80 percent.4 Cardioversion is also indicated in patients with hypotension, angina, heart failure, or other evidence of severe compromise caused by atrial fibrillation.5
Medical cardioversion of atrial fibrillation may be achieved with class IA drugs (quinidine, disopyramide [Norpace], procainamide [Procanbid]) or with amiodarone (Cordarone). In the past, quinidine was frequently used for both cardioversion and maintenance of sinus rhythm in patients who had undergone electrical cardioversion. However, because of the proarrhythmic action of class IA agents and their detrimental effects on left ventricular function, these drugs are now used less often than amiodarone for primary therapy of atrial fibrillation.4
Amiodarone therapy is successful in 86 percent of patients who have had atrial fibrillation for less than two years.4,9 Treatment is also effective in 40 to 60 percent of patients with long-standing atrial fibrillation that has been resistant to other agents and to electrical cardioversion.4 Amiodarone can be given in a dosage of 200 mg a day, which is lower than the dosages that have been associated with thyroid abnormalities and pulmonary fibrosis. Although there is little risk of toxicity when amiodarone is given in a low dosage, it is prudent to monitor patients for the development of thyroid, pulmonary, hepatic, and cardiac side effects.
Findings on the usefulness of various agents for the conversion of atrial fibrillation, based on the evidence-based practice program of the Agency for Healthcare Research and Quality, are summarized in Table 1.10 Although drugs such as digitalis preparations and sotalol (Betapace) are sometimes used for rate control, they are not effective for converting atrial fibrillation to sinus rhythm.10,11
| Drug and class | Usual oral dosing | Odds ratio for conversion compared with placebo (95% CI)* |
|---|---|---|
| Flecainide (Tambocor): class IC | 50 mg every 12 hours; increase by 50 mg per day every 4 days to maximum of 300 mg per day. | 24.7 (CI: 9.0 to 68.3) |
| Ibutilide (Corvert) given IV, followed by dofetilide (Tikosyn) given orally: both class III | Ibutilide: 0.01 mg per kg IV over 10 minutes; if first dose is not effective, give second infusion 10 minutes later (maximum dose: 1 mg). | 29.1 (CI: 9.8 to 86.1) |
| Dofetilide: 0.1 to 0.5 mg every 12 hours | ||
| Disopyramide (Norpace): class IA | 100 to 200 mg every 6 to 8 hours | 7.0 (CI: 0.3 to 153) |
| Amiodarone (Cordarone): class III | 800 to 1,600 mg per day for 7 to 14 days; then 200 to 400 mg per day as maintenance | 5.7 (CI: 1.0 to 33.4) |
| Propafenone (Rythmol): class IC | 150 mg every 12 hours; if needed, increase dose every 3 to 4 days to maximum of 300 mg every 12 hours. | 4.6 (CI: 2.6 to 8.2) |
| Quinidines: class IA | Quinidine sulfate (Quinidex): 400 mg every 6 hours | 2.9 (CI: 1.2 to 7.0) |
| Quinidine gluconate (Quinaglute): 648 mg every 8 to 12 hours |
If external electrical cardioversion is unsuccessful and antiarrhythmic drug therapy fails, other measures can be used. However, these approaches are usually reserved for use in patients who cannot tolerate atrial fibrillation and patients who have associated systolic dysfunction. Techniques include internal electrical cardioversion through the application of electrical current to pulmonary veins via a transcatheter cathode4 and radiofrequency ablation of the atrioventricular node with insertion of a ventricular pacemaker.12 In addition, an implantable atrial defibrillator can be used to provide rapid cardioversion in patients with atrial fibrillation that cannot be controlled with medications.13
Rate Control in Chronic Atrial Fibrillation
In patients in whom rhythm conversion is not indicated or those who have new-onset atrial fibrillation with a rapid ventricular response, treatment may be needed to control the ventricular rhythm. Excessive ventricular rates may result in diminished cardiac output because of poor filling time, and in ischemia because of increased myocardial oxygen demand. Medications used for ventricular rate control in patients with atrial fibrillation are listed in Table 2.14
| Drug | Dosing | Side effects and complications | |
|---|---|---|---|
| Calcium channel blockers | |||
| Diltiazem (Cardizem) | Acute IV: 0.25 mg per kg over 2 minutes; then 0.35 mg per kg after 15 minutes if needed; then 10 mg per hour in drip if needed | Acute: heart block, CHF, hypotension (∼3%) | |
| Oral maintenance: 180 to 240 mg per day | Long-term: constipation | ||
| Verapamil (Calan) | Acute IV: bolus of 5 to 10 mg over 2 minutes; may repeat 10 mg in 15 to 30 minutes | Acute: heart block, CHF, hypotension (∼5% to 10%) | |
| Oral maintenance: 240 to 320 mg per day | Long-term: constipation | ||
| Beta blockers | |||
| Propranolol (Inderal) | Acute IV: 1 to 3 mg at 1 mg per minute; repeat in 2 minutes if needed. | Acute: heart block, bronchospasm, CHF | |
| Oral maintenance: 10 to 30 mg every 6 to 8 hours | Long-term: fatigue, depression | ||
| Esmolol (Brevibloc) | Acute IV: 0.5 mg per kg over 1 minute; then 0.05 mg per kg per minute by IV drip for 4 minutes | Acute: hypotension (20% to 50%), heart block, CHF | |
| Digoxin (Lanoxin) | Acute IV: 0.25 to 0.50 mg; then 0.25 mg every 4 to 6 hours to total of 1.0 mg | Heart click, visual disturbances, delirium, hallucinations | |
| Oral maintenance: 0.125 to 0.25 mg per day | |||
Acute management of ventricular rates can usually be achieved with intravenously administered diltiazem (Cardizem), given in an initial bolus of 15 to 20 mg (0.25 mg per kg) over two minutes, or with an intravenously administered beta blocker such as propranolol (Inderal), given in a dose of 0.5 to 1 mg (up to 3 to 5 mg if needed).
