
A more recent article on community-acquired pneumonia in adults is available.
Am Fam Physician. 2004;69(7):1699-1707
Atypical organisms such as Mycoplasma pneumoniae, Chlamydia pneumoniae, and Legionella pneumophila are implicated in up to 40 percent of cases of community-acquired pneumonia. Antibiotic treatment is empiric and includes coverage for both typical and atypical organisms. Doxycycline, a fluoroquinolone with enhanced activity against Streptococcus pneumoniae, or a macrolide is appropriate for outpatient treatment of immunocompetent adult patients. Hospitalized adults should be treated with cefotaxime or ceftriaxone plus a macrolide, or with a fluoroquinolone alone. The same agents can be used in adult patients in intensive care units, although fluoroquinolone monotherapy is not recommended; ampicillin-sulbactam or piperacillin-tazobactam can be used instead of cefotaxime or ceftriaxone. Outpatient treatment of children two months to five years of age consists of high-dose amoxicillin given for seven to 10 days. A single dose of ceftriaxone can be used in infants when the first dose of antibiotic is likely to be delayed or not absorbed. Older children can be treated with a macrolide. Hospitalized children should be treated with a macrolide plus a beta-lactam inhibitor. In a bioterrorist attack, pulmonary illness may result from the organisms that cause anthrax, plague, or tularemia. Sudden acute respiratory syndrome begins with a flu-like illness, followed two to seven days later by cough, dyspnea and, in some instances, acute respiratory distress.
Community-acquired pneumonia (CAP) affects approximately 4.5 million adults in the United States annually.1 About one third of these adults require hospitalization.1 The mortality rate among hospitalized patients with CAP varies each year and can reach 35 percent.2 While Streptococcus pneumoniae causes up to 70 percent of CAP cases, atypical pathogens are responsible for 30 to 40 percent of cases3 and may be copathogens in other cases. Even with a knowledge of some of the common characteristics of infections with atypical organisms (Table 1),4 determining the specific pathogen on the basis of clinical, radiologic, and laboratory findings is difficult and usually done retrospectively, if at all.
Atypical Pathogens
MYCOPLASMA PNEUMONIAE
Mycoplasma pneumoniae causes a wide range of respiratory infections, including pneumonia, tracheobronchitis, and upper respiratory tract infection. Only 3 to 10 percent of persons infected with M. pneumoniae develop pneumonia.5 Because M. pneumoniae infection becomes more common with increasing age, it is particularly important to consider this agent in elderly patients.6
M. pneumoniae infection occurs throughout the year but can cause periodic outbreaks within small communities. Transmission is by person-to-person contact, and infection spreads slowly, most often within closed populations (e.g., households, schools, businesses).
M. pneumoniae is the pathogen most often associated with atypical pneumonia. Onset is insidious, over several days to a week. Constitutional symptoms, which usually are present, include headache exacerbated by a cough, malaise, myalgias, and sore throat. The cough is usually dry, paroxysmal, and worse at night.
The clinical course of pneumonia caused by M. pneumoniae is usually mild and self-limited. The mortality rate is approximately 1.4 percent.2 However, pulmonary complications can be significant and include effusion, empyema, pneumothorax, and respiratory distress syndrome.
| Diagnostic feature | Mycoplasma pneumonia | Chlamydia pneumoniaepneumonia | Legionella species pneumonia | |
|---|---|---|---|---|
| History | ||||
| Abdominal pain | – | – | + | |
| Confusion | +/– | – | + | |
| Diarrhea | +/– | – | + | |
| Ear pain | +/– | – | – | |
| Headache (mild) | + | – | – | |
| Myalgias | + | +/– | + | |
| Pleuritic pain | +/– | – | + | |
| Sore throat | + | + | – | |
| Physical signs | ||||
| Cardiac involvement | +/– | – | – | |
| Lobar consolidation | +/– | – | +/– | |
| Hemoptysis | – | – | + | |
| Pharyngitis (nonexudative) | + | + | – | |
| Rash | +/– | – | – | |
| Raynaud's phenomenon | +/– | – | – | |
| Chest radiograph | Patchy infiltrate | Funnel-shaped or circumscribed infiltrate | Patchy consolidation | |
| Laboratory test results | ||||
| Cold agglutinins | + | – | – | |
| Hyponatremia | – | – | + | |
| Leukocytosis | +/– | – | + | |
| Microscopic hematuria | – | – | + | |
| Transaminase elevation | – | – | + | |
M. pneumoniae infection may be associated with several extrapulmonary manifestations. Skin manifestations include erythema multiforme, erythema nodosum, maculopapular and vesicular eruptions, and urticaria. Neurologic derangements include aseptic meningitis, cerebral ataxia, encephalitis, Guillain-Barré syndrome, and transverse myelitis. The production of cold agglutinins can result in hemolytic anemia, especially when M. pneumoniae titers are high. Finally, complications such as myocarditis, pancreatitis, pericarditis, and polyarthritis can occur.
