
A more recent article on pancreatic cancer is available.
Am Fam Physician. 2006;73(3):485-492
Author disclosure: Nothing to disclose.
Although only 32,000 new cases of adenocarcinoma of the pancreas occur in the United States each year, it is the fourth leading cause of cancer deaths in this country. The overall five-year survival rate is 4 percent, and localized, resectable disease has only a 17 percent survival rate. Risk factors include smoking, certain familial cancer syndromes, and familial chronic pancreatitis. The link between risk of pancreatic cancer and other factors (e.g., diabetes, obesity) is less clear. Most patients present with obstructive jaundice caused by compression of the bile duct in the head of the pancreas. Epigastric or back pain, vague abdominal symptoms, and weight loss also are characteristic of pancreatic cancer. More than one half of cases have distant metastasis at diagnosis. Computed tomography is the most useful diagnostic and staging tool. Ultrasonography, magnetic resonance imaging, and endoscopic retrograde cholangiopancreatography may provide additional information. The majority of tumors are not surgically resectable because of metastasis and invasion of the major vessels posterior to the pancreas. Resectable tumors are treated with the Whipple procedure or the pylorus-preserving Whipple procedure. Adjuvant fluorouracil-based chemotherapy may prolong survival. For nonresectable tumors, chemotherapy with gemcitabine prolongs survival. Other agents are being studied. Radiation combined with chemotherapy has slowed progression in locally advanced cancers. Throughout the illness and during end-of-life care, patients need comprehensive symptom control.
The American Cancer Society estimated that 31,860 Americans would be diagnosed with pancreatic cancer in 2004, and that 31,270 would die from the disease.1 Pancreatic cancer accounts for only 2 percent of all new cancers in the United States, but it is the fourth leading cause of cancer deaths. At the time of diagnosis, more than one half of pancreatic cancers have metastasized, and only 8 percent are localized. The overall five-year survival rate is 4 percent. Localized cancers have a 17 percent survival rate. Survival rates have not improved during the past 25 years.1
Pancreatic cancer rarely occurs in persons younger than 50 years, and the risk increases with age. The incidence of pancreatic cancer is declining slowly in white men, but it is increasing in other groups, possibly because of changes in smoking patterns. Women account for 57 percent of new cases.1 Smoking,2 diabetes,3 and obesity4 increase risk. A link between alcohol or coffee consumption and pancreatic cancer has not been verified.5 Physical activity; high fruit and vegetable intake6; and, possibly, nonsteroidal anti-inflammatory drugs reduce the risk.7 Up to 10 percent of patients report a family history of pancreatic cancer.8 Patients with rare familial cancer syndromes or hereditary chronic pancreatitis have a substantially increased risk.9 Research on overexpression of specific oncogenes10 and reduced activity of tumor suppressor genes may provide a better understanding of the pathogenesis of pancreatic cancer and lead the way to more effective screening tests.11,12
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Dual-phase helical computed tomography is the best initial imaging test for diagnosis and staging of suspected pancreatic carcinoma. | C | 21,26,27 |
| Patients undergoing resection for pancreatic cancer should be offered referral to high-volume hospitals (i.e., performing more than 16 Whipple procedures per year) where there is less risk of perioperative mortality. | B | 33 |
| Adjuvant chemotherapy with fluorouracil and leucovorin improves survival rates and should be offered to patients with resectable pancreatic cancer. | B | 28 |
| Gemcitabine (Gemzar) is recommended as first-line chemotherapy for patients with metastatic pancreatic cancer. | B | 42 |
| Fluorouracil-based chemoradiation therapy is recommended for patients with locally advanced pancreatic cancer. | B | 45–47 |
| Endoscopicguided palliative intervention for pancreatic cancer, including celiac plexus neurolysis for pain and stenting for biliary or gastric outlet or duodenal obstruction, is effective and avoids the risks of surgery. | B | 49–51 |
Clinical Presentation
Almost all pancreatic cancers are adenocarcinomas of the ductal epithelium, and symptoms primarily are caused by mass effect rather than disruption of exocrine or endocrine function. The clinical features depend on the size and location of the tumor as well as its metastases. Jaundice, pain, and weight loss are classic symptoms of pancreatic cancer. Nonspecific early symptoms often are unrecognized; therefore, most pancreatic cancers are advanced at diagnosis (Table 1).13 More than two thirds of pancreatic cancers occur in the head of the pancreas (Figure 1) and usually present as steadily increasing jaundice caused by biliary duct obstruction. Painless obstructive jaundice traditionally is associated with surgically resectable cancers.14 Obstruction of the bile duct causes jaundice with disproportionately increased levels of conjugated bilirubin and alkaline phosphatase in the blood. The urine is dark because of the high level of conjugated bilirubin and the absence of urobilinogen. The stool is pale because of the lack of stercobilinogen in the bowel. In addition to jaundice, rising bilirubin levels can cause severe pruritus. As hepatic function becomes compromised, patients experience fatigue, anorexia, and bruising caused by loss of clotting factors.
