
Am Fam Physician. 2000;62(2):357-368
A more recent article on basal cell and cutaneous squamous cell carcinomas is available.
Patient information: See related handouts on safe-sun guidelines and skin protection, provided by an AAFP staff patient education writer.
The incidence of skin cancer is increasing by epidemic proportions. Basal cell cancer remains the most common skin neoplasm, and simple excision is generally curative. Squamous cell cancers may be preceded by actinic keratoses—premalignant lesions that are treated with cryotherapy, excision, curettage or topical 5-fluorouracil. While squamous cell carcinoma is usually easily cured with local excision, it may invade deeper structures and metastasize. Aggressive local growth and metastasis are common features of malignant melanoma, which accounts for 75 percent of all deaths associated with skin cancer. Early detection greatly improves the prognosis of patients with malignant melanoma. The differential diagnosis of pigmented lesions is challenging, although the ABCD and seven-point checklists are helpful in determining which pigmented lesions require excision. Sun exposure remains the most important risk factor for all skin neoplasms. Thus, patients should be taught basic “safe sun” measures: sun avoidance during peak ultraviolet-B hours; proper use of sunscreen and protective clothing; and avoidance of suntanning.
One in six Americans develops skin cancer at some point. Skin cancer accounts for one third of all cancers in the United States. Most patients with skin cancer develop nonmelanoma skin cancer. This group of cancers includes basal cell carcinoma, the most common neoplasm worldwide, and squamous cell carcinoma. Fortunately, mortality associated with nonmelanoma skin cancer is unusual. However, malignant melanoma accounts for 75 percent of all deaths associated with skin cancer.
Melanoma, the eighth most common malignancy in the United States, is the cancer with the most rapidly increasing incidence. One in 1,500 Americans born in 1935 were likely to develop melanoma, compared with one in 105 persons born in 1993.1 In contrast to nonmelanoma skin cancer, which typically affects older persons, the frequency of melanoma peaks between 20 and 45 years of age.
Mortality rates are higher in men than in women. This higher rate may occur because lesions tend to develop in less easily observed areas, such as the back, in men. Mortality is also increased in blacks for this reason, as is the propensity to develop more aggressive tumors and to be diagnosed at later stages.2
The rising incidence of skin cancer over the past several decades may be primarily attributed to increased sun exposure associated with societal and lifestyle shifts in the U.S. population and to depletion of the protective ozone layer.3
Screening for Skin Cancer
While early detection and treatment of skin cancer can improve patient outcomes,4 convincing data regarding the benefit of mass screening programs are lacking.5 In addition, the ability to identify potentially malignant lesions varies with physician training.6 Thus, except for very high-risk persons with a history of skin cancer or atypical mole syndrome, for whom periodic screening is universally recommended, there is considerable debate about who should be screened, who should perform the screening and how often screening should be performed (Table 1).7 Part of the screening process should include an assessment of patient risk (Table 2).1,8–12
| Organization | Recommendation |
|---|---|
| American Academy of Dermatology; Skin Cancer Foundation; and American Cancer Society | Annual complete skin examination for all patients |
| Annual complete skin examination for all patients | |
| Age 20 to 39 years: complete skin examination every three years | |
| Age 40 years and older: annual complete skin examination | |
| U.S. Preventive Services Task Force and American Academy of Family Physicians Policy Recommendation for Periodic Health Examination* | There is insufficient evidence to recommend for or against routine complete skin examination. |
| Physicians should be alert to potentially malignant lesions when examining patients for other reasons, especially when they have risk factors for melanoma, and should consider referring patients with marker lesions (i.e., atypical nevi) to a skin cancer specialist. |
