
Am Fam Physician. 2004;70(8):1481-1488
A more recent article on basal cell and cutaneous squamous cell carcinomas is available.
Rates of squamous cell and basal cell carcinomas have been increasing, possibly as a result of increased exposure to ultraviolet radiation. Primary care physicians can expect to diagnose six to seven cases of basal cell carcinoma and one to two cases of squamous cell carcinoma each year. Basal cell carcinomas may be plaque-like or nodular with a waxy, translucent appearance, often with ulceration and telangiectasia. They rarely metastasize and are treated with excision, cryotherapy, electrodesiccation and cautery, imiquimod, 5-fluorouracil, or photodynamic therapy (the latter is not approved for this purpose by the U.S. Food and Drug Administration), although surgery results in the fewest recurrences. Actinic keratoses are scaly keratotic patches that often are more easily felt than seen. They are amenable to any of the destructive techniques described above, with the exception of photodynamic therapy. Squamous cell carcinomas arise from keratotic patches and become more nodular and erythematous with growth, sometimes including keratin plugs, horns, or ulceration. Because they may metastasize, they often are treated with excisional biopsy.
| Key clinical recommendation | Label | References |
|---|---|---|
| Based on expert opinion and scant, poor-quality evidence, physicians should recommend the use of sunscreen to high-risk patients to prevent skin cancer. | C | 19,20 |
| The U.S. Preventive Services Task Force recommends that physicians remain alert for skin lesions with malignancy during physical examinations performed for other purposes. | C | 22 |
The incidence of basal cell and squamous cell carcinomas, which also are called nonmelanoma skin cancer, has been increasing steadily over the past 30 years.1,2 The reasons for this increase appear to be largely sociologic and include increasing amounts of sun exposure and use of artificial tanning beds. Primary care physicians can expect to diagnose six to seven cases of basal cell carcinoma and one to two cases of squamous cell carcinoma each year.3 The major risk factor for the development of skin cancer is exposure to ultraviolet (UV) radiation. In addition, ozone depletion may increase UV radiation exposure and thus increase rates of skin cancer,4,5 but other factors also increase risks (Table 1).
| Cumulative sun exposure in older patients |
| Gamma radiation exposure (cancer therapy, psoriasis treatment, past acne treatment) |
| History of actinic keratosis, squamous cell carcinoma, or basal cell carcinoma |
| HPV infection (in the genitourinary area) |
| Inorganic arsenic exposure (formerly used in medical treatments) |
| Old burn scars |
| Smoking |
| Ultraviolet radiation exposure (exposure before age 14 is especially significant) |
| Uncircumcised penis (for squamous cell carcinoma of the penis; occurs mainly in developing countries) |
Natural History
Basal cell and squamous cell carcinomas develop within the epidermis (Figure 1). Basal cell carcinomas are derived from the basal layer of keratinocytes, which is the deepest cell layer of the epidermis. Squamous cell carcinomas arise from the more superficial layers of keratinocytes. Keratinocyte damage is a response to repeated exposure to UV radiation, occurring especially in susceptible persons. One study6 found that a single tanning session was enough to damage DNA in keratinocytes. The damage results in actinic keratoses, solar lentigo, and dermatoheliosis (i.e., “photoaging”).7
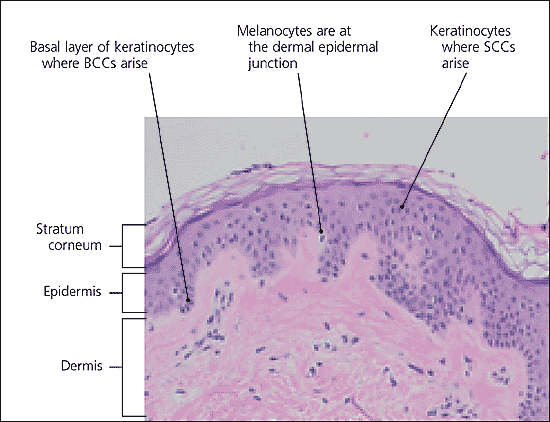
Although not universally accepted at this time, a growing body of evidence suggests that actinic keratoses should be regarded as very early squamous cell carcinomas, based on the pathology of actinic keratoses.8–11 These lesions can progress to carcinoma in situ and then to invasive cancer. Although metastasis is rare in basal cell carcinoma, local tissue effects can be destructive and disfiguring. Squamous cell carcinomas metastasize about 3 to 4 percent of the time.12
A small percentage of squamous cell carcinomas do not follow the above “natural history.” Skin cancers may arise in skin wounds, and immunosuppressed persons may have a higher incidence of cancer in general.13 Genetic syndromes in which there is a defective mechanism for the repair of damaged DNA (e.g., xeroderma pigmentosum) can put patients at increased risk. Such cancers, arising atypically, often have a higher incidence of metastasis.
