Am Fam Physician. 2020;102(6):339-346
Related editorial: Keratinocyte Carcinomas: Should We Screen for Them?
Author disclosure: No relevant financial affiliations.
Keratinocyte carcinoma, traditionally referred to as nonmelanoma skin cancer, includes basal cell and cutaneous squamous cell carcinoma and is the most common skin cancer malignancy found in humans. The U.S. Preventive Services Task Force recommends counseling about minimizing exposure to ultraviolet radiation for people aged six months to 24 years with fair skin types to decrease their risk of skin cancer. Routine screening for skin cancer is controversial. The U.S. Preventive Services Task Force concludes that current evidence is insufficient to assess the balance of benefits and harms of a routine whole-body skin examination to screen for skin cancer. Basal cell carcinoma commonly appears as a shiny, pearly papule with a smooth surface, rolled borders, and arborizing telangiectatic surface vessels. Cutaneous squamous cell carcinoma commonly appears as a firm, smooth, or hyperkeratotic papule or plaque, and may have central ulceration. Initial tissue sampling for diagnosis is a shave technique if the lesion is raised, or a punch biopsy of the most abnormal-appearing area of skin. High-risk factors for recurrence and metastasis include prior tumors, ill-defined borders, aggressive histologic patterns, and perineural invasion. Mohs micrographic surgery has the lowest recurrence rate among treatments but is best considered for large, high-risk tumors or tumors in sensitive anatomic locations. Smaller, lower-risk tumors are treated with surgical excision, electrodesiccation and curettage, or cryotherapy. Topical imiquimod and fluorouracil are also treatment options for superficial basal cell carcinoma and squamous cell carcinoma in situ. There are no clear guidelines for follow up after an index keratinocyte carcinoma, but monitoring for recurrence is important because the five-year risk of subsequent skin cancer is 41%. After more than one diagnosis, the five-year risk increases to 82%.
Keratinocyte carcinoma, referred to as nonmelanoma skin cancer, comprises basal cell carcinoma (BCC) and cutaneous squamous cell carcinoma (CSCC). BCC is the most common skin cancer malignancy and affects more than 3.3 million people annually in the United States.1 CSCC is the second most common skin cancer, with up to 400,000 U.S. cases and more than 3,000 disease-related deaths annually.2 Lifetime risk of keratinocyte carcinoma in the United States is at least 20% and is greater than 30% for White patients.3
Reported incidence rates of BCC vary depending on factors such as geographic latitude and sun exposure. Although BCCs can develop early in life, sporadically, and with certain genetic syndromes, age is an independent risk factor. The incidence of BCC increases after age 40. Incidence in younger people is increasing and may be the result of increased sun exposure.4 The proportion of keratinocyte carcinomas represented by CSCC has increased to 30% or greater, primarily in older patients, and is attributed to cumulative ultraviolet light exposure.3
Risk Factors and Prevention
Intermittent sun exposure (e.g., recreational tanning, occupational exposure, childhood sunburns) is the predominant risk factor for BCC. Cumulative sun exposure plays a critical role in CSCC carcinogenesis. The presence of any nevus on an extremity is associated with a higher risk of BCC. One study found that people with 15 or more nevi on the extremities had a 40% greater likelihood of BCC compared with those without any nevi on the extremities. No association between nevus count and CSCC has been observed.5
In addition to cumulative sun exposure, other risk factors for CSCC include chronically diseased or injured skin (e.g., ulcers, sinus tracts), exposure to ionizing or ultraviolet B radiation, immunosuppression (including HIV and drug-induced immunosuppression following organ transplantation), and xeroderma pigmentosum. Organ transplant recipients are 65 times more likely to develop CSCC compared with age-matched control patients. Lesions appear an average of two to four years after transplantation with increasing frequency over time.6
The U.S. Preventive Services Task Force (USPSTF) recommends behavior therapy aimed at minimizing exposure to ultraviolet radiation to decrease the risk of skin cancer for people aged six months to 24 years with fair skin types (i.e., ivory or pale skin, light eye color, red or blond hair, freckles, and those who sunburn easily). The USPSTF also recommends selective counseling for adults 25 years and older with fair skin types, noting that there is limited benefit to counseling all adults. There is insufficient evidence to include people without a fair skin type in the recommendation statement.7 The USPSTF stated the evidence is insufficient to assess the balance of benefits and harms for counseling adults about skin self-examination and routine screening of healthy adults for skin cancer.8
A 2016 Cochrane review found no difference in the incidence of BCC or CSCC with daily sunscreen use compared with occasional use.9 The single randomized trial included in the review demonstrated a 39% reduction in the number of CSCC tumors in the group using sunscreen daily compared with the never-used group.10 No studies evaluated other sun-protection measures, including sun-protective clothing, sunglasses, or hats, or seeking the shade when outdoors.9 Tanning bed use imparts a session-dependent increase in the risk of BCC, with a near-doubling of the risk if tanning bed use begins before age 24.11
Pathophysiology
BASAL CELL CARCINOMA
BCC develops from basal keratinocytes of the epidermis, hair follicles, and eccrine sweat ducts. BCC requires surrounding stroma for support during growth; therefore, the risk of metastasis by blood or lymphatics is less than 1%.