| Risk factor | Nonmelanoma skin cancer | Melanoma | |
|---|---|---|---|
| Age | More common with increasing age | Peak frequency in early adulthood, but age-related incidence rises with increasing age. | |
| Chemicals and exposures | Use of coal-tar products, tobacco and psoralens (e.g., PUVA therapy) increase risk. | Radiation exposure increases risk. | |
| Family history | No influence on risk | Occurrence of melanoma in a first- or second-degree relative confers increased risk. Familial atypical mole–melanoma syndrome (FAMMS) confers even higher risk. | |
| Gender | Substantially more common in males | Slight male predominance | |
| Geographic location | Higher incidence in whites living near the equator because of greater ultraviolet (UV) light exposure per unit time | Higher incidence in whites living near the equator because of greater UV light exposure per unit time | |
| Medical conditions | Chronic osteomyelitis sinus tracts, burn scars, chronic skin ulcers, xeroderma pigmentosum and human papillomavirus infection all increase risk. | Xeroderma pigmentosum, immunosuppression, other malignancies and previous nonmelanoma skin cancer all increase risk. | |
| Nevi | No influence on risk | Nonfamilial dysplastic nevi, a large number of benign pigmented nevi, and giant pigmented congenital nevi confer increased risk. Nondysplastic nevi are markers for risk, not precursor lesions. | |
| Occupation | Higher incidence in outdoor workers | Higher incidence in indoor workers, as well as those with higher education and income. | |
| Previous history of skin cancer | 36 to 52 percent chance of a new skin cancer of any kind within five years of index case | Previous melanoma is associated with increased risk. | |
| Race | More common in whites | More common in whites | |
| Skin type/ethnicity | Increased incidence in those with fair complexions; those who burn easily, tan poorly and freckle; those who have red, blonde or light brown hair; and those of Celtic ancestry | Increased incidence in those with fair complexions; those who burn easily, tan poorly and freckle; those who have red, blonde or light brown hair; and those of Celtic ancestry | |
| Sun exposure | |||
| Cumulative | Single greatest risk factor; 80 percent of lifetime sun exposure is obtained before 18 years of age. | Probably does not influence risk | |
| Episodic | Probably does not influence risk | Intense, intermittent exposure and blistering sunburns in childhood and adolescence are associated with increased risk. | |
When screening is performed, the examiner must systematically inspect the entire skin surface. The patient should completely disrobe and remove concealing cosmetics. Daylight is the ideal light source, but full-spectrum halogen light or combined incandescent light (which accentuates red tones) and fluorescent light (which accentuates blue and yellow tones) is also satisfactory.13 Photographs may improve the quality of documentation and detection of lesion changes over time.14
Identification and Treatment of Specific Skin Neoplasms
ACTINIC KERATOSES
Actinic keratoses, sometimes called solar keratoses, often arise on chronically sun-damaged body areas such as the face, ears, arms and hands. They may provide an indication of a person's cumulative ultraviolet light exposure and, therefore, that person's risk for all types of skin cancer. Actinic keratoses may also be associated with other exposures15 (Table 2).1,8–12
Actinic keratoses are often ill-defined and irregular, ranging from 1 mm to several centimeters in size. They may be macular or papular, and generally have a scaly appearance (Figure 1). Patients often have multiple lesions. The lesions are usually pale brown or flesh-colored but may be yellow, reddish-brown or even dark brown or black following trauma. The differential diagnosis for actinic keratoses includes benign inflammatory disorders with secondary reactive keratinocytic atypia, Bowen's disease (squamous cell carcinoma in situ), discoid lupus erythematosus, lichen planus and superficial basal cell carcinoma. The rate of malignant transformation of individual actinic keratoses to squamous cell carcinoma is less than one per 1,000 per year,16 but treatment of lesions is indicated to decrease the chance of progression to squamous cell carcinoma. Skin biopsy is occasionally required to rule out squamous cell carcinoma.
