
A more recent article on Barrett esophagus is available.
Am Fam Physician. 2004;69(9):2113-2118
Gastroesophageal reflux disease (GERD) is a condition commonly managed in the primary care setting. Patients with GERD may develop reflux esophagitis as the esophagus repeatedly is exposed to acidic gastric contents. Over time, untreated reflux esophagitis may lead to chronic complications such as esophageal stricture or the development of Barrett’s esophagus. Barrett’s esophagus is a premalignant metaplastic process that typically involves the distal esophagus. Its presence is suspected by endoscopic evaluation of the esophagus, but the diagnosis is confirmed by histologic analysis of endoscopically biopsied tissue. Risk factors for Barrett’s esophagus include GERD, white or Hispanic race, male sex, advancing age, smoking, and obesity. Although Barrett’s esophagus rarely progresses to adenocarcinoma, optimal management is a matter of debate. Current treatment guidelines include relieving GERD symptoms with medical or surgical measures (similar to the treatment of GERD that is not associated with Barrett’s esophagus) and surveillance endoscopy. Guidelines for surveillance endoscopy have been published; however, no studies have verified that any specific treatment or management strategy has decreased the rate of mortality from adenocarcinoma.
Barrett’s esophagus was first described in 1950 by Norman Barrett, who reported a case of chronic peptic ulcer in the lower esophagus that was covered by epithelium.1 Barrett’s esophagus can be defined simply as columnar metaplasia of the esophagus. Patients who have columnar epithelium that measures 3 cm or more from the gastroesophageal junction are said to have traditional, or “long-segment,” Barrett’s esophagus, while patients with a measure less than 3 cm have “short-segment” Barrett’s esophagus.2 In 1998, the American College of Gastroenterology (ACG) defined Barrett’s esophagus as “a change in the esophageal epithelium of any length that can be recognized at endoscopy and is confirmed to have intestinal metaplasia by biopsy of the tubular esophagus and excludes intestinal metaplasia of the cardia.”3
Gastroesophageal reflux disease (GERD) is a condition commonly evaluated and managed in the primary care setting. Surveys suggest that approximately 20 percent of U.S. adults have symptoms of GERD at least once a week.4 A subgroup of patients with GERD develop severe complications that include erosive esophagitis, stricture formation, Barrett’s esophagus, and adenocarcinoma of the esophagus. Because Barrett’s esophagus is thought to be associated with the development of adenocarcinoma, it is imperative that primary care physicians be familiar with Barrett’s esophagus, its association with GERD, and its diagnosis and management.
The overall prevalence of Barrett’s esophagus in the general population is difficult to estimate, because approximately 25 percent of persons with Barrett’s esophagus have no symptoms of reflux.5 It is known, however, that the incidence of adenocarcinoma of the esophagus has risen sharply in the past few decades. The results of one study6 note that the incidence of adenocarcinoma in white men has increased by more than 350 percent since the mid-1970s. Identification of patients at risk for adenocarcinoma of the esophagus is fairly poor. In fact, only 5 percent of patients who had resection of esophageal adenocarcinoma were known to have Barrett’s esophagus before the resection, highlighting the fact that current screening techniques are relatively ineffective.7 Perhaps by increasing awareness of Barrett’s esophagus, we can better target screening of high-risk patients.
Pathophysiology and Diagnosis
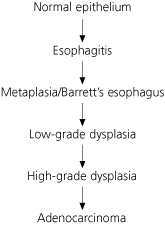
Progression from GERD to adenocarcinoma is thought to follow a stepwise process. It is believed that exposure of the esophageal epithelium to acid damages the lining, causing chronic esophagitis. The damaged area then heals in a metaplastic process in which abnormal columnar cells replace squamous cells. This abnormal intestinal columnar epithelium, which is called specialized intestinal metaplasia, can then progress to dysplasia, ultimately leading to carcinoma8 (Figure 1). The incidence of Barrett’s esophagus progressing to adenocarcinoma is estimated to be 0.5 per 100 patient-years (i.e., one in 200 patients developing carcinoma per year).9
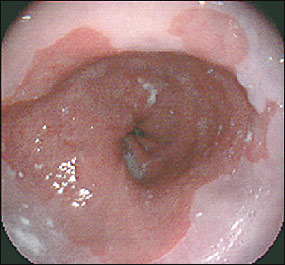
Barrett’s esophagus is diagnosed by endoscopy and histology. The line at which the columnar epithelium transitions to the squamous epithelium (i.e., the squamocolumnar junction) is known as the Z-line. Normally, the Z-line corresponds to the gastroesophageal junction. In patients with Barrett’s esophagus, the columnar epithelium extends proximally up the esophagus (Figure 2). As mentioned previously, long-segment Barrett’s esophagus measures 3 cm or more from the gastroesophageal junction, while short-segment Barrett’s esophagus is less than 3 cm from the gastroesophageal junction. It is unknown whether the natural course or pathogenesis varies between these two entities or whether short-segment Barrett’s esophagus progresses to long-segment disease. It makes sense that more metaplasia would predispose to more cancer, and one study10 has shown this to be the case. However, current guidelines recommend managing long- and short-segment Barrett’s esophagus in the same manner.3
Screening
