
A more recent article on hyperthyroidism is available.
Am Fam Physician. 2005;72(4):623-630
Patient information:A handout on treating hyperthyroidism, written by the authors of this article.
Author disclosure: Nothing to disclose.
The proper treatment of hyperthyroidism depends on recognition of the signs and symptoms of the disease and determination of the etiology. The most common cause of hyperthyroidism is Graves’ disease. Other common causes include thyroiditis, toxic multinodular goiter, toxic adenomas, and side effects of certain medications. The diagnostic workup begins with a thyroid-stimulating hormone level test. When test results are uncertain, measuring radionuclide uptake helps distinguish among possible causes. When thyroiditis is the cause, symptomatic treatment usually is sufficient because the associated hyperthyroidism is transient. Graves’ disease, toxic multinodular goiter, and toxic adenoma can be treated with radioactive iodine, antithyroid drugs, or surgery, but in the United States, radioactive iodine is the treatment of choice in patients without contraindications. Thyroidectomy is an option when other treatments fail or are contraindicated, or when a goiter is causing compressive symptoms. Some new therapies are under investigation. Special treatment consideration must be given to patients who are pregnant or breastfeeding, as well as those with Graves’ ophthalmopathy or amiodarone-induced hyperthyroidism. Patients’ desires must be considered when deciding on appropriate therapy, and close monitoring is essential.
Clinical hyperthyroidism, also called thyrotoxicosis, is caused by the effects of excess thyroid hormone and can be triggered by different disorders. Etiologic diagnosis influences prognosis and therapy. The prevalence of hyperthyroidism in community-based studies has been estimated at 2 percent for women and 0.2 percent for men.1 As many as 15 percent of cases of hyperthyroidism occur in patients older than 60 years.2
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| The choice of radioactive iodine, antithyroid medication, or surgery for hyperthyroidism should be based on the cause and severity of the disease as well as on the patient’s age, goiter size, comorbid conditions, and treatment desires. | C | 16 |
| Total thyroidectomy is recommended only for patients with severe disease or large goiters in whom recurrences would be more problematic. | C | 22,23 |
| Nonselective beta blockers such as propranolol (Inderal) should be prescribed for symptom control because they have a more direct effect on hypermetabolism. | C | 25 |
Clinical Presentation
Hyperthyroidism presents with multiple symptoms that vary according to the age of the patient, duration of illness, magnitude of hormone excess, and presence of comorbid conditions. Symptoms are related to the thyroid hormone’s stimulation of catabolic enzymopathic activity and catabolism, and enhancement of sensitivity to catecholamines. Common symptoms and signs are listed in Table 1,3 with attention to the differences in clinical presentation between younger and older patients. Older patients often present with a paucity of classic signs and symptoms, which can make the diagnosis more difficult.4 Thyroid storm is a rare presentation of hyperthyroidism that may occur after a stressful illness in a patient with untreated or undertreated hyperthyroidism and is characterized by delirium, severe tachycardia, fever, vomiting, diarrhea, and dehydration.5
Etiology
| Cause | Pathophysiology | Gland size* | Nodularity | Tenderness |
|---|---|---|---|---|
| Toxic adenoma | Autonomous hormone production | Decreased | Single nodule | Nontender |
| Toxic multinodular goiter | Autonomous hormone production | Increased | Multiple nodules | Tender |