A number of medications, including calcium channel blockers, beta blockers, and digoxin (Lanoxin), are effective for maintaining ventricular rates within acceptable ranges. Because calcium channel blockers are associated with better exercise tolerance, they may be preferable to beta blockers.15 Digoxin is associated with a high degree of exercise intolerance; therefore, it should be reserved for use in patients who are relatively immobile, who cannot tolerate other treatment options, or who have significant ventricular dysfunction.
Paroxysmal Supraventricular Tachycardias
Based on duration, supraventricular tachycardias are usually categorized as paroxysmal, persistent, or chronic. Paroxysmal supraventricular tachycardia (PSVT) is the most common of these arrhythmias and the one that is most often encountered in the primary care setting. Longer-duration supraventricular tachycardias can be treated similarly to PSVT, but cardiology consultation is often required to identify the electrophysiologic mechanism responsible for sustaining the arrhythmia. In contrast to ventricular tachycardias (discussed in part II of this article) and atrial fibrillation, PSVT is usually a narrow-complex tachycardia with a regular rate.
MECHANISMS
Atrioventricular Nodal Reentry Causing PSVT
Atrioventricular nodal reentry, the most common mechanism of PSVT, occurs when two pathways exist with different conduction rates. A premature atrial complex that is blocked in the fast pathway and redirected through the slow pathway usually triggers the tachycardia (Figure 1). The electrical signal proceeds down the slow pathway and then reenters the fast pathway in a retrograde direction. By the time the signal has propagated down the slow pathway and back around on the fast pathway, the slow pathway is no longer refractory and is ready to conduct the signal again, completing a continuous circuit.
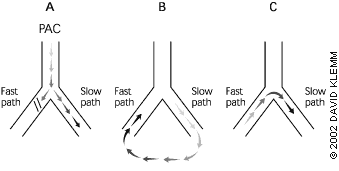
Reentrant tachycardias usually produce a narrow-complex tachycardia with no discernible P wave. The rate is usually between 160 and 190 beats per minute. In a less common form of atrioventricular nodal reentrant tachycardia, the circulating wavefront proceeds in an antegrade fashion down the fast pathway and in a retrograde fashion up the slow pathway. In this form, inverted P waves (Figure 2) are clearly visible in lead II of the electrocardiogram (ECG).
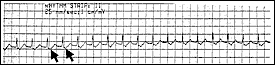
It is important to note that atrioventricular nodal reentrant tachycardia can result in a wide-complex tachycardia if the patient has preexisting bundle branch block.
Accessory Pathways Causing PSVT
Accessory pathways (Wolff-Parkinson-White syndrome) and other bypass tracts can cause PSVT. In patients with Wolff-Parkinson-White syndrome, a shortened PR interval and a slurred upstrike to the QRS complex “delta wave” on the resting ECG indicate the presence of an accessory pathway (Figure 3).
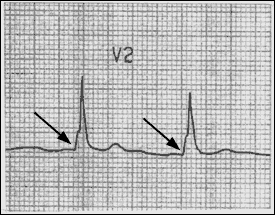
It should be noted that the resting ECG may be normal in some patients with Wolff-Parkinson-White syndrome, because of the inability of the accessory pathway to conduct in the antegrade direction. The usual mechanism of PSVT in this setting is antegrade conduction down the normal pathways through the atrioventricular node and retrograde conduction through the accessory pathway.
