
A more recent article on chronic kidney disease is available.
Am Fam Physician. 2005;72(9):1723-1732
Patient information: See related handout on chronic kidney disease, written by the authors of this article.
Author disclosure: nothing to disclose.
Chronic kidney disease affects approximately 19 million adult Americans, and its incidence is increasing rapidly. Diabetes and hypertension are the underlying causes in most cases of chronic kidney disease. Evidence suggests that progression to kidney failure can be delayed or prevented by controlling blood sugar levels and blood pressure and by treating proteinuria. Unfortunately, chronic kidney disease often is overlooked in its earliest, most treatable stages. Guidelines from the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (KDOQI) recommend estimating glomerular filtration rate and screening for albuminuria in patients with risk factors for chronic kidney disease, including diabetes, hypertension, systemic illnesses, age greater than 60 years, and family history of chronic kidney disease. The glomerular filtration rate, calculated by using a prediction equation, detects chronic kidney disease more accurately than does the serum creatinine level alone; the glomerular filtration rate also is used for disease staging. In most clinical situations, analysis of random urine samples to determine the albumin-creatinine or protein-creatinine ratio has replaced analysis of timed urine collections. When chronic kidney disease is detected, an attempt should be made to identify and treat the specific underlying condition(s). The KDOQI guidelines define major treatment goals for all patients with chronic kidney disease. These goals include slowing disease progression, detecting and treating complications, and managing cardiovascular risk factors. Primary care physicians have an important role in detecting chronic kidney disease early, in instituting measures to slow disease progression, and in providing timely referral to a nephrologist.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| All adults with risk factors for chronic kidney disease should be screened with a serum creatinine determination for GFR estimation and analysis of a random urine sample for proteinuria. | C | 1,5,6 |
| Instead of a timed urine collection, a random urine sample for the microalbumin-creatinine or protein-creatinine ratio should be used to quantify proteinuria. | C | 1,24,25 |
| Interventions proved to slow the progression of chronic kidney disease include blood pressure control, glycemic control, and reduction of proteinuria with an angiotensin-converting enzyme inhibitor or angiotensin-II receptor blocker. | A | 1,24,33,34,37 |
| A low-density lipoprotein goal of less than 100 mg per dL (2.60 mmol per L) is recommended for patients with chronic kidney disease, because these patients are statistically at highest risk for cardiovascular disease. | C | 37 |
| A blood pressure goal of 130/80 mm Hg is recommended in patients with normal urinary albumin concentrations, and a blood pressure goal of 125/75 mm Hg is recommended in patients with proteinuria equal to or greater than 1 g per 24 hours. | B | 1,30 |
Approximately 19 million Americans older than 20 years have chronic kidney disease, and an additional 435,000 have end-stage renal disease (Table 11 ). The incidence of end-stage renal disease, with its annual mortality rate of 24 percent, has doubled every decade since 1980.2 Chronic kidney disease is 100 times more prevalent than end-stage renal disease, and its incidence is increasing at an even faster rate.
| Chronic kidney disease | |
| Kidney damage for three or more months based on findings of abnormal structure (imaging studies) or abnormal function (blood tests, urinalysis) | |
| or | |
| GFR below 60 mL per minute per 1.73 m2 for three or more months with or without evidence of kidney damage | |
| End-stage renal disease (kidney failure) | |
| GFR below 15 mL per minute per 1.73 m2 | |
| or | |
| Need for kidney replacement therapy (dialysis or transplant) | |
Early treatment of chronic kidney disease and its complications may delay or prevent the development of end-stage renal disease. Consequently, detection of chronic kidney disease should be a priority for family physicians. However, data from national screening programs suggest that chronic kidney disease often is not detected, even when patients have access to primary care.3,4
The Kidney Disease Outcomes Quality Initiative (KDOQI) from the National Kidney Foundation (NKF) has developed guidelines for the detection and evaluation of chronic kidney disease.5,6 These guidelines define the disease and its stages and outline treatment goals for each stage. This article focuses on the detection of chronic kidney disease and the initial evaluation of affected patients.