CHLAMYDIA PNEUMONIAE
Chlamydia pneumoniae is an obligate intracellular organism capable of persistent latent infection. Humans are the only known reservoir. Transmission results from contact with respiratory secretions, with an incubation period of several weeks.
By the age of 20 years, one half of persons in the United States have detectable levels of antibody to C. pneumoniae.7 The antibody is present in 75 percent of elderly persons.7 C. pneumoniae infection is more likely to occur in older patients with comorbid diseases than in those who are otherwise healthy.8
Patients with C. pneumoniae infection often present with sore throat, headache, and a cough that can persist for months if treatment is not initiated early.9 Sputum is usually scant or nonexistent, and a low-grade fever is usually present. Chest radiographs tend to show less extensive infiltrates than are seen with other causes of pneumonia, although significant infiltrates have been reported.10
Most cases of C. pneumoniae infection are mild, but severe disease can occur, necessitating admission to an intensive care unit. The mortality rate has been estimated to be 9 percent, and death usually is associated with secondary infection and underlying comorbid disease.2
LEGIONELLA PNEUMOPHILA
Like C. pneumoniae, Legionella species are intracellular organisms. Legionella pneumophila is the most pathogenic species, and several serotypes have been identified. Serotype 1 has been associated with most reported human cases of pneumonia caused by L. pneumonphila.11
Infection occurs from exposure to legionellae organisms in the environment. Person-to-person spread has not been reported. Legionellae are found most commonly in freshwater and man-made water systems. The pathogens also can be found in moist soil, especially near streams and ponds. Man-made systems for heating and cooling water can be prime environments for the proliferation of legionellae, because of conditions such as temperatures between 32°C (89.6°F) and 45°C (113°F), stagnation of water, and the presence of scale sediment and amebas.12 Condensers, cooling towers, respiratory therapy equipment, showers, water faucets, and whirlpools have been associated with outbreaks of legionellosis.13
Risk factors for the development of legionellosis include overnight stays outside the home, recent home plumbing work, renal or liver failure, diabetes, malignancy, and other conditions that compromise the immune system.14
| Outpatient treatment | ||
| Doxycycline (Vibramycin) | ||
| or | ||
| Fluoroquinolone (gatifloxacin [Tequin], levofloxacin [Levaquin], moxifloxacin [Avelox]) with enhanced activity against Streptococcus pneumoniae | ||
| or | ||
| Macrolide (azithromycin [Zithromax], clarithromycin [Biaxin], erythromycin) | ||
| Inpatient treatment | ||
| Patient not in intensive care unit | ||
| Extended-spectrum cephalosporin (cefotaxime [Claforan], ceftriaxone [Rocephin]) plus a macrolide | ||
| or | ||
| Fluoroquinolone alone | ||
| Patient in intensive care unit | ||
| Cefotaxime or ceftriaxone plus a macrolide | ||
| or | ||
| Cefotaxime or ceftriaxone plus a fluoroquinolone | ||
| or | ||
| Beta-lactam (ampicillin-sulbactam [Unasyn], piperacillin-tazobactam [Zosyn]) plus a macrolide | ||
| or | ||
| Beta-lactam plus a fluoroquinolone | ||
| Other circumstances | ||
| Structural lung disease: antipseudomonal agent (piperacillin [Pipracil], piperacillin-tazobactam, a carbapenem, or cefepime [Maxipime]) plus a fluoroquinolone (including high-dose ciprofloxacin [Cipro]) | ||