The ECG in an atrial arrhythmia with an accessory pathway usually shows a narrow-complex tachycardia at rates of 160 to 240 beats per minute. Delta waves are absent because the normal pathways are used for ventricular activation. Inverted P waves may be seen in the inferior leads. In a much less common form of PSVT, antegrade conduction is down the bypass tract and results in a wide-complex tachycardia.
Increased Automaticity Causing PSVT
Increased automaticity usually occurs when the atrium is enlarged, as in patients with chronic lung disease, congestive heart failure, or electrolyte and acid-base disturbances. Usually, the stretched atria fire irregularly, producing multiple premature beats that emanate from different areas of the atria. Because the foci for the ectopic beats are in multiple sites, the P waves vary in morphology, giving rise to the term “multifocal atrial tachycardia.”
The diagnosis of multifocal atrial tachycardia depends on the identification of an irregular rhythm with three or more different P-wave morphologies. The rate is usually between 130 and 180 beats per minute. Treatment is directed at correcting the underlying cause. Antiarrhythmic drugs are usually not helpful.
MANAGEMENT
In most patients, PSVT is benign and self-limited. However, some patients can have angina, hypotension, and intense anxiety. The first step in the management of PSVT is to determine whether the patient is hemodynamically stable. If PSVT is sustained and there is any indication of instability (i.e., angina, shortness of breath, decreased level of consciousness, hypotension, or congestive heart failure), electrical cardioversion should be performed urgently.
| Physiologic interventions | |
| Rest | |
| Valsalva maneuvers: gag reflex, ice packs, etc. | |
| Carotid massage* | |
| Avoidance of inciting factors: caffeine, tobacco, alcohol, pseudoephedrine, stress, etc. | |
| Medications | |
| Drugs with direct effect on atrioventricular node or accessory pathway: amiodarone (Cordarone), sotalol (Betapace), class IC drugs (flecainide [Tambocor], propafenone [Rythmol], etc.) | |
| Drugs that work primarily on atrioventricular node: adenosine (Adenocard), calcium channel blockers, beta blockers, digoxin (Lanoxin) | |
| Drugs that work primarily on accessory pathway: class IA drugs (quinidine, disopyramide [Norpace], etc.) | |
| Radiofrequency ablation | |
| Electronic pacing | |
Vagal maneuvers to increase parasympathetic tone and slow conduction through the atrioventricular node should be the first approach. Patients should be taught some of these maneuvers for use in future episodes. They should also be instructed to avoid inciting factors, such as caffeine, tobacco, alcohol, pseudoephedrine, and stress. Carotid sinus massage can be attempted, but its role hasbecome more limited because of the effectiveness of drug therapy and the risk of embolism from carotid pressure in some patients.
The goal of pharmacologic management is to slow or block atrioventricular nodal conduction. Agents used for this purpose include adenosine (Adenocard), calcium channel blockers (verapamil [Calan] or diltiazem), and beta blockers (e.g., esmolol [Brevibloc]).
Adenosine is an ultra–short-acting agent that is cleared quickly (half-life of 1 to 6 seconds). This agent is given intravenously in an initial dose of 6 mg, which is followed by one or two 12-mg boluses. Adenosine works by reducing conductance along the slow antegrade pathway. Side effects include flushing, dyspnea, and chest pain. Because of the short half-life of adenosine, these effects are usually very brief and do not ordinarily result in complications.
One advantage of adenosine is that it lacks the negative inotropic effects of calcium channel blockers. Adenosine can also decrease the sinus rate transiently and produce a “rebound” sinus tachycardia. Adenosine should not be used in patients with heart transplants, because such patients may be too sensitive to its effects.17
Calcium channel blockers can also be used to disrupt a reentrant pathway. Verapamil can be given in a 5- to 10-mg bolus over 2 minutes, followed by 10 mg in 15 to 30 minutes if the initial dose does not convert the arrhythmia.18 Verapamil and other calcium channel blockers should not be used in patients with an undiagnosed wide-complex tachycardia, because of the risk of fatal hypotension or ventricular fibrillation if the arrhythmia is actually ventricular tachycardia and not PSVT.19
Intravenously administered diltiazem is also effective.20 Initial treatment consists of a bolus of 0.25 mg per kg administered over two minutes. A repeat bolus of 0.35 mg per kg given over two minutes can be administered 15 minutes later.
Esmolol, a short-acting beta blocker, can be given in an intravenous bolus of 0.5 mg per kg over 1 minute or in an infusion at a rate of 0.5 mg per kg per minute after an initial loading dose of 0.5 mg per kg. An advantage of esmolol over other beta blockers is its short half-life (four to five minutes), compared with the much longer half-lives (three hours or more) of most other beta blockers. Because of a similar depressive effect on left ventricular contractility, esmolol should be used with caution if initial treatment with a calcium channel blocker is not successful.