| Subacute thyroiditis | Leakage of hormone from gland | Increased | None | Tender |
| Lymphocytic thyroiditis, postpartum thyroiditis, medication-induced thyroiditis | Leakage of hormone from gland | Moderately increased | None | Nontender |
| Graves’ disease (thyroid-stimulating antibody) | Increased glandular stimulation (substance causing stimulation) | Increased | None | Nontender |
| Iodine-induced hyperfunctioning of thyroid gland (iodide ingestion, radiographic contrast, amiodarone [Cordarone]) | Increased glandular stimulation (substance causing stimulation) | Increased | Multiple nodules or no nodules | Nontender |
| Functioning pituitary adenoma (thyroid-stimulating hormone); trophoplastic tumors (human chorionic gonadotropin) | Increased glandular stimulation (substance causing stimulation) | Increased | None | Nontender |
| Factitial hyperthyroidism | Exogenous hormone intake | Decreased | None | Nontender |
| Struma ovarii; metastatic thyroid cancer | Extraglandular production | Decreased | None | Nontender |
GRAVES’ DISEASE
Graves’ disease is the most common cause of hyperthyroidism, accounting for 60 to 80 percent of all cases.8 It is an autoimmune disease caused by an antibody, active against the thyroid-stimulating hormone (TSH) receptor, which stimulates the gland to synthesize and secrete excess thyroid hormone. It can be familial and associated with other autoimmune diseases. An infiltrative ophthalmopathy accompanies Graves’ disease in about 50 percent of patients.9
TOXIC MULTINODULAR GOITER
Toxic multinodular goiter causes 5 percent of the cases of hyperthyroidism in the United States and can be 10 times more common in iodine-deficient areas. It typically occurs in patients older than 40 years with a long-standing goiter, and has a more insidious onset than Graves’ disease.10
TOXIC ADENOMA
Toxic adenomas are autonomously functioning nodules that are found most commonly in younger patients and in iodine-deficient areas.10
THYROIDITIS
Subacute
Subacute thyroiditis produces an abrupt onset of thyrotoxic symptoms as hormone leaks from an inflamed gland. It often follows a viral illness. Symptoms usually resolve within eight months. This condition can be recurrent in some patients.11
Lymphocytic and Postpartum
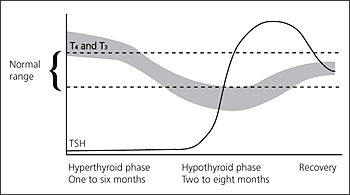
Lymphocytic thyroiditis and postpartum (subacute lymphocytic) thyroiditis are transient inflammatory causes of hyperthyroidism that, in the acute stage, may be clinically indistinguishable from Graves’ disease. Postpartum thyroiditis can occur in up to 5 to 10 percent of women in the first three to six months after delivery. A transient hypothyroidism often occurs before resolution (Figure 112).11
TREATMENT-INDUCED HYPERTHYRIODISM
Iodine-induced
Iodine-induced hyperthyroidism can occur after intake of excess iodine in the diet, exposure to radiographic contrast media, or medications. Excess iodine increases the synthesis and release of thyroid hormone in iodine-deficient patients and in older patients with preexisting multinodular goiters.5
Amiodarone-induced
Amiodarone- (Cordarone-) induced hyperthyroidism can be found in up to 12 percent of treated patients, especially those in iodine-deficient areas, and occurs by two mechanisms. Because amiodarone contains 37 percent iodine, type I is an iodine-induced hyperthyroidism (see above). Amiodarone is the most common source of iodine excess in the United States. Type II is a thyroiditis that occurs in patients with normal thyroid glands. Medications such as interferon and interleukin-2 (aldesleukin) also can cause type II.5
Thyroid hormone-induced
Factitial hyperthyroidism is caused by the intentional or accidental ingestion of excess amounts of thyroid hormone. Some patients may take thyroid preparations to achieve weight loss.