Prevention
The most important aspect of skin cancer management is prevention. Sun should be avoided during midday hours, when UV intensity is greatest. During exposure, sunscreen and protective clothing should be employed. Sunscreen should be applied generously in quantity and frequency. Many sunscreen users fail to apply sunscreen appropriately or to reapply it after swimming, sweating, or toweling off.14
Clothing provides a degree of sun protection, but some harmful rays can penetrate clothing; cloth with a tight weave provides greater protection. Patients with higher risk factors for skin cancer may consider the use of clothing that provides more sun protection. A hat should be worn, preferably one with a 3-inch brim to shield the ears, neck, and nose. The results of a Colorado study15 show that skiers and winter sports enthusiasts should be aware that the amount of UVB radiation increases by 8 to 10 percent per 1,000 feet of elevation; erythema developed in only six minutes on unprotected skin at 11,000 feet under midday spring sun exposure (Table 2).16–18
| Recommendation | Pitfall |
|---|---|
| Wear sunscreen | Person fails to apply sunscreen appropriately16 |
| Person increases time of exposure17 | |
| Wear high-SPF sunscreen | Persons using high-SPF sunscreens have less erythema and tend to increase sun exposure time18 |
| Wear protective clothing | Sun penetrates thin or loosely woven fabrics |
| Wear hat | Brim is not adequate to protect nose, ears, and neck |
Skin cancer education and prevention programs have met with only marginal success. Outcome data on skin cancer reduction through use of sunscreen have shown limited success at best.19,20 In addition, efforts to increase awareness of the danger of sun exposure have had no measurable effect on behavior, even in medical students.21
Screening
The key to diagnosing skin cancer is to have a high degree of suspicion. The U.S. Preventive Services Task Force studied whether a thorough screening skin examination affects outcomes and found no evidence to recommend for or against screening.22 Nevertheless, the Task Force members state that “clinicians should remain alert for skin lesions with malignant features noted in the context of physical examinations performed for other purposes,” and recognize that “even without formal screening programs, mortality from basal cell and squamous cell carcinoma is low compared with mortality from melanoma, but early detection and treatment may reduce morbidity and disfigurement from these cancers.”
Clinical Evaluation
Patients often present with skin lesions, complaining of increasing size, a raised lesion, bleeding, poor healing, discoloration, or discomfort such as pain or itching. Close scrutiny of the arms, face, and upper chest can uncover many lesions and give the physician clues to the need for a more thorough skin examination.
PHYSICAL FINDINGS—BASAL CELL CARCINOMAS
The hallmark of basal cell carcinoma is a waxy, translucent, or pearly appearance. Commonly, these lesions have central ulceration and a raised pale border (Figure 2). The border may be highlighted by applying traction on the skin around the lesion (Figure 3). Telangiectasias are common (Figure 4) and lead to friability, poor healing, and frequent bleeding. Atrophy is possible (Figure 5), and the borders can be indistinct.