| Beta-lactam allergy: fluoroquinolone with or without clindamycin (Cleocin) | ||
| Aspiration pneumonia: fluoroquinolone with or without clindamycin, metronidazole (Flagyl), or a beta-lactam | ||
Legionnaires' disease may present with a wide spectrum of symptoms ranging from mild cough and low-grade fever to high fever, altered mental status, and respiratory failure.15 Nonspecific symptoms may occur early in the disease and include headache, muscle aches, anorexia, and malaise.15 Diarrhea and other gastrointestinal symptoms are present in 20 to 40 percent of cases.15 Leukocytosis is a common laboratory finding, and the sputum Gram stain often shows an abundance of inflammatory cells without a predominance of organisms.11
Among cases of CAP with atypical causes, legionnaires' disease has the most severe clinical course, and illness can become progressively more severe if the infection is not treated appropriately and early. Although extrapulmonary manifestations are rare, legionellosis has been implicated in cases of myocarditis, pericarditis, and prosthetic valve endocarditis, as well as glomerulonephritis, pancreatitis, and peritonitis.15 When CAP is caused by Legionella species, the mortality rate is 14 percent.2
Therapy
Therapy for pneumonia is empiric because specific pathogens usually are not identified at the time treatment is initiated. Several classes of antibiotics are effective against atypical pathogens. However, because C. pneumoniae and Legionella species are intracellular organisms and M. pneumoniae lacks a cell wall, beta-lactams are not effective.
Erythromycin and, in some cases, tetracycline have been traditional choices for the treatment of pneumonia caused by atypical pathogens. There are few (if any) clinical trials demonstrating the efficacy of erythromycin for Legionella infection. However, erythromycin and tetracycline are effective against M. pneumoniae and have been shown to reduce symptom duration in C. pneumoniae infection.5,8
Newer macrolides such as azithromycin (Zithromax) and clarithromycin (Biaxin) have good activity against M. pneumoniae, C. pneumoniae, and Legionella species, and generally are better tolerated than erythromycin.16–20 Doxycycline (Vibramycin) also is effective,21 typically is associated with fewer gastrointestinal side effects, and is a less expensive alternative.
The Infectious Diseases Society of America (IDSA)26 has published a comprehensive, evidence-based guideline to the management of CAP in adults who are immunocompetent. Empiric treatment recommendations are based on whether patients are treated as outpatients or inpatients (Table 2).26 The decision to hospitalize can be guided by the mortality prediction rule shown in Figure 1.27
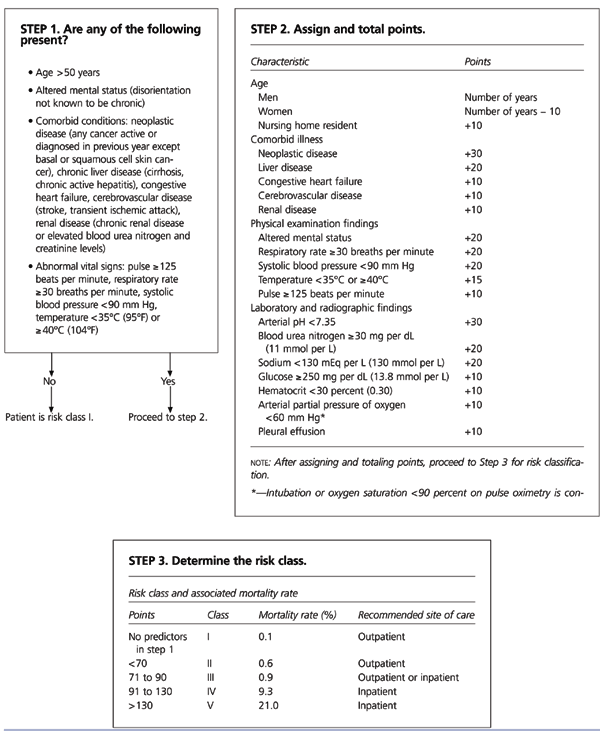
Blood cultures do not have to be performed before out-patient therapy is started. However, the IDSA26 recommends performing blood cultures in hospitalized patients, if possible before antibiotics are administered. Sputum Gram stain and culture also are recommended in these patients. Antibiotic therapy should be initiated within four hours of hospitalization.28
Treatment Challenges
FAILURE OF OUTPATIENT MANAGEMENT
Patients treated with antibiotics may fail outpatient management for a number of reasons, such as antibiotic resistance, poor compliance with or intolerance of oral antibiotics, obstructing lesions (e.g., foreign body, cancer), empyema, and incorrect diagnosis.26 Table 326,29 lists alternate diagnoses that may mimic CAP.