TUMORS
Rare causes of hyperthyroidism include metastatic thyroid cancer, ovarian tumors that produce thyroid hormone (struma ovarii), trophoblastic tumors that produce human chorionic gonadotrophin and activate highly sensitive TSH receptors, and TSH-secreting pituitary tumors.5
Diagnostic Workup
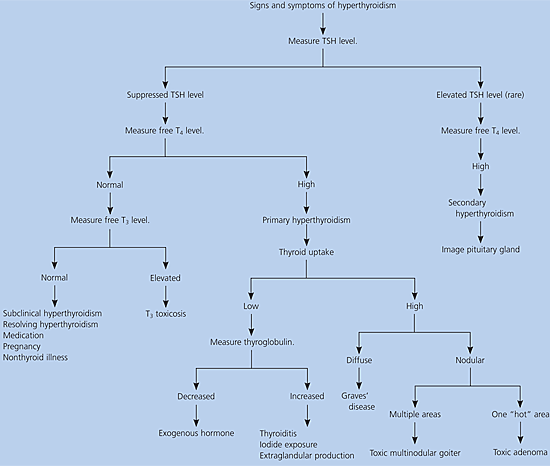
A diagnostic approach to patients who present with signs and symptoms of hyperthyroidism is summarized in Figure 2.5,13 Measurement of the TSH level is the only initial test necessary in a patient with a possible diagnosis of hyperthyroidism without evidence of pituitary disease. Further testing is warranted if the TSH level is abnormal. An undetectable TSH level is diagnostic of hyperthyroidism. Antithyroid antibodies are elevated in Graves’ disease and lymphocytic thyroiditis but usually are not necessary to make the diagnosis.14 Thyroid-stimulating antibody levels can be used to monitor the effects of treatment with antithyroid drugs in patients with Graves’ disease.15 Radionuclide uptake and scan easily distinguishes the high uptake of Graves’ disease from the low uptake of thyroiditis and provides other useful anatomic information. Nonspecific laboratory findings can occur in hyperthyroidism, including anemia, granulocytosis, lymphocytosis, hypercalcemia, transaminase elevations, and alkaline phosphatase elevation.5
Treatment
The treatment of hyperthyroidism depends on the cause and severity of the disease, as well as on the patient’s age, goiter size, comorbid conditions, and treatment desires. The goal of therapy is to correct the hypermetabolic state with the fewest side effects and the lowest incidence of hypothyroidism. Beta blockers and iodides are used as treatment adjuncts. Antithyroid drugs, radioactive iodine, and surgery are the main treatment options for persistent hyperthyroidism (Table 3).5,8,9,14–24 Each therapy can produce satisfactory outcomes if properly used.16
| Treatment | Mechanism of action | Indications | Contraindications and complications |
|---|---|---|---|
| Beta blockers | Inhibit adrenergic effects | Prompt control of symptoms; treatment of choice for thyroiditis; first-line therapy before surgery, radioactive iodine, and antithyroid drugs; short-term therapy in pregnancy | Use with caution in older patients and in patients with pre-existing heart disease, chronic obstructive pulmonary disease, or asthma |
| Iodides | Block the conversion of T4 to T3 and inhibit hormone release | Rapid decrease in thyroid hormone levels; preoperatively when other medications are ineffective or contraindicated; during pregnancy when antithyroid drugs are not tolerated; with antithyroid drugs to treat amiodarone- (Cordarone-) induced hyperthyroidism | Paradoxical increases in hormone release with prolonged use; common side effects of sialadenitis, conjunctivitis, or acneform rash; interferes with the response to radioactive iodine; prolongs the time to achieve euthyroidism with antithyroid drugs |
| Antithyroid drugs (methimazole [Tapazole] and PTU) | Interferes with the organification of iodine; PTU can block peripheral conversion of T4 toT3 in large doses | Long-term treatment of Graves’ disease (preferred first-line treatment in Europe, Japan, and Australia); PTU is treatment of choice in patients who are pregnant and those with severe Graves’ disease; preferred treatment by many endocrinologists for children and for adults who refuse radioactive iodine; pretreatment of older and cardiac patients before radioactive iodine or surgery; both medications considered safe for use while breastfeeding | High relapse rate; relapse more likely in smokers, patients with large goiters, and patients with positive thyroid-stimulating antibody levels at end of therapy; major side effects include polyarthritis (1 to 2 percent), agranulocytosis (0.1 to 0.5 percent); PTU can cause elevated liver enzymes (30 percent), and immunoallergic hepatitis (0.1 to 0.2 percent); methimazole can cause rare cholestasis and rare congenital abnormalities; minor side effects (less than 5 percent) include rash, fever, gastrointestinal effects, and arthralgia |
