
A more recent article on seborrheic dermatitis is available.
Am Fam Physician. 2006;74(1):125-132
Author disclosure: Nothing to disclose.
Seborrheic dermatitis affects the scalp, central face, and anterior chest. In adolescents and adults, it often presents as scalp scaling (dandruff). Seborrheic dermatitis also may cause mild to marked erythema of the nasolabial fold, often with scaling. Stress can cause flare-ups. The scales are greasy, not dry, as commonly thought. An uncommon generalized form in infants may be linked to immunodeficiencies. Topical therapy primarily consists of antifungal agents and low-potency steroids. New topical calcineurin inhibitors (immunomodulators) sometimes are administered.
Seborrheic dermatitis can affect patients from infancy to old age.1–3 The condition most commonly occurs in infants within the first three months of life and in adults at 30 to 60 years of age. In adolescents and adults, it usually presents as scalp scaling (dandruff) or as mild to marked erythema of the nasolabial fold during times of stress or sleep deprivation. The latter type tends to affect men more often than women and often is precipitated by emotional stress. An uncommon generalized form in infants may be linked to immunodeficiencies.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Infants with generalized seborrheic dermatitis, diarrhea, and failure to thrive should be evaluated for immunodeficiencies. | C | 14 |
| The first-line therapy for seborrheic dermatitis of the scalp should be topical steroids. | C | 2,20,34 |
| Topical calcineurin inhibitors (e.g., tacrolimus ointment [Protopic], pimecrolimus cream [Elidel]) are recommended for seborrheic dermatitis of the face and ears. | B | 26–28 |
| Once-daily ketoconazole (Nizoral) combined with two weeks of once-daily desonide (Desowen) is recommended for seborrheic dermatitis of the face. | B | 22 |
Seborrheic dermatitis and pityriasis capitis (cradle cap) are common in early childhood. According to one survey of 1,116 children,4 the overall age- and sex-adjusted prevalence of seborrheic dermatitis was 10 percent in boys and 9.5 percent in girls. The highest prevalence occurred in the first three months of life, decreasing rapidly by one year of age, and slowly decreasing over the next four years.4 Most patients (72 percent) had minimal to mild seborrheic dermatitis. Pityriasis capitis occurred in 42 percent of the children examined (86 percent had a minimal to mild case).4 Prevalence estimates for older persons are consistently higher than estimates for the general population.5
Etiology
Despite the high prevalence of seborrheic dermatitis, little is known about its etiology. However, several factors (e.g., hormone levels, fungal infections, nutritional deficits, neurogenic factors) are associated with the condition. The possible hormonal link may explain why the condition appears in infancy, disappears spontaneously, then reappears more prominently after puberty. A more causal link seems to exist between seborrheic dermatitis and the proliferation of Malassezia species (e.g., Malassezia furfur, Malassezia ovalis) found in normal dimorphic human flora.6–8 Yeasts of this genus predominate and are found in seborrheic regions of the body that are rich in sebaceous lipids (e.g., head, trunk, upper back). A causal relationship is implied because of the ability to isolate Malassezia in patients with seborrheic dermatitis and by its therapeutic response to antifungal agents.9 A similar link has been suggested in studies of patients with seborrheic dermatitis that is associated with acquired immunodeficiency syndrome (AIDS).10,11 Seborrheic dermatitis also may be associated with nutritional deficiencies, but there is no firm linkage.
An altered essential fatty acid pattern may be important in the pathogenesis of infantile seborrheic dermatitis. Serum essential fatty acid patterns from 30 children with the condition suggested a transient impaired function of the delta-6 desaturase enzyme.12 A neurogenic theory for the development of seborrheic dermatitis may account for its association with parkinsonism and other neurologic disorders, including postcerebrovascular accidents, epilepsy, central nervous system trauma, facial nerve palsy, and syringomyelia induced by neuroleptic drugs with extrapyramidal effects.7 It may be confined to the syringomyelia-affected area or to the paralyzed side in a patient with hemiplegia. However, no neurotransmitters have been identified in this context.
Classification
Adolescent and adult seborrheic dermatitis usually starts as mild greasy scaling of the scalp with erythema and scaling of the nasolabial folds (Figure 1) or the postauricular skin. The scaling often is concurrent with an oily complexion and appears in areas of increased sebaceous gland activity (e.g., auricles, beard area, eyebrows, trunk [flexure and inframammary areas; Figure 2]). Sometimes the central face is involved (Figure 3). Blepharitis, with meibomian gland occlusion and abscess formation, otitis externa, and coexistent acne vulgaris or pityriasis versicolor, may be evident.