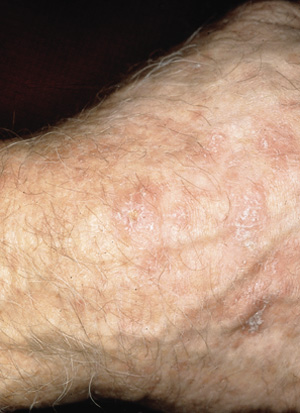
Cryotherapy with liquid nitrogen is the treatment of choice for most cases of actinic keratosis. Curettage may also be used and may be used in conjunction with cryosurgery or electrodessication. Surgical excision is rarely required but may be useful in excluding squamous cell carcinoma as a possible cause in lesions that are larger than 0.5 cm in diameter.15
Chemical destruction of superficial lesions may be used when there are many lesions, particularly on the face and head. A pyrimidine antagonist that inhibits DNA synthesis, 5-fluorouracil (5-FU), is most commonly used. This agent is supplied in several topical formulations, including 1, 2 and 5 percent solutions and 1 and 5 percent creams. Areas other than the head and neck require the higher concentrations because of greater skin thickness. In conventional regimens, 5-FU is applied twice daily for two to five weeks. Adverse effects include true hypersensitivity, secondary bacterial and herpetic infection, and postinflammatory pigmentation changes. This therapy is often associated with significant discomfort related to an intense inflammatory response. Pulsed dosing regimens aimed at reducing skin irritation have met with mixed success.17 Topical corticosteroids may reduce inflammation but also make the treatment end point difficult to discern.18 Other therapies used occasionally for treatment of actinic keratoses include carbon dioxide laser, topical tretinoin, chemexfoliation (chemical peeling) and facial dermabrasion.
SQUAMOUS CELL CARCINOMA
Squamous cell carcinoma is the second most common skin cancer, comprising 20 percent of all cases of nonmelanoma skin cancer. This is the most common tumor in elderly patients, and it is usually the result of a high lifetime cumulative dose of solar radiation. However, other irritants and exposures may lead to squamous cell carcinoma (Table 2).1,8–12 Up to 60 percent of squamous cell carcinomas occur at the site of a previous actinic keratosis.19 Changes in an actinic keratosis that suggest evolution to squamous cell carcinoma include pain, erythema, ulceration, induration, hyperkeratosis and increasing size.
As many as 50 to 60 percent of squamous cell carcinomas occur on the head and neck (Figure 2). Other common sites include the hands and forearms, upper trunk and lower legs. Squamous cell carcinomas typically appear as exophytic tumors that may grow moderately rapidly over a period of months and range from a few millimeters to centimeters in size. They may appear nodular or papular, and may be reddish-brown, pink or flesh-colored. Larger squamous cell carcinomas may appear crusted, erythematous or eroded. In contrast to basal cell carcinoma, a definitive edge is difficult to demonstrate when a squamous cell carcinoma lesion is stretched.16
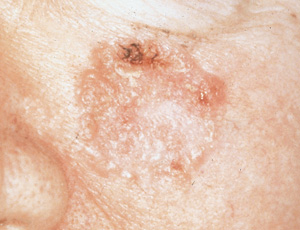
The differential diagnosis of squamous cell carcinoma includes actinic keratosis, amelanotic melanoma, basal cell carcinoma, benign tumors, keratoacanthoma, healing traumatic wounds, spindle cell tumor and warts. Histologic confirmation by a full-thickness skin biopsy (incisional or excisional) is mandatory before definitive treatment. Well-differentiated lesions less than 2 cm in diameter can be treated with surgical excision, with a cure rate approaching 99 percent. Typically, an elliptical excision with margins of 4 to 6 mm is employed.19
Squamous cell carcinomas may grow aggressively and are associated with a 2 to 6 percent risk of metastasis. Risk factors for metastasis include poor cell differentiation, increasing lesion depth and location on the lip or ear. The most common locations for metastatic spread are the regional lymph nodes, lungs and liver. Once metastasis occurs, the five-year cure rate for squamous cell carcinoma is 34 percent. Recurrence and metastasis typically occur within three years of initial treatment.