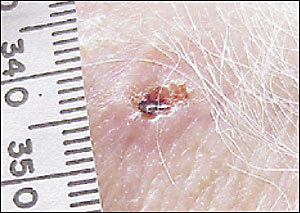
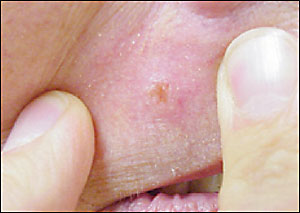
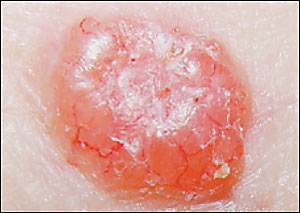
Pigmented basal cell carcinomas are less common and are easily confused with melanoma. They are caused by the presence of melanin within the lesion.23 These lesions have the same structural features described above but, because of the pigmentation, can be mistaken for melanoma. In melanoma or pigmented basal cell carcinomas, treatment is based on tissue diagnosis.
PHYSICAL FINDINGS—ACTINIC KERATOSES AND SQUAMOUS CELL CARCINOMAS
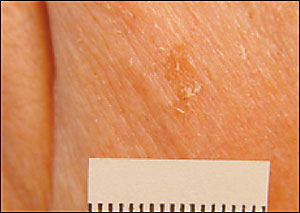
Actinic keratosis may present as a pinhead-sized area of white scale or as a rough patch several centimeters in diameter (Figure 6). Sometimes it presents as a white scale over a pink macule or papule. Often it can be felt more easily than seen. Patients may mention that they have been picking off the scale, but that it keeps returning.
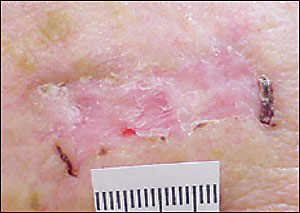
Squamous cell carcinomas arising from actinic keratoses are scaly, as are actinic keratoses, but tend to grow thicker, and the pink macular to papular area develops into an erythematous raised base. Sometimes the lesion develops an overlying keratin horn (Figure 7). The lesions may take the form of a patch, plaque, or nodule, sometimes with scaling or an ulcerated center. The borders often are irregular and bleed easily. Unlike basal cell carcinomas, the heaped-up edges of the lesions are fleshy rather than clear in appearance.
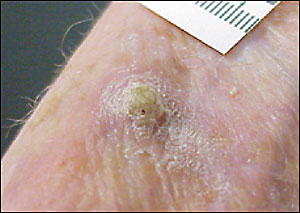
Biopsy
The question of malignancy and cell type of a lesion is often in doubt (Table 3 lists some differential diagnoses). Enlarging or symptomatic lesions that are not clearly benign should be excised (complete removal) or biopsied (partial removal, usually by shave or punch) to determine appropriate treatment. Suspicion of melanoma always warrants full-thickness complete excision unless precluded by the size of the lesion. The extent of local reexcision and consideration of lymph node biopsy are based on the lesion depth on histologic examination. Figure 8 provides a decision tree to guide the type of biopsy chosen.
| Condition | Key to recognition |
|---|---|
| Keratoacanthoma | Rapid growth, spontaneous remission, central plug: excise to confirm because primary lesion usually resolves very slowly, with scarring. |
| Seborrheic keratosis | Pigmented, “stuck-on” appearance, hereditary |
| Extramammary Paget’s disease | Red or white plaque; eroded, anogenital location is most common. |
| Lichen planus | Purple polygonal patches |
| Nummular eczema | History of chronic atopy; lesion responds to eczema treatment |
| Psoriasis | Silvery-white scale, characteristic locations, nail pitting |
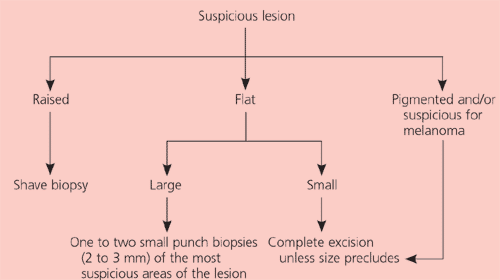
Raised lesions (i.e., most basal cell and squamous cell carcinomas) can be sampled easily with a simple superficial shave biopsy. Hemostasis can be obtained with electro- or chemical cautery. If histology confirms malignancy, definitive treatment is pursued as outlined below.