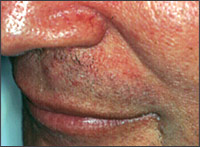
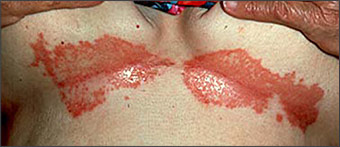
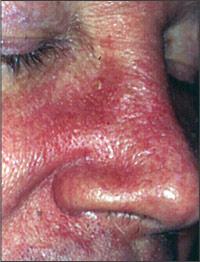
Two types of seborrheic dermatitis may appear on the chest—a common petaloid type and a rarer pityriasiform type.2 The former starts as small, reddish-brown follicular and perifollicular papules with greasy scales. These papules become patches that resemble the shape of flower petals or a medallion (medallion seborrheic dermatitis). The pityriasiform type often has generalized macules and patches that resemble extensive pityriasis rosea. These patches rarely produce an eruption so generalized that it causes erythroderma.
In infants, seborrheic dermatitis may present as thick, greasy scales on the vertex of the scalp (cradle cap).2,3 The condition is not pruritic in infants, as it is in older children and adults. Typically, acute dermatitis (characterized by oozing and weeping) is absent. The scales may vary in color, appearing white, off-white, or yellow. Infants with large, dry scales often have psoriasiform seborrheic dermatitis. This presentation often is the only sign of seborrheic dermatitis in infants and usually occurs in the third or fourth week after birth. However, the scalp, central face, forehead, and ears may have fine, widespread scaling. The dermatitis may become generalized. The flexural folds may be involved, often with a cheesy exudate that manifests as a diaper dermatitis that also may become generalized. Generalized seborrheic dermatitis is uncommon in otherwise healthy children and usually is associated with immunodeficiencies. Immunocompromised children with generalized seborrheic dermatitis often have concomitant diarrhea and failure to thrive5–8 (Leiner’s disease); therefore, infants with these symptoms should be evaluated for immunodeficiencies.13–15
Differential Diagnosis
A number of disorders are similar to seborrheic dermatitis (Table 1). One study11 showed that 47 percent of patients with AIDS had recalcitrant eruptions similar to seborrheic dermatitis that may be generalized in children and adults (Figure 4). Highly active antiretroviral therapy may reduce incidence in patients with AIDS.
| Atopic dermatitis |
| Candidiasis |
| Dermatophytosis |
| Langerhans cell histiocytosis |
| Psoriasis |
| Rosacea |
| Systemic lupus erythematosus |
| Tinea infection |
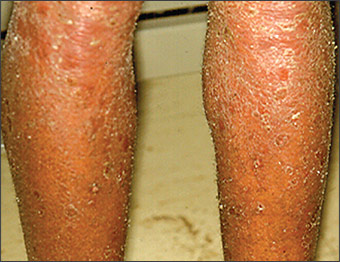
Seborrheic dermatitis also may resemble atopic dermatitis, tinea capitis, and, rarely, cutaneous lymphoma or Langerhans cell histiocytosis. Atopic dermatitis in adults characteristically appears in antecubital and popliteal fossae. Tinea capitis, tinea faciei, and tinea corporis may have hyphae on potassium hydroxide cytologic examination; candidiasis produces pseudohyphae. Seborrheic dermatitis of the groin may resemble dermatophytosis, psoriasis, candidiasis, and, sometimes, Langerhans cell histiocytosis. Rosacea may produce a facial erythema resembling seborrheic dermatitis. Although rosacea tends to include central facial erythema, it may involve only the forehead.
Infants may have atopic dermatitis that is prevalent in certain body areas (e.g., scalp, face, diaper areas, extensor limb surfaces), suggesting seborrheic dermatitis.18 However, in infants, seborrheic dermatitis has axillary patches, lacks oozing and weeping, and lacks pruritus. The distinction is a clinical one because elevated immunoglobulin E (IgE) levels associated with atopic dermatitis are a nonspecific finding. Rarely, infants are affected by histologic-specific scaling, seborrheic dermatitis-like eruptions on the scalp with fever, and other systemic signs of acute Langerhans cell histiocytosis (Letterer-Siwe disease). Scabetic eczema occasionally resembles widespread seborrheic dermatitis. Riboflavin, biotin, and pyridoxine deficiencies have been associated with seborrheic dermatitis-like eruptions in infants.19 Concomitant disorders (e.g., psoriasis, scabetic eczema, superficial fungal infection) may complicate seborrheic dermatitis, especially in patients with AIDS.