Mohs' micrographic surgery involves gradual lesion excision using serial frozen section analysis and precise mapping of excised tissue until a tumor-free plane is reached. Mohs' micrographic surgery is used when tissue removal must be kept to a minimum for cosmetic reasons or to maximize function. It is the treatment of choice for difficult and high-risk squamous cell carcinomas, including lesions that are larger than 2 cm in diameter; located in areas where deep invasion is more likely or tumor extent is hard to assess, such as the nasolabial folds, eyelids and periauricular areas (facial “H zone”); rapidly growing; recurrent or incompletely excised; ill-defined; located in an area of previous irradiation; or located where perineural invasion is likely. Cure rates of 99 percent have been reported.20
Cryotherapy and the combination of curettage and desiccation are reserved for treatment of superficial tumors, lesions less than 2 cm in diameter and lesions located on the trunk and extremities. Radiation therapy may be employed when preservation of function and cosmesis are critical, when patients refuse surgery, when metastasis is present or when an adjunct to surgery is required for high-risk tumors. Because of the long-term risk of radiation-induced carcinoma, radiation therapy is used only in patients older than 60 years.16
BASAL CELL CARCINOMA
Basal cell carcinoma is the most common skin neoplasm. Basal cell carcinomas may be classified as one of six types: nodular, the most common type (Figure 3a); pigmented (Figure 3b); cystic; sclerosing or morpheaform (Figure 3c); superficial (Figure 3d); and nevoid. The sclerosing type may extend beyond clinically assessed borders and is the most difficult type to adequately treat. Basal cell carcinomas are usually located on the face or the backs of the hands. They typically grow slowly and generally spread only locally. Metastasis is quite rare.
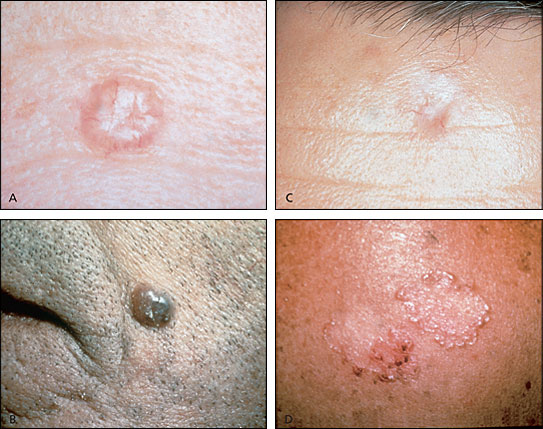
While a preliminary diagnosis of basal cell carcinoma may be made on the basis of appearance, incisional or excisional biopsy is required for definitive diagnosis. Cure rates of 95 to 99 percent can be achieved for low-risk lesions using simple excision with margins of 2 to 5 mm.21,22 A lesion is considered low risk if it is less than 1.5 cm in diameter; has not previously been treated; is not in a difficult-to-treat area, like the H zone of the face; and is nodular or cystic.21
Treatment of basal cell carcinomas with cryotherapy can also be successful, but healing may take weeks, and success depends on the skill of the cryotherapist. Curettage with electrodesiccation or freezing may also be used for tumors less than 1.5 cm in diameter. These modalities are associated with lower cure rates when used to treat sclerosing and recurrent tumors.22 Mohs' micrographic surgery is the treatment of choice for most sclerosing basal cell carcinomas, as well as for large tumors and those located in areas that are difficult to treat. Radiation therapy produces cure rates of 90 to 95 percent but has the same limitations as those outlined for squamous cell carcinoma treatment.22 Other therapies used occasionally include oral retinoids; topical tretinoin; carbon dioxide and neodynium-YAG lasers; topical 5-FU; and, for inoperable and metastatic tumors, chemotherapy.21–23
MALIGNANT MELANOMA
There are four types of malignant melanoma.18 The superficial spreading type is the most common among whites and accounts for 70 percent of all melanomas. It usually occurs in adults and may develop anywhere on the body but appears with increased frequency on the upper backs of both men and women and on the legs of women (Figure 4).
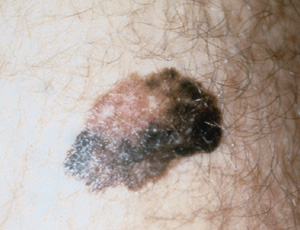
Nodular melanoma (accounting for 15 to 30 percent of all melanomas) is a dome-shaped, pedunculated or nodular lesion that may occur anywhere on the body. It is commonly dark brown or reddish brown but may occasionally be amelanotic. Nodular melanomas tend to rapidly invade the dermis from the onset with no apparent horizontal growth phase. These tumors are frequently misdiagnosed, because they may resemble blood blisters, hemangiomas, dermal nevi or polyps (Figure 5).