Detection of Chronic Kidney Disease
WHICH PATIENTS TO SCREEN
The KDOQI guidelines1,6 recommend assessing all patients for kidney-disease risk factors. Further screening is performed in patients with identified risk factors. Although screening methods for chronic kidney disease have not been evaluated in randomized controlled trials,7 the high prevalence of the disease in at-risk populations, the ease of screening, and the availability of effective treatments during early asymptomatic stages of the disease provide sufficient rationale for screening.8 Nonetheless, screening rates for patients with known risk factors for chronic kidney disease are as low as 20 percent.3,4
High-risk groups that should be screened for chronic kidney disease include patients who have a family history of the disease and patients who have diabetes, hypertension, recurrent urinary tract infections, urinary obstruction, or a systemic illness that affects the kidneys.1 A recent analysis9 suggested that screening all patients older than 60 years is cost-effective even when other risk factors for chronic kidney disease are absent; screening low-risk patients younger than 60 years does not appear to be cost-effective.
Diabetes is the most common cause of kidney disease. From 40 to 60 percent of patients who progress to end-stage renal disease have diabetes. Other underlying conditions in patients with end-stage renal disease include hypertension (15 to 30 percent), glomerulonephritis (less than 10 percent), and cystic kidney (2 to 3 percent). Unknown causes account for the remaining patients with end-stage renal disease.2
HOW TO SCREEN
Screening patients at risk for chronic kidney disease relies on the detection of functional abnormalities using readily available, inexpensive laboratory tests. The measured serum creatinine level is used to calculate an estimated glomerular filtration rate (GFR). Screening for proteinuria often alerts the physician to the presence of chronic kidney disease before changes in the GFR become apparent.
Current KDOQI guidelines1,5,6 recommend screening for kidney disease with a serum creatinine measurement for use in GFR estimation and analysis of a random urine sample for albuminuria. Significant kidney disease can present with decreased GFR or proteinuria, or both. An analysis7 of data from the third National Health and Nutrition Examination Survey (NHANES III) showed that 20 percent of persons with diabetes, and 43 percent of persons with hypertension and a GFR below 30 mL per minute per 1.73 m2, had no proteinuria. Therefore, an estimate of the GFR and a screening method for proteinuria are required.1
Selected patients with risk factors for kidney disease should be screened with renal ultrasonography. Indications for this study include suspected urinary tract obstruction, recurrent urinary tract infections, vesicoureteral reflux, and a family history of polycystic kidney disease.1
ESTIMATING THE GFR
| Stage | GFR (mL per minute per 1.73 m2) |
|---|---|
| 1 | ≥90 |
| 2 | 60 to 89 |
| 3 | 30 to 59 |
| 4 | 15 to 29 |
| 5 | < 15 or dialysis |
The standard for GFR measurement is the clearance rate of inulin, a substance that passes through the kidney unchanged. Creatinine clearance, as measured by a 24-hour urine collection, usually overestimates the GFR because of the active secretion of creatinine by the kidney and can vary with muscle mass.11
Significant kidney dysfunction may be present despite a normal serum creatinine level. An estimation of the GFR based on the serum creatinine level correlates better with direct measures of the GFR and detects more cases of chronic kidney disease than does the serum creatinine level alone. Furthermore, patients with the same serum creatinine level may have different estimated GFRs. For example, a 45-year-old black man whose serum creatinine level is 1 mg per dL (88 μmol per L) has normal kidney function with an estimated GFR of 130 mL per minute per 1.73 m2, whereas a 65-year-old white woman with the same serum creatinine level has an estimated GFR of 59 mL per minute per 1.73 m2, or stage 3 kidney disease.
Based on an analysis7 of NHANES III data, 20 percent of persons with diabetes, and 14.2 percent of persons with hypertension but no diabetes, have a GFR below 60 mL per minute per 1.73 m2. The prevalence of GFRs below 60 mL per minute per 1.73 m2 increases steadily with age; 22.5 percent of nondiabetic, nonhypertensive octogenarians have a GFR below this level.
Clinically useful GFR estimates are calculated from the measured serum creatinine level12,13 after adjustments for age, sex, and race. A GFR of 100 mL per minute per 1.73 m2 is considered normal for women, and 120 mL per minute per 1.73 m2 is a normal GFR for men.1 The two most commonly used formulas for GFR estimation are shown in Table 3.12–18 These methods have been studied in a variety of populations.12,14–18 Validation studies12 performed in middle-aged patients with chronic kidney disease showed that the Modification of Diet in Renal Disease (MDRD) study equation was more accurate than the Cockcroft-Gault equation, which calculates creatinine clearance. In a recent study,18 however, the MDRD study equation was found to systematically underestimate the GFR in patients without chronic kidney disease.