Histology
Skin biopsies may effectively distinguish seborrheic dermatitis from similar disorders. Seborrheic dermatitis should have neutrophils in the scale crust at the margins of follicular ostia. AIDS-associated seborrheic dermatitis more commonly presents as parakeratosis, a few individually necrotic keratinocytes within the epidermis, and plasma cells in the dermis. Yeast cells sometimes are visible within keratinocytes on special stains. If hyphae are present, dermatomycosis is the diagnosis. Shorter hyphae with spores (“spaghetti and meatball pattern”) are present with tinea versicolor.8
Treatment
| Therapy | Usage | |
|---|---|---|
| Anti-inflammatory (immunomodulatory) agents | ||
| Steroid shampoo | ||
| Fluocinolone (Synalar) | Two times per week | |
| Topical steroids | ||
| Fluocinolone | Daily | |
| Betamethasone valerate lotion (Beta-Val) | Daily | |
| Desonide cream (Desowen) | Daily | |
| Topical calcineurin inhibitors | ||
| Tacrolimus ointment (Protopic)* | Daily | |
| Pimecrolimus cream (Elidel)* | Daily | |
| Keratolytics | ||
| Salicylic acid shampoo | Two times per week | |
| Tar shampoo | Three times per week | |
| Zinc pyrithione shampoo (also has antifungal properties) | Two times per week | |
| Antifungals | ||
| Ketoconazole shampoo (Nizoral) | Three times per week | |
| Selenium sulfide shampoo (Selsun) | Two times per week | |
| Alternative medication | ||
| Tea tree oil shampoo | Daily | |
ANTI-INFLAMMATORY (IMMUNOMODULATORY) AGENTS
The conventional treatment for adult seborrheic dermatitis of the scalp starts with topical steroids or a calcineurin inhibitor. These therapies may be administered as a shampoo, such as fluocinolone (Synalar), topical steroid solutions, lotions applied to the scalp, or creams applied to the skin.30,31,34–36 Adults with seborrheic dermatitis typically use topical steroids once or twice daily, often in addition to a shampoo. Low-potency topical steroids may effectively treat infantile or adult seborrheic dermatitis of the flexural areas or persistent recalcitrant seborrheic dermatitis in adults.2,20 A topical azole preparation may be combined with a desonide regimen (one dose daily for two weeks) for facial seborrheic dermatitis.22
Topical calcineurin inhibitors (e.g., tacrolimus ointment [Protopic], pimecrolimus cream [Elidel]) have fungicidal and anti-inflammatory properties without the risk of cutaneous atrophy, which is associated with topical steroids.26–28 Calcineurin inhibitors also are good therapies when the face and ears are affected. However, one week of daily use is necessary before benefits are apparent.
KERATOLYTICS
Older modalities for treating seborrheic dermatitis may have had keratolytic but not specific antifungal properties.1,2 Keratolytics that are widely used to treat seborrheic dermatitis include tar, salicylic acid, and zinc pyrithione shampoos. Pyrithione zinc has nonspecific keratolytic and antifungal properties3,21 and can be applied two or three times per week. Patients should leave these shampoos on the hair for at least five minutes to ensure that it reaches the scalp. Patients also may use it on other affected sites, such as the face. Infantile seborrheic dermatitis of the scalp requires a gentle approach3 (e.g., a mild, nonmedicated shampoo).
ANTIFUNGALS
Most antifungal agents attack Malassezia associated with seborrheic dermatitis.1,2 A once-daily ketoconazole gel preparation (Nizoral) combined with a two-week, once-daily regimen of desonide (Desowen), may be useful for facial seborrheic dermatitis.22 Shampoos containing selenium sulfide (Selsun) or an azole often are used.1,2,20,21 These shampoos can be applied two or three times per week. Ketoconazole (cream or foaming gel)31,32 and oral terbinafine (Lamisil) also may be beneficial.23 Other topical antifungal agents include ciclopirox (Loprox)33,36 and fluconazole (Diflucan).29 Patients also may use a 2 % ketoconazole or a fluconazole shampoo.29,30,35 Some azoles (e.g., itraconazole [Sporanox], ketoconazole) also have anti-inflammatory properties.37
ALTERNATIVE MEDICATIONS
Natural therapies are becoming increasingly popular. Tea tree oil (Melaleuca oil) is an essential oil from a shrub native to Australia. The therapy appears to be effective and well tolerated when used daily as a 5% shampoo.25