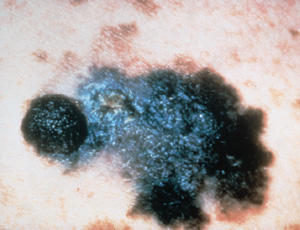
Lentigo maligna melanoma (which accounts for 4 to 10 percent of all melanomas) originates from lentigo maligna. Untreated lentigo maligna tends to exhibit horizontal or radial growth with epidermal involvement for many years (often decades) before it enters the vertical growth phase and invades the dermis to become lentigo maligna melanoma. This change is often indicated clinically by the development of focal papular or nodular areas (Figure 6).
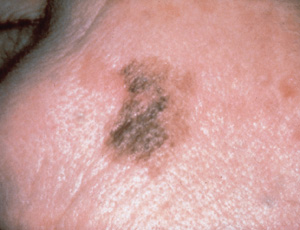
Acral lentiginous melanoma (2 to 8 percent of all melanomas) occurs on the palmar and plantar surfaces, the digits and the subungual areas (Figure 7). Unlike other subtypes of melanoma, it has a similar incidence in all ethnic groups.
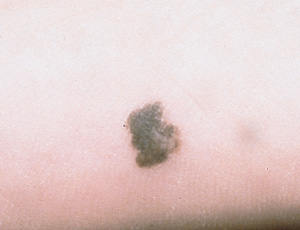
Melanomas that do not fit easily into any of these subtypes are simply labeled malignant melanoma.
Evaluation of Pigmented Lesions
Common benign lesions that mimic melanoma include seborrheic keratoses, benign acquired nevi and solar lentigenes. Other lesions that mimic melanoma are more ominous. Large congenital nevi (greater than 20 cm in diameter) are clearly associated with an increased risk of melanoma,24 and controversy exists regarding the potential for malignant transformation in smaller congenital nevi. Lentigo maligna (Hutchinson's melanotic freckle), discussed above, is commonly located in sun-damaged areas on the heads and necks of elderly persons. Because lentigo maligna is considered by many authorities to be a melanoma in situ, prompt excision is generally indicated.
Atypical or dysplastic nevi may lead to melanoma. These macular to papular lesions may be present in great numbers in affected patients and up to 6 mm or larger in size. They have a cobblestone surface with a variable mixture of tan, brown, and red or pink coloration, and characteristically ill-defined borders (Figure 8). Dysplastic nevi with substantial asymmetry, excessive pigmentary variegation and focal black or gray areas suggestive of partial regression should be biopsied. In the atypical mole syndrome, an autosomal dominant familial disorder, affected persons have numerous dysplastic nevi and often develop melanoma at a young age. Patients with atypical mole syndrome require periodic screening and preventive education.25
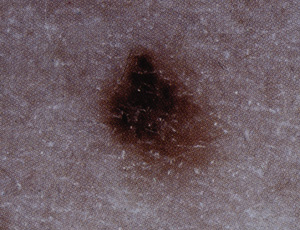
Clinical application of the ABCD checklist may improve detection of suspicious lesions26 (Table 3). Although melanomas may lack one or more of these features, their presence, especially in combination, should raise suspicion of malignancy. The ABCD checklist has been reported to have a sensitivity of 92 to 100 percent and a specificity of 98 percent for the detection of melanoma.11 It may also be prudent to lower the threshold for biopsy when a patient is worried that a nevus may be cancerous or reports recent changes in the nevus such as increased size, alteration of shape or color, itching, burning, bleeding or pain. The revised seven-point checklist, which incorporates these findings, has a reported sensitivity of 79 to 100 percent (Table 4).6 Its low specificity of 30 to 37 percent reflects the fact that most melanomas are asymptomatic.6 Dermoscopic evaluation of suspicious lesions may also help clarify the need for biopsy. In dermoscopy, a hand-held microscope with a 10-power lens is pressed on the skin lesion after the application of oil. The presence or absence of subsurface features suggestive of melanoma guides the need for biopsy.27
| Checklist item | Reassuring elements | Nonreassuring elements |
|---|---|---|
| A = Asymmetry Suggestive of melanoma if the lesion is bisected and the halves are not identical | ||
| Symmetric (benign) | Asymmetric (malignant melanoma) | |
| B = Border irregularity Suggestive of melanomaif the border is uneven or ragged | ||
| Borders are even (benign) | Borders are irregular (malignant melanoma) | |