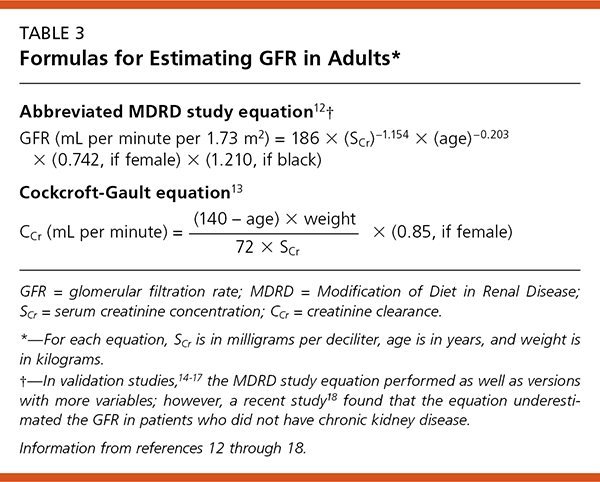
In most situations and as long as kidney function is stable, a calculated GFR can replace measurement of a 24-hour urine collection for creatinine clearance. A user-friendly GFR calculator is available online at http://www.kidney.org/professionals/kdoqi/gfr_page.cfm. Determination of creatinine clearance using a 24-hour urine collection is still required to assess kidney function in patients with the conditions listed in Table 4.3,12–17
| Method | Situations for use |
|---|---|
| MDRD study equation for estimating GFR* | Patients with diabetic kidney disease† |
| Patients with chronic kidney disease in middle-age (average age: 51 years)† | |
| Black patients with hypertensive chronic kidney disease† | |
| Patients with a kidney transplant† | |
| Cockcroft-Gault equation for estimating creatinine clearance* | Older patients (performs better than the MDRD study equation) |
| 24-hour urine collection for creatinine clearance | Pregnant women |
| Patients with extremes of age and weight | |
| Patients with malnutrition | |
| Patients with skeletal muscle diseases | |
| Patients with paraplegia or quadriplegia | |
| Patients with a vegetarian diet and rapidly changing kidney function |
DETECTING AND QUANTITATING PROTEINURIA
Proteinuria is associated with more rapid progression of chronic kidney disease and a greater likelihood of developing end-stage renal disease. Consequently, detection and quantitation of proteinuria are essential to the diagnosis and treatment of chronic kidney disease.
Albumin, the predominant protein excreted by the kidney in most types of renal disease, is detected readily by urine dip-stick testing. In some conditions, immunoglobulins also may be excreted in urine. The protein-creatinine ratio in an early-morning random urine sample correlates well with 24-hour urine protein excretion and is much easier to obtain.1
An analysis7 of NHANES III data showed that 8.3 percent of 14,622 adults had microalbuminuria (i.e., excretion of 30 to 300 mg of albumin per 24 hours) and 1 percent had macroalbuminuria (i.e., excretion of more than 300 mg of albumin per 24 hours). Albuminuria was detected in one of every three persons with diabetes, one of every seven persons with hypertension but no diabetes, and one of every six persons older than 60 years.
Microalbuminuria often heralds the onset of diabetic nephropathy. In a recent study21 of patients with type 1 diabetes, spontaneous regression of microalbuminuria occurred in some patients, suggesting that microalbuminuria may represent an initial reversible phase of kidney damage rather than the beginning of an inexorable progression to end-stage renal disease.22
The KDOQI guidelines1 and the American Diabetes Association (ADA) guidelines23 recommend screening for microalbuminuria in all patients at risk for kidney disease. Screening can be performed using a microalbumin-sensitive dipstick or analysis of a random morning urine sample to determine the microalbumin-creatinine ratio. Microalbumin dipsticks have a sensitivity of 51 to 100 percent and a specificity of 27 to 97 percent.24 The ADA25 recommends repeated sampling, but the NKF1 and others question the necessity for this. An algorithm for detecting proteinuria and microalbuminuria is provided in Figure 1.1
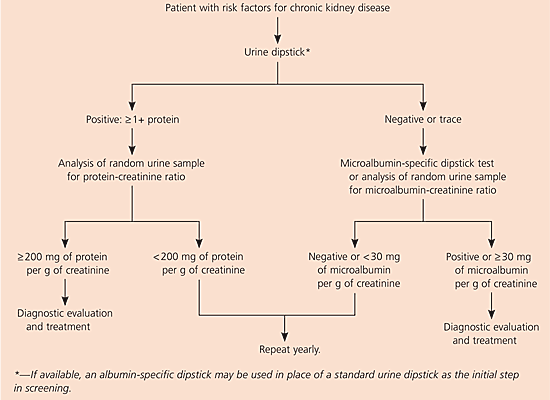
The value of screening for microalbuminuria has been questioned in patients who already are receiving ACE-inhibitor therapy26 on the basis that the results are unlikely to change management. One study27 in patients with type 2 diabetes showed that increasing the dose of an ARB to decrease or eliminate microalbuminuria provides additional benefit in slowing progression to overt nephropathy. Therefore, current research suggests that it may be beneficial to monitor patients with chronic kidney disease, including those who are taking an ACE inhibitor or ARB, for persistence of microalbuminuria or for progression to overt proteinuria. The medication dosage should be adjusted as tolerated, with the goal of eliminating albuminuria.