Small lesions with distinct borders can be excised easily in their entirety with a punch biopsy instrument (available in sizes ranging from 2 to 10 mm.) Elliptic excision (excisional biopsy) with a 3- to 4-mm margin may be selected in areas in which tissue loss is acceptable and cosmesis with a linear scar is expected to be good.24,25 If the excision is complete with clear margins, diagnosis and treatment are done in one procedure.
In the case of large lesions, incisional biopsies, a small punch biopsy (2 to 3 mm), or a shave biopsy is easy to perform. A punch biopsy should not be chosen if the physician is considering electrodesiccation and curettage as the ultimate treatment.
Treatment
Once a lesion has been identified as suspicious, the question becomes how best to treat it. The method of treatment depends somewhat on the diagnosis, lesion size, morphology, and location, as well as patient compliance. If the pathology shows melanoma, reexcision is required, and lymph node sampling might be considered. If the pathology reveals a basal cell carcinoma or squamous cell carcinoma by excisional biopsy and the margins are clear of malignancy, treatment of that lesion is complete, but the physician and patient should be aware of the risk for additional synchronous or future lesions.
Basal cell carcinomas, the most common of all cancers, usually are treated with full-thickness excision, curettage, electro-desiccation and cautery, cryotherapy, or 5-fluorouracil (Efudex), applied topically or intralesionally.26 Other treatments include photodynamic therapy (PDT),27 laser therapy, radiotherapy and, more recently (not yet approved for this purpose by the U.S. Food and Drug Administration), imiquimod (Aldara).28,29 PDT, which uses 5-aminolevulinic acid (Levulan Kerastick) as a tumor sensitizer activated by visible light treatment, can be useful in areas in which loss of tissue would be cosmetically or functionally unacceptable. This process localizes tissue destruction to the cancer without damaging surrounding or deep tissues. Pain and photosensitivity can occur in the first several weeks following treatment. Table 48,9,19,27,30–37 provides more detailed information on treatment modalities.
| Modality | Number of RCTs | Lesion types | Comments |
|---|---|---|---|
| Surgical excision | One (347 patients) | BCC, SCC, AK, potential melanoma | Most effective treatment; can confirm removal of cancer; renders the most tissue loss |
| Radiotherapy | One (347 patients) | BCC | Less effective than surgery (four-year follow-up); can cause dyspigmentation, telangiectasia |
| Curettage with electrocautery | Three | BCC, SCC, AK | Can find and treat meandering margins of lesion based on “feel” of abnormal tissue |
| Cryotherapy | Three (only two used histology) | BCC, SCC, AK | Only fair control of tissue destruction; higher recurrence rate than surgery or radiotherapy; pain, leaking, and wound infection not infrequent |
| Laser destruction | Two | BCC | Higher clinical recurrence rate than cryotherapy; better cosmetic result; no difference compared with broadband light when used with 5-aminolevulinic acid (Levulan Kerastick; see below) |
| Intralesional interferon | Four | BCC | At 20 weeks, 14 to 30 percent failure; can cause influenza-like symptoms, pain, and inflammation at injection site |
| 5-fluorouracil (Efudex) | Two (one = 13 patients and 17 lesions; one = 122 patients and four regimens using 5-fluorouracil) | AK,35 early BCC and SCC | 10 percent failure rate in BCC; good blanket therapy for affected areas. Use twice daily for two weeks or until “ugly” (i.e., marked tissue response with oozing and crusting); expensive |
| Imiquimod (Aldara) | Seven (six sponsored by product manufacturer) | AK, early BCC and SCC | Good blanket therapy for affected areas; use up to three cycles of treatment three times per week for four weeks, then rest for four weeks; expensive |
| 5-aminolevulinic acid with halogen lamp | One (83 patients and 245 treated lesions) | BCC | Used with dimethyl sulfoxide as the vehicle in patients subjected to two types of light therapy with identical outcomes. Not an FDA-approved therapy for BCC at this time.27 Is FDA approved for AK. |
| Mohs’ surgery | — | Large, aggressive, recurrent or location-sensitive BCC and SCC | Most definitive treatment but costly and time intensive |
Electrodesiccation and cautery (traditionally, three scraping and burning cycles performed at a single sitting) is most appropriate for intermediate-size lesions. In contrast to full-thickness excision, electrodesiccation and cautery usually maintains the integrity of the underlying dermis, ensuring adequate healing with no overall loss of skin surface area and with minimal scarring.