BCC has two common histologic patterns: nodular (60% to 80%) and superficial (20%). Approximately 15% of BCCs exhibit a micronodular pattern, and less than 10% have morpheaform or sclerosing, desmoplastic, or infiltrative changes.12 A mixed pattern, containing two or more histologic patterns, occurs in more than 40% of cases.13
CUTANEOUS SQUAMOUS CELL CARCINOMA
Actinic keratosis is the principal precursor to CSCC. Actinic keratosis and CSCC represent the same disease process at different stages of development, with neoplastic transformation of epidermal keratinocytes commonly triggered by ultraviolet radiation.14 CSCC spreads by local infiltration and expansion, and may follow tissue planes and conduits, such as nerves and vessels. The average risk of nodal metastasis in CSCC is approximately 3%.15 The risk of metastasis increases in patients who are immunosuppressed.16
Estimates of the rate of malignant transformation of actinic keratosis to invasive CSCC vary widely, but may be as high as 20% per year.17 At least 60% of CSCC develop from lesions previously diagnosed as actinic keratoses, and there is a clear association between the number of actinic keratosis lesions and the risk of malignant transformation. Although up to 50% of lesions spontaneously resolve over 12 months, actinic keratosis is a marker for invasive skin cancer.18
Clinical Presentation
BASAL CELL CARCINOMA
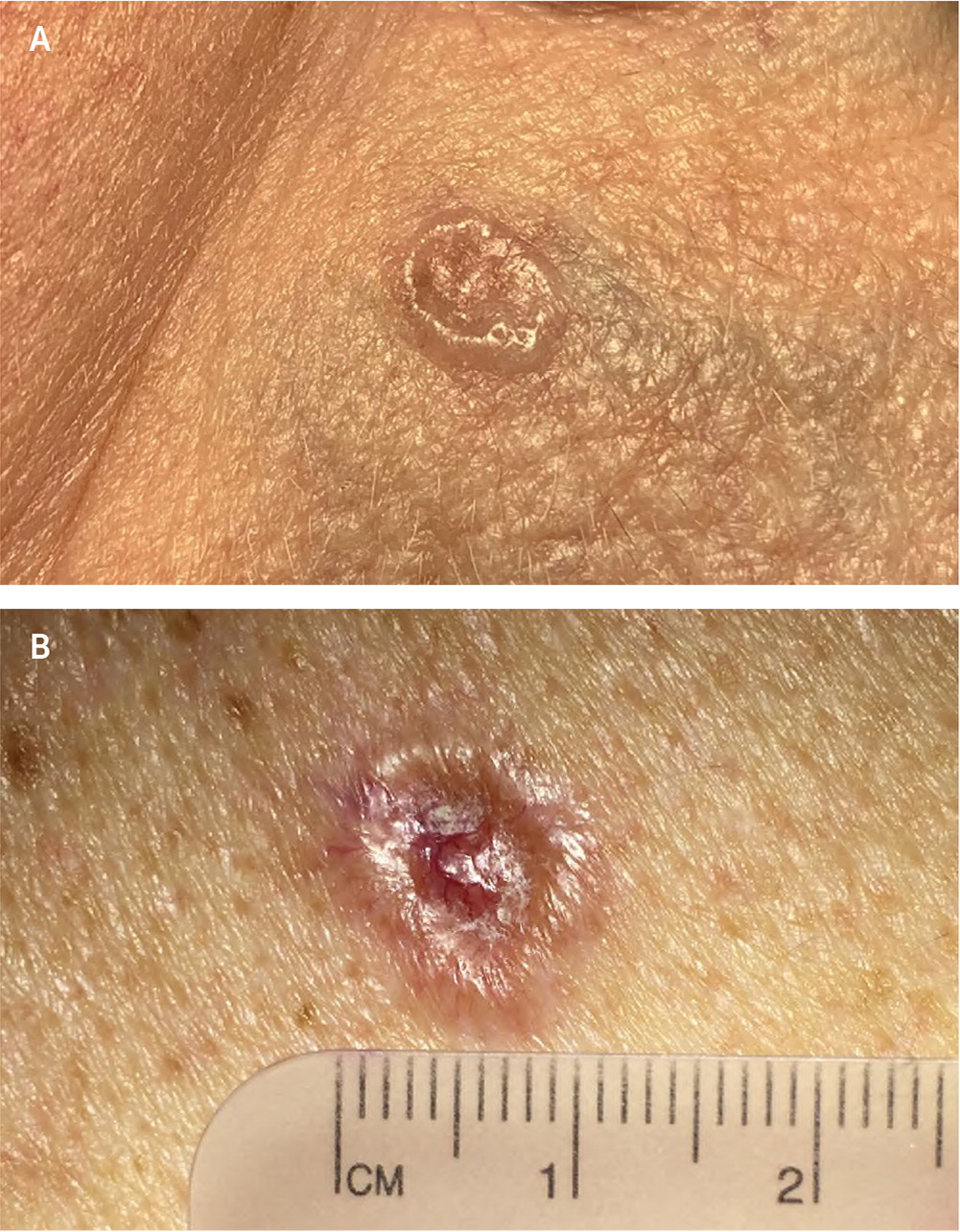
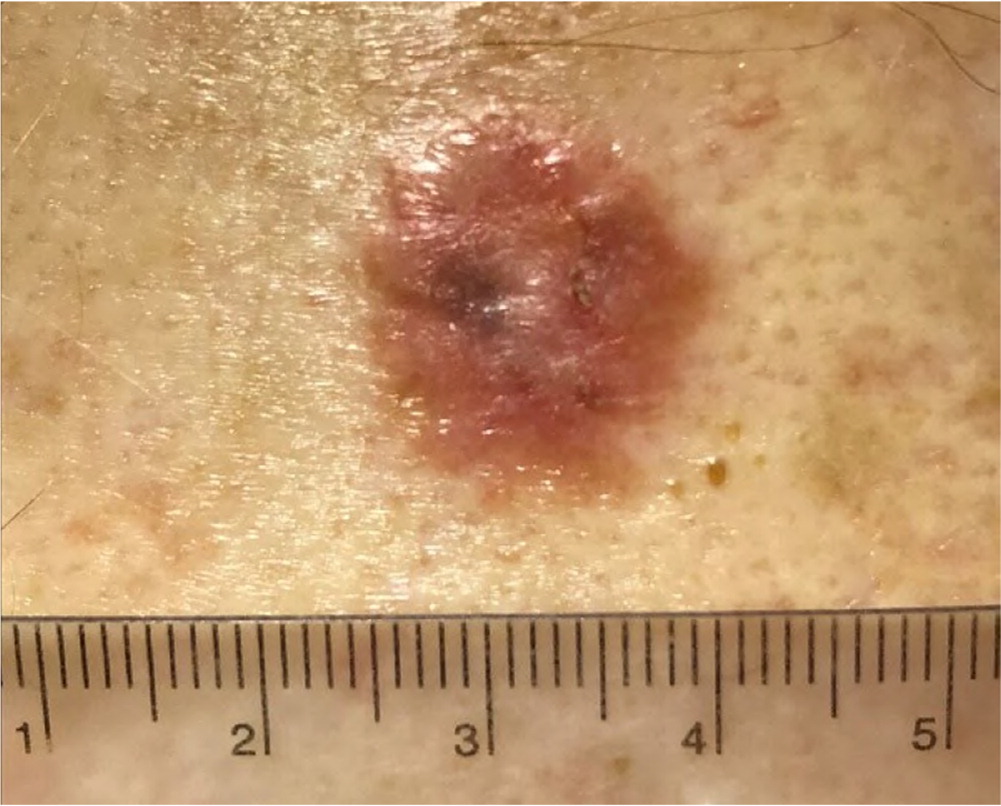