Evaluation of Patients with Chronic Kidney Disease
Once chronic kidney disease has been identified, goals include determining the stage of the disease, establishing the cause of the disease, and evaluating comorbid conditions. All patients with chronic kidney disease should undergo urinalysis and renal imaging as part of the diagnostic evaluation. Patients with long-standing diabetes, hypertension, and a clinical course consistent with chronic kidney disease secondary to these conditions may not require further evaluation.1
The evaluation of all patients is guided by the symptoms (e.g., rash, arthritis, or urinary symptoms); family history of kidney disorders (e.g., cystic kidney diseases); and known medical problems. Underlying diseases may be identified by the physical examination, with special attention given to the skin, joints, and cardiovascular system. Table 528 summarizes the common presentations and appropriate serologic evaluations for the most common causes of chronic kidney disease.
| Disorder | Clinical clues | Urine sediment | Protein-creatinine ratio | Additional tests |
|---|---|---|---|---|
| Diabetes mellitus | Diabetes for > 15 years, retinopathy | RBCs in < 25 percent of affected patients | > 30 to > 3,500 mg of protein per g of creatinine | Fasting blood sugar, A1C |
| Essential hypertension | Left ventricular hypertrophy, retinopathy | Benign | > 30 to 3,000 mg of protein per gram of creatinine | No additional tests |
| Glomerulonephritis | History and physical examination: infections; rash, arthritis; patient older than 40 years | Dysmorphic RBCs or RBC casts | > 30 to > 3,500 mg of protein per g of creatinine | C3 and C4 for all patients |
| Tests for infections: anti-ASO, ASK, HIV, HBsAg, HCV, RPR, blood cultures | ||||
| Tests if there is rash or arthritis: ANA, ANCA, cryoglobulin, anti-GBM | ||||
| Tests if patient is older than 40 years: SPEP, UPEP | ||||
| Interstitial nephritis | Medications, fever, rash, eosinophilia | WBCs, WBC casts, eosinophils | 30 to 3,000 mg of protein per g of creatinine | ACE level; SS–A, SS–B |
| Low flow states | Volume depletion, hypotension, congestive heart failure, cirrhosis, atherosclerosis | Hyaline casts, eosinophils | < 200 mg of protein per g of creatinine | FENa: < 1 percent; eosinophilia |
| Urinary tract obstruction | Urinary symptoms | Benign, or RBCs | None | KUB radiography, intravenous pyelography, spiral CT scanning, renal ultrasonography |
| Chronic urinary tract infection | Urinary symptoms | WBCs, RBCs | < 2,000 mg of protein per g of creatinine | Pelvic examination, urine culture, voiding cystourethrography, renal ultrasonography, CT scanning |
| Neoplasm, paraproteinemia | Patient older than 40 years, constitutional symptoms, anemia | RBCs, RBC casts, granular casts | False-negative result or > 30 to > 3,500 mg of protein per g of creatinine | SPEP, UPEP, calcium level, ESR |
| Cystic kidney disease | Palpable kidneys with or without family history of cystic kidney disease, flank pain | RBCs | 30 to 3,000 mg of protein per g of creatinine | Renal ultrasonography or CT scanning if there is a complex kidney cyst or mass |
| Renovascular disease | Late-onset or refractory hypertension, sudden onset of hypertension in young woman, smoking history, abdominal bruit | Benign | < 200 mg of protein per g of creatinine | Renal Doppler ultrasonography, radioisotope renal scanning, MRA, renal angiography |
| Vasculitis | Constitutionalsymptoms, peripheral neuropathy, rash, respiratory symptoms | RBCs; granular casts | > 30 to > 3,500 mg of protein per g of creatinine | C3, C4, ANA, ANCA; HBsAg, HCV, cryoglobulins, ESR, RF, SS–A, SS–B, HIV |
Several tests may help determine the underlying cause of chronic kidney disease. Tests for complements 3 and 4 are used to screen for collagen vascular disease, hepatitis C–related disease, and infection-related immune complex disease. The antineutrophil cytoplasmic antibody assay identifies vasculitis, whereas serum protein electrophoresis and urine protein electrophoresis detect multiple myeloma.