Given the high prevalence of GERD, it is physically and financially impossible to screen all patients with GERD symptoms for the development of Barrett’s metaplasia. Obviously, patients with alarm symptoms such as dysphagia, odynophagia, bleeding, or weight loss should be referred promptly for endoscopy. In patients without alarm symptoms, screening guidelines for Barrett’s esophagus are somewhat problematic. Symptoms of GERD are usually the reason a patient is referred for evaluation for Barrett’s esophagus but, as previously mentioned, many patients are asymptomatic.5
Currently, there is little evidence that screening for Barrett’s esophagus decreases the rate of mortality from adenocarcinoma.11 Several possible reasons for this finding include the older age of patients being studied and the relatively low absolute risk of adenocarcinoma of the esophagus. Current studies lack adequate power to show statistical significance. Nevertheless, the ACG states that “patients with chronic GERD symptoms are those most likely to have Barrett’s esophagus and should undergo upper endoscopy.”3 With this general guideline in mind, other elements of the risk assessment for Barrett’s esophagus and esophageal adenocarcinoma may be considered (Table 1). For instance, symptom severity alone is not an accurate marker; however, patients with a long history of reflux are at greater risk. Patients who had symptoms of GERD more than three times per week for more than 20 years have a 40-fold increased risk of developing adenocarcinoma.12
| GERD, especially of long duration |
| White or Hispanic race |
| Male sex |
| Advancing age (reaching a plateau in the 60s) |
| Smoking |
| Obesity |
The incidence of Barrett’s esophagus is much higher in whites and Hispanics, compared with rates among blacks and Asians.13 Epidemiologic data also indicate that men are at greatest risk and, although Barrett’s esophagus can be found at any age, the prevalence increases with advancing age until a plateau is reached in the 60s.14 Other possible risk factors include tobacco use and obesity. Cigarette smoking, in particular, is associated with an approximately twofold increase in adenocarcinoma compared with the rate in nonsmokers, and this risk persists for years after smoking cessation.15,16
The question of whom to screen remains. One recent study17 suggests once-in-a-lifetime endoscopy screening in 50-year-old white men with GERD. In patients without dysplasia, even those with Barrett’s esophagus, further screening was not performed. This strategy appeared to be reasonable and cost-effective because the rate of conversion to adenocarcinoma is so low.17 A reasonable interpretation of the ACG recommendations might be to perform endoscopy in a patient with a combination of risk factors—although which risk factors will depend on clinical judgment and conversations with the patient—as well as symptoms occurring three or more times per week for several years. Unfortunately, there will be many variations on these parameters until further information is available.
Treatment
Treatment of Barrett’s esophagus is aimed at decreasing reflux of acid into the esophagus. Although GERD is the primary risk factor for developing esophageal adenocarcinoma,5 it is unclear whether GERD predisposes patients to malignancy by causing Barrett’s esophagus or by affecting carcinogenesis in patients with established Barrett’s esophagus.18 Both medical and surgical therapies have proved effective in controlling symptoms.19 However, symptom control does not seem to correlate with complete acid control. A study20 of esophageal pH monitoring showed that symptom relief using conventional dosages of proton pump inhibitors (PPIs) does not necessarily correlate with suppression of acid reflux into the esophagus. Although aggressive medical treatment with PPIs and histamineH2 receptor antagonists to produce near-complete achlorhydria has been advocated, this approach remains controversial.2
| Patients with Barrett’s esophagus should undergo surveillance endoscopy with biopsies. |
| GERD should be treated before endoscopy to minimize inflammation, which can make interpretation more difficult. |
| Patients with two consecutive negative surveillance endoscopies showing no dysplasia may undergo subsequent surveillance every three years. |
| Patients with dysplasia should have the diagnosis confirmed by another expert pathologist. |
| Patients with low-grade dysplasia should receive annual surveillance endoscopy. |
| Patients with high-grade dysplasia can receive short-interval endoscopy (i.e., every three months) or intervention (e.g., esophagectomy), depending on the extent of the dysplasia. |
The efficacy of surgical therapy in preventing adenocarcinoma also remains unclear. Recent evaluations of surgical therapy such as fundoplication showed no significant decrease in the risk of adenocarcinoma compared with medical therapy.21 Given the lack of adequate data to support more aggressive measures, the ACG recommends that, in patients with Barrett’s esophagus, GERD be managed in the same manner as in patients with GERD who do not have Barrett’s esophagus: the control of symptoms of GERD and the maintenance of healed mucosa.3 Antireflux therapy (medical or surgical) should not be prescribed beyond that needed for healing signs and symptoms of reflux esophagitis.3
Surveillance