| Head of the pancreas | Body and tail of the pancreas | ||
|---|---|---|---|
| Symptoms | Patients (%) | Symptoms | Patients (%) |
| Weight loss | 92 | Weight loss | 100 |
| Jaundice | 82 | Pain | 87 |
| Pain | 72 | Nausea | 43 |
| Anorexia | 64 | Weakness | 42 |
| Dark urine | 63 | Vomiting | 37 |
| Light stool | 62 | Anorexia | 33 |
| Nausea | 45 | Constipation | 27 |
| Vomiting | 37 | Food intolerance | 7 |
| Weakness | 35 | Jaundice | 7 |
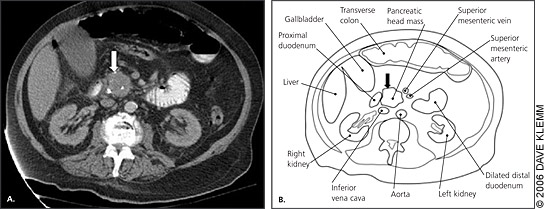
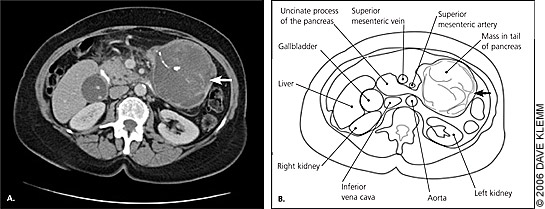
Patients with tumors in the body and tail of the pancreas generally present with nonspecific pain and weight loss. Body and tail tumors are much less likely to cause obstructive signs and symptoms. Patients may have pain in the epigastrium or back ranging from a dull ache to a severe pain. The pain may be exacerbated by eating or by lying flat. Tumors in the body and tail usually do not cause symptoms until they are large (Figure 2), and most present as locally advanced disease extending to the peritoneum and spleen.
Unexplained weight loss of about 5 lb (2.3 kg) per month may be the presenting feature of pancreatic cancer. Weight loss may be caused or exacerbated by anorexia, diarrhea, or early satiety. Obstruction of the pancreatic duct causes steatorrhea, exacerbating weight loss and malnutrition. Patients commonly become cachectic as the disease progresses.
PHYSICAL EXAMINATION
Other than jaundice, weight loss, and bruising, physical examination findings may be normal. A distended, palpable but nontender gallbladder in a jaundiced patient (Courvoisier’s sign) is 83 to 90 percent specific but only 26 to 55 percent sensitive for malignant obstruction of the bile duct.15 Although Courvoisier’s sign increases the likelihood of malignancy, absence of the sign does not rule it out. The liver may be tender and enlarged with advanced disease, and patients may present with ascites, palmar erythema, and spider angioma. Other findings associated with advanced pancreatic cancer or other abdominal malignancies include left supraclavicular lymphadenopathy (Virchow’s node) and recurring superficial thrombophlebitis (Trousseau’s sign).
Diagnostic Tests
A patient history, physical examination, and serum bilirubin and alkaline phosphatase levels can point to pancreatic cancer, but they are not diagnostic. The serum tumor marker cancer antigen (CA) 19–9 may help confirm the diagnosis in symptomatic patients16 and may help predict prognosis and recurrence after resection.17 However, CA 19–9 lacks sufficient sensitivity (50 to 75 percent) and specificity (83 percent) to effectively screen asymptomatic patients. Recent data18 suggest the serum tumor markers beta subunit of human chorionic gonadotropin (beta-hCG) and CA 72–4 are stronger independent prognostic factors than CA 19–9.