Eighty-five percent of all BCCs appear on the head and neck region.4 Nodular BCC has a variable presentation but typically is a shiny, pearly papule or nodule with a smooth surface, rolled borders, and arborizing telangiectatic surface vessels (Figures 1A and 1B). Pigmented BCC typically has a nodular histologic pattern and contains melanin, which may give the lesion a blue, brown, or black color19 (Figure 2). Pigmented BCC may be mistaken for melanoma. Superficial BCC is the least invasive of the subtypes and most commonly occur on the trunk and extremities (Figure 3). It appears as a thin scaly plaque resembling eczema or psoriasis, but often retains the characteristic raised, pearly borders of the nodular subtype.
Micronodular and infiltrative BCCs are difficult to differentiate clinically from superficial and nodular BCCs and can present as erythematous macules or thin papules or plaques. Micronodular changes often present with other histopathologic patterns and increase the lesion's risk of recurrence. Less than 10% of BCCs exhibit morpheaform or sclerosing changes that present as an infiltrated plaque with poorly defined borders, shiny surface, and may resemble a scar. These lesions are commonly found on the head and neck and are significantly more difficult to treat because of aggressive biologic behavior with local destruction and subclinical extension, and higher local recurrence rates.12
The concordance between the subtype identified on a biopsy specimen vs. excision is 60% to 80%.12,20 Small biopsy specimens with a less worrisome superficial or nodular subtype may show a more aggressive micronodular or infiltrative subtype in more than 25% of complete excisions.20 The most aggressive subtype guides management.