| Radioactive iodine | Concentrates in the thyroid gland and destroys thyroid tissue | High cure rates with singledose treatment (80 percent); treatment of choice for Graves’ disease in the United States, multinodular goiter, toxic nodules in patients older than 40 years, and relapses from antithyroid drugs | Delayed control of symptoms; posttreatment hypothyroidism in majority of patients with Graves’ disease regardless of dosage (82 percent after 25 years); contraindicated in patients who are pregnant or breastfeeding; can cause transient neck soreness, flushing, and decreased taste; radiation thyroiditis in 1 percent of patients; may exacerbate Graves’ ophthalmopathy; may require pretreatment with antithyroid drugs in older or cardiac patients |
| Surgery (subtotal thyroidectomy) | Reduces thyroid mass | Treatment of choice for patients who are pregnant and children who have had major adverse reactions to antithyroid drugs, toxic nodules in patients younger than 40 years, and large goiters with compressive symptoms; can be used for patients who are noncompliant, refuse radioactive iodine, or fail antithyroid drugs, and in patients with severe disease who could not tolerate recurrence; may be done for cosmetic reasons | Risk of hypothyroidism (25 percent) or hyperthyroid relapse (8 percent); temporary or permanent hypoparathyroidism orlaryngeal paralysis (less than 1 percent); higher morbidity and cost than radioactive iodine; requires patient to be euthyroid preoperatively with antithyroid drugs or iodides to avoid thyrotoxic crisis |
BETA BLOCKERS
Beta blockers offer prompt relief of the adrenergic symptoms of hyperthyroidism such as tremor, palpitations, heat intolerance, and nervousness. Propranolol (Inderal) has been used most widely, but other beta blockers can be used. Nonselective beta blockers such as propranolol, are preferred because they have a more direct effect on hypermetabolism.25 Therapy with propranolol should be initiated at 10 to 20 mg every six hours. The dose should be increased progressively until symptoms are controlled. In most cases, a dosage of 80 to 320 mg per day is sufficient.5 Calcium channel blockers such as diltiazem (Cardizem) can be used to reduce heart rate in patients who cannot tolerate beta blockers.17
IODIDES
Iodides block the peripheral conversion of thyroxine (T4) to triiodothyronine (T3) and inhibit hormone release. Iodides also are used as adjunctive therapy before emergency nonthyroid surgery, if beta blockers are unable to control the hyperthyroidism, and to reduce gland vascularity before surgery for Graves’ disease.9 Iodides are not used in the routine treatment of hyperthyroidism because of paradoxical increases in hormone release that can occur with prolonged use. Organic iodide radiographic contrast agents (e.g., iopanoic acid or ipodate sodium) are used more commonly than the inorganic iodides (e.g., potassium iodide). The dosage of either agent is 1 g per day for up to 12 weeks.26
ANTITHYROID DRUGS
Antithyroid drugs act principally by interfering with the organification of iodine, thereby suppressing thyroid hormone levels. Methimazole (Tapazole) and propylthiouracil (PTU) are the two agents available in the United States. Remission rates vary with the length of treatment, but rates of 60 percent have been reported when therapy is continued for two years.15 Relapse can occur in up to 50 percent of patients who respond initially, regardless of the regimen used. A recent randomized trial27 indicated that relapse was more likely in patients who smoked, had large goiters, or had elevated thyroid-stimulating antibody levels at the end of therapy.