The U.S. Preventive Services Task Force (USPSTF) does not recommend screening average-risk, asymptomatic patients with abdominal palpation, ultrasonography, or serologic tumor markers.19 Although regular screening with endoscopic ultrasonography may be cost-effective in patients with a family history of pancreatic cancer,20 the USPSTF has not addressed the question of screening these patients. The accuracy of imaging studies for suspected pancreatic malignancy is summarized in Table 2.21–25
| Imaging study | Sensitivity †(%) | Specificity ‡(%) | Percentage of patients with pancreatic cancer at 10 percent pretest probability* | Percentage of patients with pancreatic cancer at 30 percent pretest probability* | ||
|---|---|---|---|---|---|---|
| Abnormal (%) | Normal (%) | Abnormal (%) | Normal (%) | |||
| Dual-phase helical computed tomography | 98 | 54 | 19 | 0.4 | 48 | 2 |
| Transabdominal ultrasonography | 83 | 99 | 90 | 1.9 | 97 | 7 |
| Endoscopic ultrasonographyguided fine-needle aspiration | 92 | 100 | 95 | 0.9 | 99 | 3 |
| Endoscopic retrograde cholangiopancreatography | 70 | 94 | 56 | 3.4 | 83 | 12 |
| Magnetic resonance cholangiopancreatography | 84 | 97 | 76 | 1.8 | 92 | 7 |
| Positron emission tomography | 96 | 65 | 23 | 0.7 | 54 | 3 |
Although conventional computed tomography (CT) and transabdominal ultrasonography are appropriate for initial imaging, dual-phase helical CT scanning is the best option if available. Dual-phase helical CT is the most sensitive test, and it noninvasively identifies 98 percent of pancreatic cancers and distant metastases, providing diagnostic and staging information.21–26 If CT is indeterminate or negative and clinical suspicion remains high, endoscopic ultrasonography should be performed next.27 A fine-needle aspiration biopsy guided by endoscopic ultrasonography may provide tissue diagnosis in patients who are not surgical candidates.23 Patients with resectable disease who are surgical candidates can undergo definitive surgery without preoperative histologic confirmation. Magnetic resonance imaging is not used in typical clinical practice, and it is less sensitive than CT (i.e., similar in sensitivity to transabdominal ultrasonography). Once a mainstay in diagnostic imaging and tissue sampling, endoscopic retrograde cholangiopancreatography (ERCP) is used only when other modalities are inconclusive and suspicion for malignancy is high or when delineation of the biliary tree is crucial. ERCP also is appropriate when stent placement to relieve biliary obstruction is a consideration.12
Staging
Accurate staging is important in identifying surgical candidates and sparing noncandidates the risk and cost associated with surgery. Unresectable disease is defined by distant metastasis (e.g., hepatic, extra-abdominal, peritoneum, omentum, lymph nodes outside the resection zone); invasion of superior mesenteric artery, inferior vena cava, aorta, or celiac axis; or encasement or occlusion of the superior mesentericportal venous complex.12
The tumor, node, and metastasis system may be used for pancreatic cancer staging, but in clinical decision making, pancreatic cancers can be categorized as resectable, locally advanced, or metastatic (Table 3).28,29 Staging begins with a thorough history and physical examination to find evidence of metastatic disease. Initial imaging with dual-phase helical CT of the abdomen and pelvis is the best way to assess most tumors and identify distant metastases and arterial involvement.26 If the patient has high surgical risk, or if CT shows unrsectable disease, fine-needle aspiration can confirm the diagnosis, and no further staging work-up is necessary.23 If the CT scan is indeterminate, endoscopic ultrasonography can identify smaller lesions and further delineate vascular involvement.30 Staging laparoscopy generally is reserved for patients whose physicians highly suspect metastasis but have not yet identified it.12,31
| Stage | Classifications | Clinical classification | Stage distribution at diagnosis (%) | Five-year survival rate (%) |
|---|---|---|---|---|
| 0 | Tis, N0, M0 | Resectable | 7.5 | 15.2 |
| IA | T1, N0, M0 | |||
| IB | T2, N0, M0 | |||
| IIA | T3, N0, M0 | |||
| IIB | T1-3, N1*, M0 | Locally advanced | 29.3 | 6.3 |
| III | T4, any N, M0 | |||
| IV | Any T, any N, M1 | Metastatic | 47.2 | 1.6 |
Treatment
Surgical resection is the only potentially curative treatment for patients with pancreatic cancer, although many patients are not candidates for resection.