| C = Color variation Suggestive of melanoma if there is more than one shade of pigment | ||
| One shade/even color (benign) | Two or more shades/uneven color (malignant melanoma) | |
| D = Diameter Suggestive of melanoma if the diameter is greater than 6 mm | ||
| Diameter < 6 mm (benign) | Diameter > 6 mm (malignant melanoma) |
Diagnosis, Staging and Therapy
Biopsies for evaluation of suspected melanoma should generally be excisional. An elliptical excision that includes 1 to 2 mm of normal skin surrounding the lesion and extends to the subcutaneous tissue provides acceptable histopathologic margins. A larger margin is unnecessary and may entail an excessively wide definitive excision if melanoma is confirmed. Incisional and punch biopsies of the most visually concerning lesion segments, considered acceptable by some, may be problematic because the histologic diagnosis of melanoma depends on overall lesion architecture and because such areas may not contain the most histopathologically diagnostic tissue.28 Therefore, complete excision should be strongly considered even when the lesion is large or located in a cosmetically sensitive area.
Prognosis in patients with clinically localized disease is directly related to tumor thickness (Table 5).18,29 The prognosis is more favorable in women, in persons less than 50 years of age and for tumors that occur on an extremity (excluding the hands and feet). The five-year survival rate in patients with nodal spread is about 36 percent and drops to 5 percent in those with distant metastasis.6 Patients with melanoma proved by biopsy should be expeditiously referred to a dermatologist for treatment.
| Breslow's microstages | Five-year survival (%) |
|---|---|
| Stage 1: < 0.76 mm | > 98 |
| Stage 2: 0.76 to 1.49 mm | 87 to 94 |
| Stage 3: 1.50 to 3.99 mm | 66 to 83 |
| Stage 4: > 4.0 mm | < 50 |
Prevention of Skin Cancer
Primary prevention of skin cancer is based on increasing public awareness of the risks of sun exposure and providing patients with individualized guidance. Patients who are educated about risk factors for skin cancer are more likely to self-select for clinical screening30 and to bring malignant lesions to the attention of a health care provider.31 Family physicians can easily integrate education regarding “safe sun” guidelines into general preventive counseling (Table 6).1 While the role of sunscreens has been questioned, most evidence suggests that their correct use can lower the risk of skin cancer.32 Sufficient amounts must be applied at least 30 minutes before sun exposure, with reapplication after prolonged exposure or swimming. Most importantly, sunscreens will not be effective unless they are employed as only one element of a skin cancer prevention program.
| Sun exposure during the peak ultraviolet-B (UV-B) hours should be avoided or minimized. The peak UV-B period is from 10 a.m. to 4 p.m. |
| Sunscreen with a solar protection factor (SPF) of at least 15 should be generously applied. |
| In addition to use of an appropriate sunscreen, persons should wear wide-brimmed hats, sunglasses and protective clothing (e.g., tightly woven fabrics and long-sleeved shirts) when sun exposure during peak UV-B hours cannot be avoided. |
| Deliberate sun tanning and use of tanning parlors should be avoided. |
Resources for patients and health care providers are listed in Table 7.
| American Academy of Family Physicians | |
| “How to Prevent Skin Cancer” (patient education handout) | |
| Web address: https://www.aafp.org/patientinfo/980915a.html | |
| American Cancer Society | |
| 1599 Clifton Rd., NE | |
| Atlanta, GA 30329 | |
| Telephone: 1–800–227–2345 | |
| Web address: www.cancer.org | |
| The Skin Cancer Foundation | |
| P.O. Box 561 | |
| Department SEC | |
| New York, NY 10156 | |
| Telephone: 1–800-SKIN-490 | |
| www.skincancer.org/home | |
| American Academy of Dermatology | |
| 930 N. Meacham Rd. | |
| Schaumburg, IL 60173–4965 | |
| Telephone: 1–888–462–3376 | |
| Web address: www.aad.org | |