Once Barrett’s esophagus is discovered, the question of how to follow affected patients arises. Surveillance of Barrett’s esophagus is a relatively unproven area of study. As in screening, no evidence has shown that surveillance improves mortality rates.2,22,23 Proponents of surveillance endoscopy claim that previous studies included elderly patients who frequently died from unrelated causes, and that younger patients would likely benefit from surveillance.24 Others suggest that, given the low rate at which Barrett’s esophagus progresses to adenocarcinoma (0.5 percent per year), low-risk asymptomatic patients (i.e., Asians and blacks, women of any age), patients older than 75 years, and those with precarious health conditions do not need routine surveillance if the initial endoscopy showed no dysplasia.25
Published guidelines for the surveillance of Barrett’s esophagus suggest different intervals for surveillance endoscopy, depending on histologic findings of previous endoscopy.3 The ACG recommendations are summarized in Table 2.3 Recommendations for endoscopy every three years in patients without dysplasia were based on adenocarcinoma incidence rates of 1 to 2 percent per year. With more recent studies reporting the true incidence of 0.5 percent per year, a less frequent endoscopy interval of three to five years is also reasonable.2 As already mentioned, once-in-a-lifetime endoscopy may be sufficient in patients without dysplasia.17
The cost of surveillance has been analyzed. In the United Kingdom, it would cost an estimated $23,000 to detect one case of esophageal cancer in men, compared with $65,000 in women.26 A U.S. study27 estimated the cost for men and women to be approximately $38,000. To compare, detecting one case of breast cancer using mammography was estimated to cost about $55,000. It is important to note that the incidence rates used in these studies were higher than current estimates, so the cost of surveillance actually may be higher.
There are several limitations to surveillance in patients with dysplasia. The histologic properties of dysplasia may be difficult to evaluate, because inflamed and healing epithelium may be similar in appearance. In addition, sampling errors (e.g., missed areas from random sampling) and lack of interobserver agreement of histologic specimens (especially in low-grade dysplasia) make this process less than perfect. By the time high-grade dysplasia is noted, many of these patients already have invasive carcinoma.2 Future modalities include the use of other markers of cancer risk and different endoscopic techniques, such as employing cytology as an adjunct to biopsy, chromoendoscopy (i.e., use of vital dyes such as methylene blue), and spectroscopic biopsy techniques, which may improve the diagnostic accuracy of Barrett’s esophagus screening and surveillance.28
Approaches to high-grade dysplasia include esophagectomy or, possibly, surveillance, as noted in the ACG guidelines. Other societies recommend that all relatively healthy patients with high-grade dysplasia be considered for esophagectomy.2 Other options include endoscopic ablation with thermal or photodynamic therapy. Photodynamic therapy is a nonthermal means of ablating tissue, using visible light, a photosensitizer, and oxygen. The photosensitizer is absorbed preferentially by premalignant or malignant tissue, followed by light activation via laser. Photofrin (porfimer sodium) currently is the only agent approved in the United States.29 Limitations of ablative therapy include esophageal stricture and incomplete ablation of dysplastic tissue.
Helicobacter pylori and Barrett’s Esophagus
Helicobacter pylori infection tends to cause inflammation in the stomach, which may lead to gastric metaplasia and carcinoma; however, infection does not occur in the esophagus. Some data suggest that H. pylori infection actually decreases the risk of GERD, Barrett’s esophagus, and adenocarcinoma of the esophagus. One proposed mechanism is that chronic gastritis may interfere with acid production and increase gastric pH.30 Despite these associations, little is known about the relationship between H. pylori and GERD and its complications. Currently, guidelines do not recommend testing for or treating H. pylori infection in patients with GERD and its complications.31
Final Comment
Barrett’s esophagus is an acquired condition that results from injury of the squamous epithelium of the esophagus through repetitive exposure to gastric acid. Although Barrett’s esophagus is associated with adenocarcinoma of the esophagus, no studies show a decrease in mortality from adenocarcinoma in the way the condition is managed currently. Nonetheless, management guidelines, which seem to make intuitive sense, do exist. It is important to closely monitor patients with GERD and to educate them to return for medical advice if symptoms persist despite treatment; endoscopy is indicated in these patients. Once a diagnosis of Barrett’s esophagus is made, surveillance guidelines may be helpful; however, it is important to remember that no current data show that surveillance decreases the rate of mortality. Further study and future treatments may bring more answers and, perhaps, better outcomes.
| Key clinical recommendation | Strength of recommendation | References |
|---|---|---|
| Patients with chronic GERD symptoms are those most likely to have Barrett’s esophagus and should undergo upper endoscopy. | C | 3 |
| Recent evaluations of surgical therapy, such as fundoplication, showed no significant decrease in the risk of adenocarcinoma compared with medical therapy. | B | 21 |
| As in screening, no evidence has shown that surveillance favorably affects mortality. | B | 22,23 |