PARAPNEUMONIC EFFUSION AND EMPYEMA
Up to 57 percent of patients with CAP have pleural effusions on chest radiographs.30 Empyema, defined as pus in the pleural space, should be drained by chest tube, image-guided catheter, thoracoscopy, or thoracotomy.26 Even if the pleural fluid does not contain free-flowing frank pus, it should be drained when the pH level is less than 7.2 or the Gram stain is positive.26 Some experts recommend drainage for any parapneumonic effusion that measures more than 10 mm on a lateral decubitus radiograph.30
CHILDREN
An evidence-based guideline on the management of CAP in children, developed by a children's hospital, is available online.31 Pneumonia should be suspected in a child who presents with fever and tachypnea. Because infection with an atypical pathogen is unlikely in children two months to five years of age, the recommended treatment in these patients is high-dose amoxicillin (80 to 90 mg per kg daily) for seven to 10 days. A cephalosporin or macrolide is recommended in those who are allergic to penicillin. Macrolides are recommended for the treatment of CAP in children older than five years, because of the increased likelihood of infection with M. pneumoniae or C. pneumoniae in older children. Macrolides also provide coverage for S. pneumoniae.
Hospitalization should be considered for any child with CAP and is necessary if a child requires oxygen or intravenous therapy, or if treatment compliance or follow-up may be an issue. Treatment with a macrolide plus a beta-lactam (high-dose amoxicillin or parenteral ceftriaxone [Rocephin]) should be considered in children with more severe pneumonia. Children treated as outpatients should have a follow-up examination within 24 to 72 hours.31
| Acute respiratory distress syndrome |
| Atelectasis |
| Bronchiolitis obliterans with organizing pneumonia |
| Churg-Strauss syndrome |
| Collagen vascular disease |
| Congestive heart failure |
| Drug-induced lung disease |
| Idiopathic pulmonary fibrosis |
| Inflammatory lung disease |
| Interstitial pneumonitis |
| Malignant pleural effusion |
| Neoplasm |
| Occupational lung disease |
| Pulmonary embolus |
| Pulmonary hemorrhage or infarction |
| Radiation pneumonitis |
| Sarcoidosis |
| Wegener's granulomatosis |
ELDERLY PATIENTS
Elderly patients with CAP may present with few respiratory signs or symptoms of pneumonia. Instead, they may have altered mental status or a history of falls.32 Elderly patients also are more likely to have significant comorbid conditions, such as chronic obstructive pulmonary disease, congestive heart failure, or renal disease, and are particularly susceptible to silent aspiration.32,33
| Hospitalize the patient if two or more of the following are present: | |
| Respiratory rate >30 breaths per minute or 10 breaths above baseline | |
| Oxygen saturation < 90 percent on room air | |
| Systolic blood pressure < 90 mm Hg or 20 mm Hg less than baseline | |
| Oxygen requirement of 3 L per minute more than baseline | |
| Uncontrolled chronic obstructive pulmonary disease, congestive heart failure, or diabetes | |
| If previously conscious, cannot be awakened | |
| New or increased agitation | |
| With the presence of any one of the above, hospitalization also is indicated if proper physical and human resources are not available (e.g., nursing staff, physician, oxygen, suction equipment, laboratory access, intravenous fluids). | |
Pneumonia acquired in a nursing home has a high rate of morbidity and mortality (up to 44 percent).34 It is the leading infectious illness requiring transfer from a nursing home to a hospital.32 Guidelines on when to hospitalize are provided in Table 4.34 Recommended treatment regimens for patients in nursing homes are summarized in Table 5.32
IMMUNOCOMPROMISED PATIENTS
The guidelines and recommendations given throughout this article are intended for immunocompetent patients. Immunocompromised patients may develop pneumonia from organisms such as Pneumocystis carinii, Candida species, and Aspergillus species, as well as other opportunistic organisms not reviewed in this article.