CUTANEOUS SQUAMOUS CELL CARCINOMA
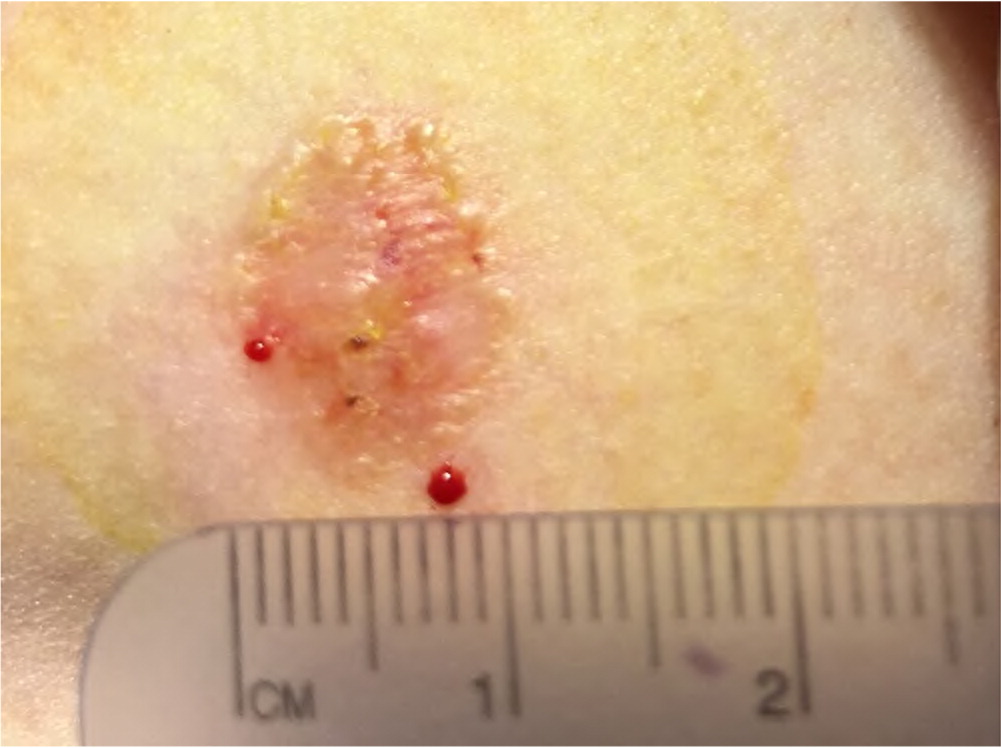
Actinic keratosis presents as a rough, scaly papule on an erythematous base, typically measuring 2 to 6 mm in diameter (Figure 4). The lesion is more easily recognized by palpation than by visual inspection. CSCC most commonly appears as a firm, smooth, or hyperkeratotic papule or plaque, often with central ulceration (Figure 5 and Figure 6). Patients may describe a nonhealing lesion that bleeds with minimal trauma.
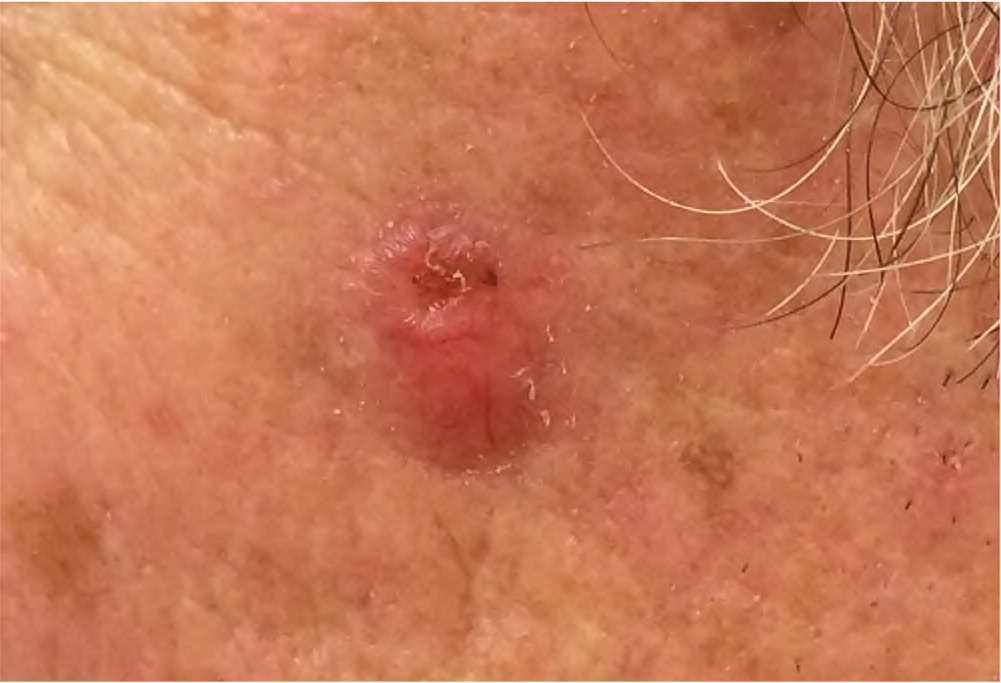
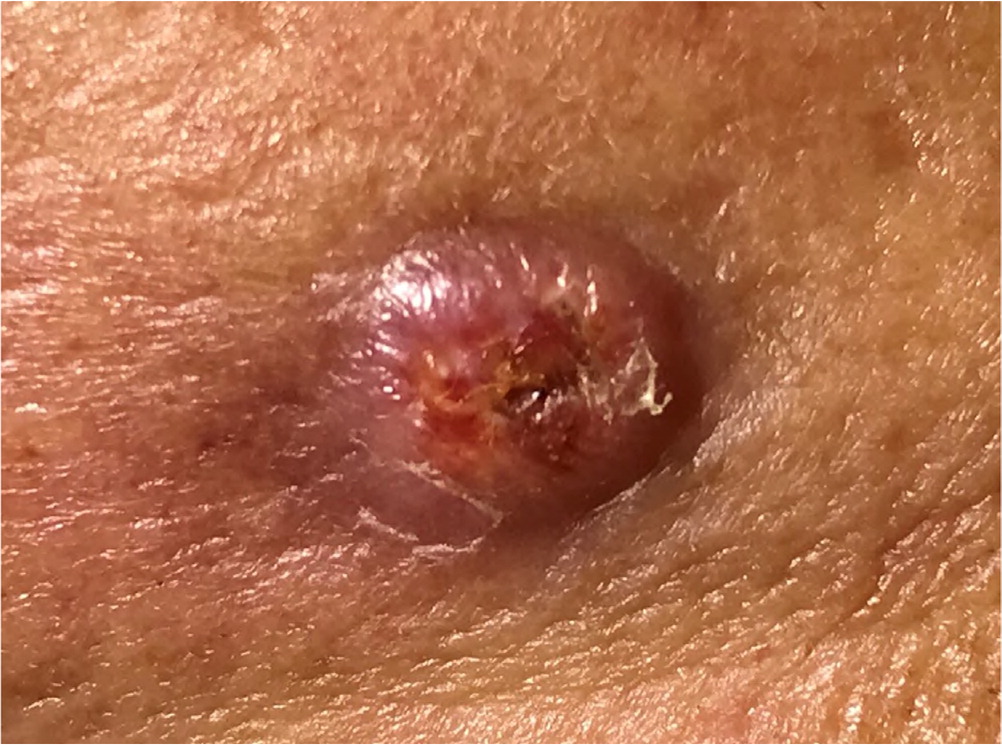
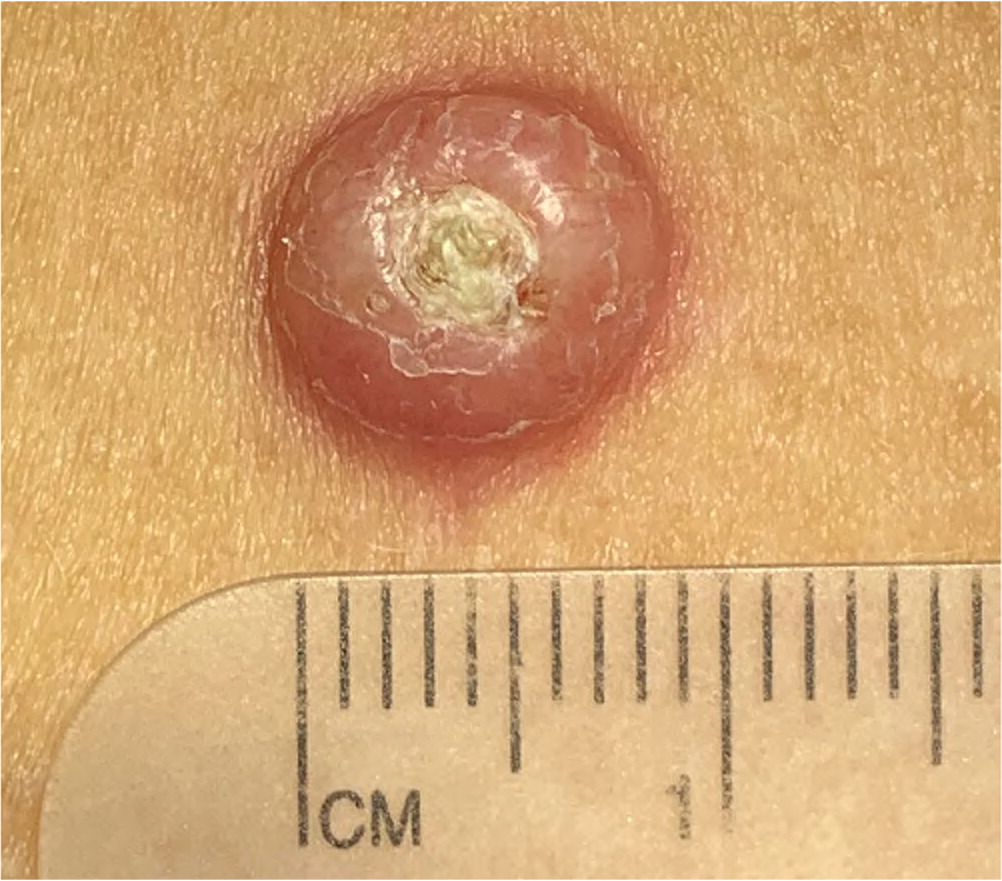
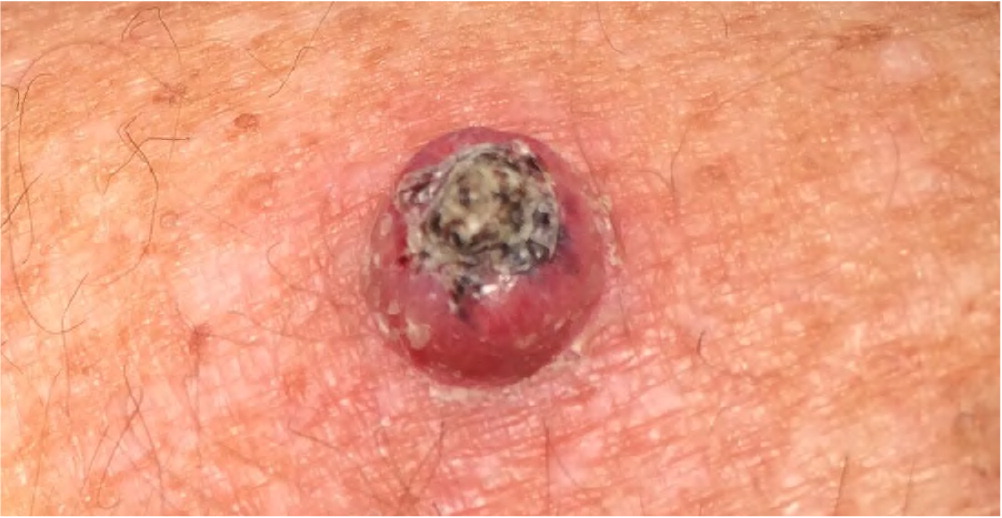
Bowen disease (i.e., squamous cell carcinoma in situ) presents as a slowly growing, scaly, red plaque that typically appears on sun-exposed skin. A cutaneous horn may begin as actinic keratosis and degenerate into squamous cell carcinoma.21 Keratoacanthoma may be difficult to distinguish from CSCC and may contain CSCC at its base. Keratoacanthoma appears as a firm, rapidly growing, erythematous papule with a central keratotic plug (Figure 7). Although it may follow an indolent course, keratoacanthoma is best characterized as a malignant lesion and should be treated as CSCC. Table 1 summarizes and compares the characteristics of BCC and CSCC.1,6

| Characteristics | Basal cell carcinoma | Cutaneous squamous cell carcinoma |
|---|---|---|
| Patient age | Uncommon in adults younger than 40; up to 20% of tumors occur in adults younger than 50 | Uncommon in adults younger than 50 |
| Patient characteristics | Fair skin, blue eyes, red or light-colored hair, inability to tan | Few, if any, identifiable phenotypic markers associated with high risk |