Methimazole
Methimazole usually is the drug of choice in nonpregnant patients because of its lower cost, longer half-life, and lower incidence of hematologic side effects. The starting dosage is 15 to 30 mg per day, and it can be given in conjunction with a beta blocker.28 The beta blockade can be tapered after four to eight weeks and the methimazole adjusted, according to clinical status and monthly free T4 or free T3 levels, toward an eventual euthyroid (i.e., normal T3 and T4 levels) maintenance dosage of 5 to 10 mg per day.9,17 TSH levels may remain undetectable for months after the patient becomes euthyroid and should not be used to monitor the effects of therapy. At one year, if the patient is clinically and biochemically euthyroid and a thyroid-stimulating antibody level is not detectable, therapy can be discontinued. If the thyroid-stimulating antibody level is elevated, continuation of therapy for another year should be considered. Once antithyroid drug therapy is discontinued, the patient should be monitored every three months for the first year, because relapse is more likely to occur during this time, and then annually, because relapse can occur years later. If relapse occurs, radioactive iodine or surgery generally is recommended, although antithyroid drug therapy can be restarted.9
Propylthiouracil
Complications
Agranulocytosis is the most serious complication of antithyroid drug therapy and is estimated to occur in 0.1 to 0.5 percent of patients treated with these drugs.28 The risk is higher in the first several months of therapy and may be higher with PTU than methimazole.5,9,15 It is extremely rare in patients taking less than 30 mg per day of methimazole.9 The onset of agranulocytosis is sometimes abrupt, so patients should be warned to stop taking the drug immediately if they develop a sudden fever or sore throat. Routine monitoring of white cell counts remains controversial, but results of one study29 showed that close monitoring of white cell counts allowed for earlier detection of agranulocytosis. In this study, patients had white cell counts every two weeks for the first two months, then monthly. In most cases, agranulocytosis is reversible with supportive treatment.15,25 Minor side effects (e.g., rash, fever, gastrointestinal symptoms) sometimes can be treated symptomatically without discontinuation of the antithyroid drug; however, if symptoms of arthralgia occur, antithyroid drugs should be discontinued because arthralgia can be a precursor of a more serious polyarthritis syndrome.28
RADIOACTIVE IODINE
In the United States, radioactive iodine is the treatment of choice for most patients with Graves’ disease and toxic nodular goiter. It is inexpensive, highly effective, easy to administer, and safe. There has been reluctance to use radioactive iodine in women of childbearing years because of the theoretical risk of cancer of the thyroid, leukemia, or genetic damage in future offspring. Long-term follow-up of patients has not validated these concerns.14,15 The treatment of hyperthyroidism in children remains controversial, but radioactive iodine is becoming more acceptable in this group.30
Dosage
The treatment dosage of radioactive iodine has been a topic of much debate. A gland-specific dosage based on the estimated weight of the gland and the 24-hour uptake may allow a lower dosage and result in a lower incidence of hypothyroidism but may have a higher recurrence rate.15 Higher-dose ablative therapy increases the chance of successful treatment and allows the early hypothyroidism that results from this regimen to be diagnosed and treated while the patient is undergoing close monitoring. Some studies8,18 have shown that the eventual incidence of hypothyroidism is similar regardless of the radioactive iodine dosage. The high-dose regimen is clearly favored in older patients, those with cardiac disease, and other groups who need prompt control of hyperthyroidism to avoid complications. Patients with toxic nodular goiter or toxic adenomas are more radio resistant and generally need high-dose therapy to achieve remission. They have a lower incidence of eventual hypothyroidism because the rest of the gland has been suppressed by the toxic nodules and protected from the effects of radioactive iodine.18,30
Graves’ Disease
In 15 percent of patients, Graves’ ophthalmopathy can develop or be worsened by the use of radioactive iodine.17,19 The use of prednisone, 40 to 80 mg per day tapered over at least three months, can prevent or improve severe eye disease in two thirds of patients.19 Lower-dose radioactive iodine sometimes is used in patients with ophthalmopathy because posttreatment hypothyroidism may be associated with exacerbation of eye disease. Cigarette smoking is a risk factor for the development and progression of Graves’ ophthalmopathy.14,19