Prevention Strategies
Despite evidence showing that pneumococcal vaccine significantly reduces the occurrence of pneumococcal pneumonia in persons who are immunocompetent, immunization rates are modest at best.35 Vaccination is more likely when it is recommended by a patient's physician or by someone in the physician's office.35 Although influenza is not reviewed in this article, it is an important contributor to CAP; therefore, patients at risk for CAP should be given annual influenza vaccination.26 In addition, long-term oral hygiene appears to reduce the incidence of pneumonia among elderly persons living in nursing homes.36
BIOTERRORISM AGENTS THAT MAY CAUSE PNEUMONIA
Several organisms that can be used in biological weapons may cause illness that presents as CAP (Table 6).26,37 It is important for physicians to be aware of potential bioterrorist tactics and risks, and the resulting health care demands. Information on bioterrorism response is available on the American Academy of Family Physicians Web site (https://www.aafp.org/btresponse.xml).
SUDDEN ACUTE RESPIRATORY SYNDROME
Periodically, an epidemiologic investigation prompted by an outbreak of pneumonia leads to the identification of a previously unrecognized organism.26 Sudden acute respiratory syndrome (SARS) is an example. SARS, which is thought to have originated in a Hong Kong apartment building, is caused by a coronavirus (SARS-CoV). During the SARS outbreak of 2002 to 2003, over 8,000 probable cases were reported from 29 different countries. Twenty-nine cases were reported in the United States.38
SARS presents with a prodrome of symptoms of a flu-like illness (fever, chills, myalgias, headache, diarrhea), followed in two to seven days by cough, dyspnea and, possibly, acute respiratory distress syndrome.26,38 The case-fatality rate for SARS is about 9.6 percent.38 No deaths in the United States have been linked to SARS.38 Information about SARS and travel guidelines are available at the Centers for Disease Control and Prevention Web site (http://www.cdc.gov/sars/index.html).
| Disease | Causative agent | Clinical features | Ancillary findings or diagnostic clue | Diagnosis | Treatment | Duration of treatment | Prophylaxis after exposure |
|---|---|---|---|---|---|---|---|
| Anthrax | Bacillus anthracis | Fever, malaise, cough, acute respiratory distress syndrome, shock | Widened mediastinum on chest radiograph | Gram stain of unspunperipheral blood, blood culture | Ciprofloxacin (Cipro) or other fluoroquinolone, doxycycline (Vibramycin), or penicillin (if susceptible) | 60 days | Ciprofloxacin, amoxicillin, or doxycycline for 60 days |
| Plague | Yersinia pestis | High fever, malaise, cough, bloody sputum, shock | Blood-tinged sputum within 24 hours of onset | Gram-negative bipolar coccobacillus on Gram stain and culture of blood, sputum, cerebrospinal fluid | Doxycycline or fluoroquinolone | 10 days | Doxycycline or fluoroquinolone for 7 days |
| Tularemia | Francisella tularensis | Fever, prostration, cough | Chestradiograph shows focal infiltrate, hilar adenopathy | Culture of blood, sputum, pharyngeal specimen (high risk to laboratory personnel) | Doxycycline | 14 days | Doxycycline or tetracycline for 14 days |
| Q Fever | Coxiella burnetii | Fever, cough, shortness of breath, weight loss, chest pain | Minimally productive cough, vague substernal chest pain or tightness | Blood culture, PCR, serology | Doxycycline, tetracycline | 5 to 7 days | Tetracycline or doxycycline for five days starting on day 8 to 12 |
| Brucellosis | Brucella species | Fever, myalgias, cough | Undulating fever, bone-associated osteoarticular symptoms | Blood culture, marrow culture, immunoassay, PCR (high risk to laboratory personnel) | Doxycycline plus rifampin(Rifadin) or streptomycin | 6 weeks | None recommended |