| Tumor location | Most tumors (85%) occur on the head and neck region, with 25% to 30% occurring on the nose; does not correlate well with areas of maximal sun exposure; approximately one-third occur on areas that receive little or no ultraviolet exposure | More common on the back of the hands and forearms; tumors on the head and neck are most common on areas that receive maximal sun exposure |
| Ultraviolet light exposure | Weaker association; exposure in childhood and adolescence more important | Stronger association; cumulative exposure more important |
Diagnosis
Multiple biopsy techniques are available for sampling lesions suspected to be carcinomas. Initial tissue sampling is typically performed using a shave technique if the lesion is raised, or using a punch biopsy of the most abnormal-appearing skin. Complete excision may be an appropriate initial diagnostic procedure for smaller lesions. Pigmented lesions and those with any features concerning for melanoma risk should always be evaluated using a full-thickness technique.
The Brigham and Women's Hospital tumor classification system can determine the stage of CSCC based on tumor risk factors. The eighth edition of the American Joint Committee on Cancer staging manual adds nodal and metastasis classification, but the Brigham and Women's Hospital system may provide superior prognostication for patients with localized CSCC.2 The National Comprehensive Cancer Network has developed a stratification framework that offers practical guidance for clinicians in distinguishing BCC and CSCC at low vs. high risk of recurrence (Table 2).1,2