Use with OtherTreatments
Using antithyroid drugs to achieve a euthyroid state before treatment with radioactive iodine is not recommended for most patients, but it may improve safety for patients with severe or complicated hyperthyroidism. Limited evidence supports this approach.8,14,17 It is unclear whether antithyroid drugs increase radioactive iodine failure rates.20,31,32 If used, they should be withdrawn at least three days before radioactive iodine and can be restarted two to three days later. The antithyroid drug is continued for three months after radioactive iodine, then tapered. Beta blockers are used to control symptoms before radioactive iodine and can be continued throughout treatment if needed. Iodine-containing medications need to be discontinued several weeks before therapy.21
Safety Precautions
Most of the radioactive iodine is eliminated from the body in urine, saliva, and feces within 48 hours; however, double flushing of the toilet and frequent hand washing are recommended for several weeks. Close contact with others, especially children and pregnant women, should be avoided for 24 to 72 hours.21 Additional treatments with radioactive iodine can be initiated as early as three months, if indicated.33
SURGERY
Gradually, radioactive iodine has replaced surgery for the treatment of hyperthyroidism, but it still may be indicated in some patients and is considered underused by some researchers. A subtotal thyroidectomy is performed most commonly. This surgery preserves some of the thyroid tissue and reduces the incidence of hypothyroidism to 25 percent, but persistent or recurrent hyperthyroidism occurs in 8 percent of patients.22 Total thyroidectomy is reserved for patients with severe disease or large goiters in whom recurrences would be highly problematic, but carries an increased risk of hyperparathyroidism and laryngeal nerve damage.22,23
NEW POSSIBILITIES
Newer treatment options under investigation include endoscopic subtotal thyroidectomy,34 embolization of the thyroid arteries,35 plasmapheresis,36 and percutaneous ethanol injection of toxic thyroid nodules.37 Autotransplantation of cryopreserved thyroid tissue may become a treatment option for postoperative hypothyroidism.38 Nutritional supplementation with L-carnitine39 has been shown to have a beneficial effect on the symptoms of hyperthyroidism, andl-carnitine may help prevent bone demineralization caused by the disease.
Prognosis and Follow-up
The prognosis for a patient with hyperthyroidism is good with appropriate treatment. Indications for referral and admission are listed in Table 4.6 Even with aggressive treatment, some manifestations of the disease may be irreversible, including ocular, cardiac, and psychologic complications. Patients treated for hyperthyroidism have an increased all-cause mortality risk, as well as increased risk of mortality from thyroid, cardiovascular and cerebrovascular diseases, and hip fractures.5,15,40 Morbidity can be attributed to the same causes, and patients should be screened and treated for osteoporosis and atherosclerotic risk factors. Patients who have been treated previously for hyperthyroidism have an increased incidence of obesity41 and insulin resistance.42 The effect of hyperthyroidism on endothelial function may be an independent risk factor for thromboembolism.43
| Indication for referral | Type of referral |
|---|---|
| Radioactive iodine therapy | Endocrinologist or radiologist |
| Amiodarone- (Cordarone-) induced hyperthyroidism | Endocrinologist and cardiologist |
| Graves’ ophthalmopathy | Endocrinologist and ophthalmologist |
| Obstruction | Surgeon |
| Pregnancy | Endocrinologist or surgeon (if contraindications to antithyroid drugs) |
| Cosmesis | Surgeon |
| Breastfeeding | Endocrinologist |
| Failed drug therapy or refusal to take radioactive iodine | Surgeon |
| Visual impairment caused by ophthalmopathy | Hospital admission with urgent ophthalmology consult |
| Severe cardiovascular symptoms Such as congestive heart failure, Rapid atrial fibrillation, or angina | Hospital admission with endocrine and cardiac consultation |
Patients should be monitored closely after any treatment for hyperthyroidism, especially during the first three months. After the first year, patients should be monitored annually even if they are asymptomatic. Patient education concerning the risk of relapse and possible late-onset hypothyroidism is imperative.