If punch biopsy was performed previously, electrodesiccation and cautery is more difficult. Curetting inevitably opens up the site of the punch hole, and there will be a defect into the subcutaneous layer. If a shave biopsy was performed, the electrodesiccation and cautery usually will not go through the dermis, and there will be good healing with time.
Cryotherapy has been used to treat basal cell carcinomas, but targeting tissue destruction is less controlled. Two 30-second freeze-thaw cycles should be employed; this regimen results in 95 percent cure rates on facial lesions.38
With electrodesiccation and cautery, cryotherapy, 5-fluorouracil, and imiquimod therapy, complete lesion removal cannot be confirmed. 5-fluorouracil and imiquimod are well suited for early, superficial, or diffuse but mild disease, in which the likelihood of deep structure invasion is low, and surgical treatment might yield a cosmetically poor result.
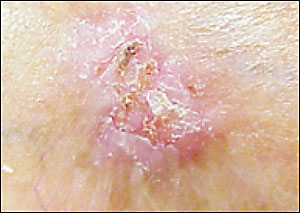
Basosquamous carcinomas, with microscopic features of both basal cell and squamous cell carcinomas (Figure 9), or morphea-like basal cell carcinomas should be excised completely because of their more aggressive nature. This may be done in the primary care office, based on the location of the lesion and the skill of the physician.
There is scant evidence showing which treatments for squamous cell carcinoma are effective. Squamous cell carcinomas usually are treated with the same modalities as basal cell carcinomas. Because these lesions have metastatic potential, surgical removal or Mohs’ micrographic surgery should be considered over destructive techniques.36 Excision allows the physician to confirm that the surgical margins are free of disease.
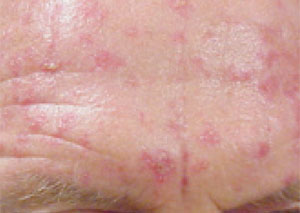
Actinic keratoses are considered by many to be an early form of squamous cell carcinoma, although disagreement still exists in the dermatologic community.39 Cryotherapy is an effective and well-accepted therapy for actinic keratoses. However, actinic keratoses may be diffuse, with clinically unapparent lesions in adjacent skin. Regional therapy with 5-fluorouracil or imiquimod is highly successful in the treatment of early apparent and unapparent lesions. 5-Fluorouracil can be used twice daily for approximately two weeks until marked inflammation develops (Figure 10). Imiquimod is applied three times per week at bedtime for four weeks and washed off each morning. Repeated cycles may be necessary. For the 5 to 20 percent of lesions that do not respond to this treatment, more aggressive therapy (usually cryotherapy) can be used.
Regardless of the initial treatment choice, all patients should be followed closely; the treated area specifically should be watched for recurrence for up to five years, and other lesions should be looked for in these high-risk patients. If any lesion fails to respond to initial treatment, surgical resection is appropriate.
Referral
Patients with any nonmelanoma skin cancer greater than 2 cm, lesions with indistinct margins, recurrent lesions, and those close to important structures, including the eyes, nose, and mouth, should be considered for referral for complete excision via Mohs’ micrographic surgery, with possible plastic repair. The Mohs’ surgeon can confirm the complete removal of the lesion by immediately reviewing the pathology during a staged excision which, in these high-risk settings, can require removal of much more tissue than might have been clinically apparent. If a difficult repair is anticipated or a poor cosmetic result is expected, referral is appropriate.