| Parameters | Low risk | High risk |
|---|---|---|
| Basal cell and squamous cell carcinoma (clinical) | ||
| Location* and size† | Low-risk location and < 20 mm Moderate-risk location‡ and < 10 mm — | Low-risk location and ≥ 20 mm Moderate-risk location and ≥ 10 mm High-risk location§ |
| Borders | Well defined | Poorly defined |
| Primary vs. recurrent | Primary | Recurrent |
| Immunosuppression | No | Yes |
| Site of prior radiation therapy | No | Yes |
| Basal cell carcinoma (pathologic) | ||
| Growth pattern | Superficial, nodular|| | Aggressive¶ |
| Perineural involvement | No | Yes |
| Squamous cell carcinoma (pathologic) | ||
| Degree of differentiation | Well or moderately differentiated | Poorly differentiated |
| High-risk histologic subtype** | No | Yes |
| Depth (thickness or Clark level)†† | < 2 mm or I, II, III | ≥ 2 mm or IV, V |
| Perineural, lymphatic, or vascular involvement | No | Yes |
Treatment
BASAL CELL CARCINOMA
Treatment of BCC with Mohs micrographic surgery has the lowest recurrence rate. However, because of cost and limited availability, it is best considered for larger tumors (i.e., greater than 2 cm on the trunk or extremities), more invasive histologic subtypes (i.e., micronodular, infiltrative, and morpheaform), or tumors at sites with a higher risk of recurrence.22 The recurrence rate for tumors treated with Mohs surgery is 4.4% at 10 years, whereas standard surgical excision has a 12.2% recurrence rate at 10 years.23 The slow growth rate of BCC results in recurrences that are commonly diagnosed more than five years following definitive treatment.