Other antiarrhythmic drugs, including quinidine, procainamide, flecainide (Tambocor), and amiodarone, may be used in patients who do not respond to initial medications. However, selective radiofrequency ablation is rapidly becoming the treatment of choice in this situation.
Long-term control of recurrent PSVT caused by atrioventricular nodal reentry may be achieved with pharmacologic therapy or radiofrequency ablation. Patients who have infrequent, well-tolerated recurrences may manage these episodes with self-administered physiologic maneuvers.
Radiofrequency ablation is now used early in the management of patients with PSVT caused by an accessory pathway (Wolff-Parkinson-White syndrome), atrioventricular nodal reentrant tachycardia, or atrial tachycardia.21 The success rate for radiofrequency ablation is 95 percent in patients with an accessory pathway or atrioventricular nodal reentrant tachycardia, and approximately 80 percent in patients with atrial tachycardia.21
Other Atrial Arrhythmias
SINUS ARRHYTHMIA
Sinus arrhythmia is usually a normal event in young persons and athletes. In fact, it occurs with such high frequency that it may considered a normal variant rather than a true arrhythmia.
There are two forms of sinus arrhythmia. In the “respiratory” form, the RR interval shortens during inspiration and slows during expiration. Breath-holding eliminates the variation. In the “nonrespiratory” form, the same phasic variation is seen in the RR interval but is not related to respirations. This form of sinus arrhythmia occurs in elderly patients, patients with digoxin overdose, and patients with increased intracranial pressure.
Sinus arrhythmia is usually asymptomatic. Sometimes, however, the long pauses can cause dizziness or syncope. Treatment is usually unnecessary.
WANDERING ATRIAL PACEMAKER
Patients with wandering atrial pacemaker are usually not symptomatic. The condition is most often an isolated finding on the ECG and requires no treatment. Sometimes it is noted on physical examination as an irregularly irregular rhythm.
With wandering atrial pacemaker, the ECG shows variable P-wave morphology and PR intervals. The atrial impulses conduct in a 1:1 fashion and usually control the rhythm for several beats before shifting to another focus. The normal heart rate in wandering atrial pacemaker differentiates this condition from multifocal atrial tachycardia.
PREMATURE ATRIAL COMPLEXES
A premature atrial complex is generated from an ectopic focus in the atria. Therefore, the P wave is usually different in morphology from the usual sinus P wave. The impulse conducts along the normal pathways, generating a narrow QRS complex followed by a pause. Sometimes the premature atrial complex is not conducted and can mimic heart block (Figure 4).
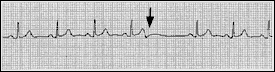
Premature atrial complexes are found in a variety of settings, including the excessive consumption of caffeine or alcohol and the use of sympathomimetic drugs. These complexes can also be present in patients with structural heart disease.
Patients with premature atrial complexes are usually asymptomatic and require no treatment. A beta blocker given in a low dosage can be tried in patients with uncomfortable symptoms, but no studies of efficacy have been reported. Patients should be counseled to decrease their intake of caffeine, tobacco, and alcohol, and their use of over-the-counter sympathomimetic substances, which are often present in cold medicines and weight-loss preparations.
It is important to note that premature atrial complexes sometimes precipitate supraventricular tachycardia, atrial flutter, or atrial fibrillation.
Sinus Nodal Arrhythmias
SINUS PAUSE AND SINOATRIAL EXIT BLOCK
Sinus pause or arrest occurs when the sinoatrial node fails to discharge. The ECG shows a pause in the sinus rhythm, with no preceding P wave. Patients usually have no symptoms, but if the pause is prolonged, they may have lightheadedness, palpitations, syncope, and falls. In sinus arrest, the length of the pause has no relationship to the PP interval. Sinoatrial exit block is recognized by the pauses being multiples of PP intervals.
Sinus node dysfunction is usually caused by drugs such as digoxin, quinidine, or procainamide. It can also be caused by ischemia, myocarditis, or fibrosis.
SICK SINUS SYNDROME
The term “sick sinus syndrome” encompasses a number of abnormalities, including sinus bradycardia, sinus arrest or exit block, combinations of sinoatrial and atrioventricular nodal conduction disturbances, and atrial tachyarrhythmias. More than one of these arrhythmias may be recorded in the same patient (bradycardia-tachycardia syndrome).
The abnormalities in sick sinus syndrome are usually due to ischemia, fibrosis, or drug-induced or autonomic dysfunction. Signs and symptoms are related to cerebral hypoperfusion and reduced cardiac output.
Treatment of recurrent symptomatic bradycardia or prolonged pauses requires implantation of a permanent pacemaker.24