RESECTABLE LESIONS
About 15 to 20 percent of patients with pancreatic adenocarcinoma have resectable disease at the time of diagnosis.12 The classic Whipple procedure (Figure 3) involves removal of the head and uncinate process of the pancreas, duodenum, proximal 6 in (15 cm) of jejunum, gallbladder, common bile duct, and distal stomach, with anastomosis of the common hepatic duct and the remaining pancreas and stomach to the jejunum.32 The perioperative mortality rate of patients undergoing this procedure has improved significantly over the past three decades. Surgical teams performing more than 16 procedures per year report significantly lower perioperative mortality rates than centers with less experience (3.8 versus 7.5 to 17.6 percent).33
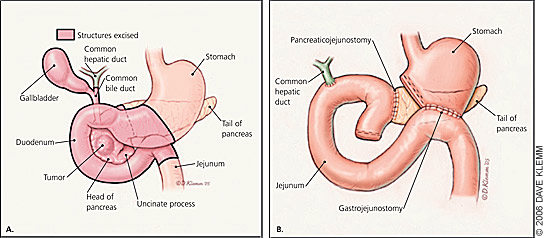
Pyloruspreserving pancreaticoduodenostomy appears to offer the same long-term survival benefits as the standard Whipple procedure with shorter operative time and reduced blood loss, decreasing the need for blood transfusions.34 Risks associated with both procedures include delayed gastric emptying, pancreatic fistula, anastomotic leaks, wound infection, intraabdominal abscess, hemorrhage, diabetes, and pancreatic exocrine insufficiency.34 Distal pancreatectomy is performed in patients with resectable cancer in the body or tail of the pancreas. The spleen usually is removed as well. The resectability rate for body and tail lesions is less than one half of that for head lesions35 because diagnosis usually occurs late in the disease process after local invasion has occurred. Five-year survival for resection of body or tail lesions is similar to that of resection for pancreatic head lesions.35 Five-year survival rates after surgical resection range from 10 to 30 percent.36–41 Negative prognostic factors include poorly differentiated histology, positive resection margins, lymph node involvement, and a tumor larger than 0.8 in (2 cm).36–38
Randomized clinical trials36,39–41 evaluating the effectiveness of adjuvant chemoradiotherapy and chemotherapy after surgical resection have been heavily criticized and have had inconsistent results. Recent data,36 however, suggest adjuvant chemotherapy with leucovorin and fluorouracil may increase survival, but adjuvant chemoradiotherapy offers no survival benefit and may decrease survival when administered before chemotherapy. Trials are underway to study postoperative chemotherapy with f luorouracil and leucovorin or gemcitabine (Gemzar) and chemotherapy with fluorouracil-based chemoradiation combined with gemcitabine or fluorouracil.12
METASTATIC LESIONS
Researchers have studied many single- and multiple-agent chemotherapeutic regimens for patients with metastatic disease, and more studies are ongoing; however, few studies have shown survival or clinical benefit. The use of gemcitabine as first-line therapy has a 12-month survival advantage and improves or stabilizes pain, performance status, and weight compared with fluorouracil monotherapy.42 Although the combination of leucovorin and fluorouracil is effective as adjuvant chemotherapy in resectable disease, it does not seem to be any more effective than fluorouracil monotherapy for treatment of unresectable disease.43–44
LOCALLY ADVANCED LESIONS
External beam and intraoperative radiation therapy decrease local progression in patients with unresectable, locally advanced disease, but neither affects survival or metastasis.45 Therefore, radiation therapy alone does not effectively treat patients with locally advanced pancreatic cancer outside of palliation. Combined radiation therapy and fluorouracil-based chemotherapy offer significant survival improvement compared with radiation therapy alone (40 versus 10 percent survival after one year, number needed to treat = 3) and are routinely used unless a patient is enrolled in an investigational study of another treatment regimen.12,45–47 Radiation with gemcitabine increases toxicity rates but does not significantly impact survival compared with radiation and fluorouracil.48 Regardless of stage, the potential benefits of therapy for pancreatic cancer must be balanced against the significant side effects, costs, and quality-of-life factors.
PALLIATIVE CARE
Palliative treatment of patients with pancreatic cancer is important, and involving hospice early is appropriate. Patients should be monitored closely for depression and treated when it arises. Othercomplications that require palliative intervention include pain; gastric outlet or duodenal obstruction; and bile duct obstruction and subsequent jaundice, cachexia, and malabsorption caused by exocrine pancreatic insufficiency.
Exocrine pancreatic insufficiency and subsequent malabsorption should be treated with pancreatic enzyme replacement (30,000 IU) of pancrelipase before, during, and after a meal, with increased titration as needed. Weight loss unrelated to malabsorption generally is multifactorial and may be treated with appetite stimulants (e.g., megestrol [Megace], dronabinol [Marinol], corticosteroids) and a high-calorie diet or nutritional supplements.
Pain from pancreatic cancer can be managed with opioid analgesics, radiation therapy, chemotherapy, or celiac plexus neurolysis (i.e., chemical splanchnicectomy of the celiac plexus with alcohol). Celiac plexus neurolysis eases pain without the side effects of opioids and can be administered intraoperatively, percutaneously, or by endoscopic ultrasonography. Endoscopic ultrasonography–guided neurolysis is effective and has minimal risk of the potentially serious complications associated with the surgical or percutaneous approaches.49
Biliary decompression for palliation of jaundice can be achieved surgically through choledochojejunostomy or cholecystojejunostomy. These procedures can be performed at the same time as gastrojejunostomy, which can relieve gastric outlet or duodenal obstruction. Biliary decompression also can be achieved endoscopically using expandable wire stents. Endoscopic placement of metal stents has a much lower risk than with surgery and less stent occlusion than with plastic stent use.12,50 This method relieves obstructive symptoms in 97 percent of patients and has morbidity and mortality rates of 12 and 3 percent, respectively. Complications include bleeding, infection, and pancreatitis.50 Similarly, metal stent placement can effectively manage duodenal obstruction in 81 percent of patients. Metal stents cost less and require a shorter hospital stay than surgical treatment.51