BCC and CSCC are characterized by asymmetric subclinical extension of the tumor beyond the clinically visible lesion. National Cancer Care Network guidelines recommend the excision of low-risk primary BCC with a 4-mm margin of uninvolved skin around the tumor.1 Incomplete excision of the primary tumor (i.e., pathology demonstrating tumor at the surgical margin) should be followed by immediate reexcision or Mohs micrographic surgery because it is difficult to control recurrent BCC.24
Electrodesiccation and curettage is an appropriate choice for low-risk primary, nonfibrosing tumors. Tumor recurrence rates at five years range from less than 2% to more than 20%, depending on lesion characteristics and practitioner skill.25
Consider cryotherapy for low-risk BCC when more effective therapies are contraindicated or impractical. Biopsy should be performed before the procedure to determine tumor depth because cryotherapy is not indicated for tumors that are more than 3-mm deep.26 Reported recurrence rates for cryosurgery range from less than 2% to up to 20%, depending on lesion characteristics and duration of follow-up.13
If surgical excision of BCC is not feasible, contraindicated, or not preferred by the patient, radiotherapy is an additional treatment option. Radiotherapy requires multiple sessions, and postradiation changes can include dyspigmentation and radiodystrophy. Tumor recurrence postradiation may be more difficult to treat.22
Topical imiquimod (Aldara), an immunomodulator, is approved by the U.S. Food and Drug Administration (FDA) for treatment of superficial BCC, but not for other BCC subtypes. Rates of clinical and histologic cure at one year range from 60% to 80%. Monotherapy with topical fluorouracil, an antimetabolite, is also FDA-approved for the treatment of superficial BCC.27 These treatments are optimal for patients with small tumors in low-risk locations who are unable to tolerate more definitive therapies.1
CUTANEOUS SQUAMOUS CELL CARCINOMA
National Cancer Care Network guidelines recommend the excision of low-risk primary CSCC with a 4- to 6-mm margin of uninvolved skin around the tumor.Mohs micrographic surgery is an appropriate option for high-risk tumors or tumors in sensitive anatomic locations.Electrodesiccation and curettage or cryotherapy may be considered for smaller, low-risk lesions. If surgical therapy is not feasible or preferred, consider radiotherapy when tumors are low risk; however, radiotherapy is associated with a lower cure rate. Additionally, postradiation cosmesis can worsen with time and radiotherapy is not usually considered for patients younger than 55.2
There is little evidence from randomized controlled trials comparing the effectiveness of interventions for primary CSCC. Moderate-quality evidence supports the use of topical options (i.e., imiquimod, and fluorouracil) for the treatment of CSCC in situ but are not approved by the FDA. Cryosurgery may be considered for low-risk CSCC when more effective therapies are contraindicated or impractical.4