Renal ultrasonography helps establish the diagnosis and prognosis by documenting the size of the kidneys. Normal size indicates kidney disease that may be amenable to medical treatment. Small kidneys suggest irreversible disease. Asymmetry in kidney size suggests renovascular disease.
Imaging studies that may be useful in identifying the cause of chronic kidney disease are listed in Table 6.1,28 Renal biopsy is indicated when the cause cannot be determined by the history and laboratory evaluation, when the patient’s signs and symptoms suggest parenchymal disease, and when the differential diagnosis includes diseases that require different treatments or that have different prognoses.11 Biopsy more commonly is required in patients with chronic kidney disease that is not related to diabetes, and biopsy often is indicated in adult patients with nephrotic syndrome or suspected glomerulonephritis. Based on an international survey29 of nephrologists, rates of biopsy vary widely in practice.
| Imaging study | What the study helps identify |
|---|---|
| Plain-film radiography of kidneys, ureters, and bladder | Ureter or bladder stones |
| Renal ultrasonography | Kidney size, obstructive kidney disease, polycystic kidney disease |
| Renal Doppler ultrasonography | Renovascular disease, renal vein thrombosis |
| Radioisotope renal scanning | Individual kidney function, renovascular disease, obstructive uropathy |
| Computed tomography | Kidney mass or complex cyst |
| Magnetic resonance angiography | Renovascular disease |
| Renal angiography | Renovascular disease, renal artery thrombosis/thromboembolism, polyarteritis nodosa |
| Retrograde ureterography | Upper urinary tract obstruction |
The management of chronic kidney disease depends on the specific treatment of the underlying cause, the stage of the kidney disease, and the presence or absence of proteinuria. Treatment goals for all patients include slowing disease progression, detecting and managing complications, and preventing cardiovascular disease.
RATE OF DISEASE PROGRESSION
The rate of progression for chronic kidney disease depends on the underlying cause. In general, tubulointerstitial diseases progress more slowly than do glomerular diseases, diabetic and hypertensive nephropathy, and polycystic kidney disease.11 Rates of progression also vary widely among patients with the same type of kidney disease.
In rapidly progressing kidney disease, the GFR may decrease by as much as 10 to 20 mL per minute per 1.73 m2 per year. In more slowly progressing disease, the GFR may decrease by as little as 2 mL per minute per 1.73 m2 per year. Plotting the GFR against time is helpful in estimating the rate of disease progression and the time to kidney failure, and it helps predict the need for kidney replacement therapy (Figure 21 ).
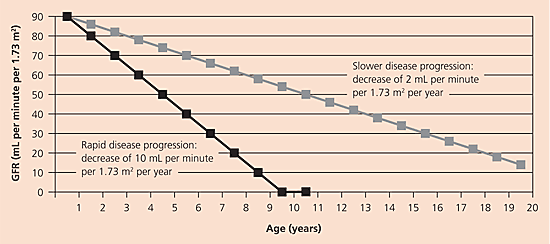
Three interventions have been proved to slow the progression of kidney disease: blood pressure control,30 glycemic control in patients with diabetes,1 and reduction of proteinuria with an ACE inhibitor or ARB.19,20,31,32 Other interventions that may be beneficial include lipid-lowering measures, partial correction of anemia,1 and limiting dietary protein intake to 0.60 to 0.75 g per kg of body weight per day in patients with a GFR below 25 mL per minute per 1.73 m2.33
COMPLICATIONS
| Test | Complications detected |
|---|---|
| Hemoglobin concentration | Anemia |
| Red blood cell indexes, reticulocyte count, iron studies, fecal occult blood test | For ruling out other causes of anemia before erythropoietin therapy is started |
| Serum electrolyte levels | Hyperkalemia, hyponatremia, acidosis |
| Calcium, phosphorus, and parathyroid hormone levels | Hypocalcemia, hyperphosphatemia, secondary hyperparathyroidism |
| Serum albumin and total protein levels | Hypoalbuminemia, decreased levels of immunoglobulins in patients with nephritic levels of proteinuria or signs of malnutrition |
Clinical evaluation may detect gastrointestinal, neurologic, dermatologic, and musculoskeletal complications in the advanced stages of chronic kidney disease. Gastrointestinal symptoms may herald the onset of uremia, indicating the need for kidney replacement therapy.