Cryotherapy is the most appropriate treatment for individual actinic keratosis lesions. Multiple lesions in a defined region may be treated with field therapy using topical fluorouracil, imiquimod, topical diclofenac 3% in 2.5% hyaluronic acid, or ingenol mebutate (Picato). Complete clearance of actinic keratosis lesions is difficult to achieve and the goal of therapy is to decrease the absolute number of actinic keratosis lesions.18 Table 3 summarizes the treatments for keratinocyte carcinomas.28
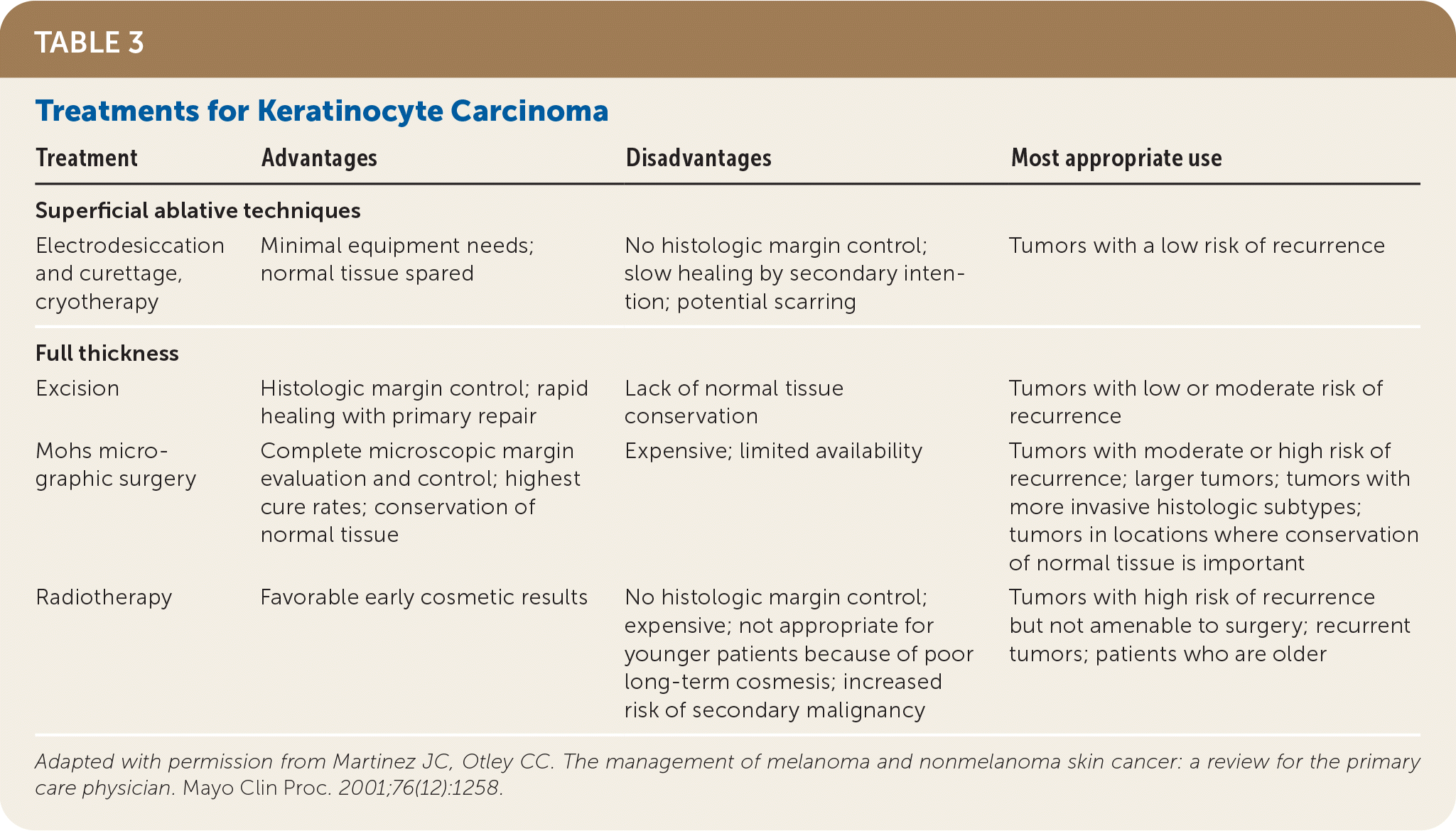
| Treatment | Advantages | Disadvantages | Most appropriate use |
|---|---|---|---|
| Superficial ablative techniques | |||
| Electrodesiccation and curettage, cryotherapy | Minimal equipment needs; normal tissue spared | No histologic margin control; slow healing by secondary intention; potential scarring | Tumors with a low risk of recurrence |
| Full thickness | |||
| Excision | Histologic margin control; rapid healing with primary repair | Lack of normal tissue conservation | Tumors with low or moderate risk of recurrence |
| Mohs micrographic surgery | Complete microscopic margin evaluation and control; highest cure rates; conservation of normal tissue | Expensive; limited availability | Tumors with moderate or high risk of recurrence; larger tumors; tumors with more invasive histologic subtypes; tumors in locations where conservation of normal tissue is important |
| Radiotherapy | Favorable early cosmetic results | No histologic margin control; expensive; not appropriate for younger patients because of poor long-term cosmesis; increased risk of secondary malignancy | Tumors with high risk of recurrence but not amenable to surgery; recurrent tumors; patients who are older |
Follow-Up
After diagnosis, screening of the patient for new primary skin cancers, including BCC, CSCC, and melanoma, should be performed at least once per year. A prospective cohort study found the five-year probability of a subsequent keratinocyte carcinoma after a first diagnosis was 40.7%. After more than one diagnosis, the five-year risk probability increased to 82%.29 Initial diagnosis of keratinocyte carcinoma also more than doubles the risk of subsequent malignant melanoma.30
The article updates previous articles by the author,31 Stulberg, et al.,32 and Jerant, et al.33
Data Sources: Medline and the Cochrane Database of Systematic Reviews was searched for the key terms basal cell carcinoma, cutaneous squamous cell carcinoma, keratinocyte carcinoma; and with limits, humans, English language. Essential Evidence Plus literature summary was reviewed, and key articles and data from review articles and guidelines were incorporated into data sources. Search dates: June 2, 2019; August 11, 2019; and May 19, 2020.