Laboratory tests detect complications such as electrolyte abnormalities, disordered calcium or phosphorus metabolism, and anemia. Patients with nephrotic-range proteinuria are at risk for hypoalbuminemia and immune dysfunction because of the loss of immunoglobulins. Periodic monitoring of the total serum protein level and the albumin level is indicated in these patients. Nutritional status should be evaluated because malnutrition adversely affects prognosis.
RISK OF CARDIOVASCULAR DISEASE
Cardiovascular disease is the most common cause of death in patients with chronic kidney disease. The risk of cardiovascular disease and associated mortality increases in proportion to the decrease in the GFR.34 Patients with albuminuria and normal GFR also are at increased risk. Evaluation for traditional cardiovascular risk factors, including smoking, high lipid levels, hypertension, and sedentary lifestyle, is essential. The KDOQI guidelines1 recommend a blood pressure goal of 130/80 mm Hg in patients with normal urinary albumin concentrations, and a blood pressure goal of 125/75 mm Hg in patients with excretion of more than 1 g of protein per 24 hours. A long-term follow-up study35 of patients with nondiabetic kidney disease and an average GFR of 32 mL per minute per 1.73 m2 found that the patients randomized to a low blood pressure target were one third less likely to develop kidney failure than were the patients randomized to a usual blood pressure goal.
The KDOQI guidelines on managing dyslipidemias36 in chronic kidney disease recommend a low-density lipoprotein cholesterol goal of less than 100 mg per dL (2.60 mmol per L) for patients with chronic kidney disease, because they are statistically at highest risk for cardiovascular disease. In these patients, the 10-year risk for mortality from cardiovascular disease exceeds 20 percent.36
Paradoxically, dialysis patients with the lowest cholesterol levels are the most likely to die of cardiovascular disease. This is because low levels of cholesterol are associated with nontraditional cardiac risk factors of malnutrition and are markers of chronic inflammation.37 Lipid disorders should be treated aggressively in patients with chronic kidney disease and end-stage renal disease.36
Additional cardiac risk factors specific to chronic kidney disease include volume overload, hyperparathyroidism, and uremia. Anemia caused by decreased erythropoietin production also may contribute to cardiovascular mortality. Treatment with exogenous erythropoietin has been shown to improve the prognosis.38
WHEN TO REFER
Nephrology referral generally is recommended for patients with a serum creatinine level of 1.5 to 2.0 mg per dL (133 to 177 μmol per L). According to one estimate, that would mean seven new patients per day for every nephrologist in the United States.39 Some indications for nephrology referral are listed in Table 8.6,11,28,38,40
| Underlying cause unclear after basic work-up |
| Renal biopsy indicated |
| Management of underlying cause beyond the scope of primary care |
| Stage 3 chronic kidney disease (GFR < 60 mL per minute per 1.73 m2): consider comanagement |
| Stage 4 chronic kidney disease (GFR < 30 mL per minute per 1.73 m2): nephrologist involvement essential |
| Rapid progression of chronic kidney disease |
| Superimposed acute kidney failure |
The value of timely referral has been demonstrated,40 but the contribution of primary care to outcomes in patients with chronic kidney disease has not been studied.41 Given the magnitude of the rapid increase in the number of cases of chronic kidney disease, primary care evaluation and timely referral are recommended. The KDOQI endorses a model of collaboration between primary care physicians and subspecialists.6 The National Kidney Disease Education Program provides tools to promote this collaboration; they may be accessed online at http://www.nkdep.nih.gov/professionals/index.