
Am Fam Physician. 2006;74(11):1883-1888
Author disclosure: Nothing to disclose.
Erythema multiforme is a skin condition considered to be a hypersensitivity reaction to infections or drugs. It consists of a polymorphous eruption of macules, papules, and characteristic “target” lesions that are symmetrically distributed with a propensity for the distal extremities. There is minimal mucosal involvement. Management involves treating the existing infectious agent or discontinuing the causal drug. Mild cases resolve without sequelae and do not require treatment. Recurrent cases have been prevented with continuous acyclovir. Patients who have no response to acyclovir may have a response to valacyclovir or famciclovir, which have greater oral bioavailability and more convenient dosing. Patients with recurrent erythema multiforme despite suppressive antiviral therapy should be referred to a dermatologist for further treatment.
Erythema multiforme is an acute, self-limited, and sometimes recurring skin condition considered to be a hypersensitivity reaction associated with certain infections and medications (Table 11,2).2,3 Previously, the condition was thought to be part of a clinical spectrum of disease that included erythema minor, erythema major (often equated with Stevens-Johnson syndrome [SJS]), and toxic epidermal necrolysis (TEN), with erythema minor being the most mild and TEN the most severe.4 An often-cited study from 1993 proposed a useful clinical classification of erythema multiforme, SJS, and TEN based on the pattern of individual skin lesions and the estimation of body surface area with detachment of the epidermis (i.e., blisters, denuded areas, or erosions) at the worst stage of the disease (Table 21,2,5,6).5 Although SJS and TEN may represent the same process with differing severity,6 erythema multiforme, with its minimal mucous membrane involvement and less than 10 percent epidermal detachment, now is accepted as a distinct condition. The remainder of this article will focus on erythema multiforme.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Oral acyclovir (Zovirax) should be given early in herpes-associated outbreaks of erythema multiforme to lessen the number and duration of lesions. | B | 29 |
| Continuous acyclovir is recommended to prevent recurrent herpes-associated erythema multiforme. | A | 29,34,35 |
| Infections* |
| Herpes simplex virus 1 and 2 |
| Mycoplasma pneumoniae |
| Fungal infections |
| Medications |
| Barbiturates |
| Hydantoins |
| Nonsteroidal anti-inflammatory drugs |
| Penicillins |
| Phenothiazines |
| Sulfonamides |
| Condition | Pattern of skin lesions | Body surface area with epidermal detachment (%) |
|---|---|---|
| Erythema multiforme | Typical targets, raised atypical targets, minimal mucous membrane involvement | Less than 10 |
| SJS | No typical targets, flat atypical targets, confluent purpuric macules on the face and trunk, severe mucosal erosions at one or more mucosal sites | Less than 10 |
| Overlapping SJS and TEN | No typical targets, flat atypical targets | 10 to 30 |
| TEN | No typical targets, flat atypical targets; begins with severe mucosal erosions and progresses to diffuse, generalized detachment of the epidermis | Greater than 30 |
Etiology and Pathophysiology
Erythema multiforme usually occurs in adults 20 to 40 years of age,6 although it can occur in patients of all ages.1 Herpes simplex virus (HSV) is the most commonly identified etiology of this hypersensitivity reaction, accounting for more than 50 percent of cases.1,3,7–10 Mycoplasma pneumoniae is another commonly reported etiology, especially in children, as is fungal infection.1,11,12 The medications most often associated with erythema multiforme are barbiturates, hydantoins, nonsteroidal anti-inflammatory drugs, penicillins, phenothiazines, and sulfonamides.2
In addition, there have been reports of erythema multiforme associated with vaccines (diphtheria-tetanus,13 hepatitis B,14 smallpox15), other viruses (varicella zoster virus,16 hepatitis C,17 cytomagalovirus,18,19 and human immunodeficiency virus20), and some newer medications (candesartan cilexetil [Atacand],21 rofecoxib [Vioxx; withdrawn from the U.S. market],22 metformin [Glucophage],23 adalimumab [Humira],24 bupropion [Well-butrin],25 and ciprofloxacin [Cipro]26).
Recurrent erythema multiforme often is secondary to HSV-1 and -2 reactivation, although the HSV may be clinically silent.10 In a study involving 63 patients with erythema multiforme, HSV DNA was detected (by polymerase chain reaction in skin biopsy specimens) in 60 percent of patients with clinically diagnosed recurrent herpes-associated erythema multiforme and in 50 percent of patients with recurrent idiopathic erythema multiforme (defined as erythema multiforme with no clinical history of HSV infection or drug ingestion).19 In another study, researchers determined the genotype of HSV in cutaneous lesions of patients with herpes-associated erythema multiforme; 66.7 percent of cases were attributed to HSV-1, 27.8 percent to HSV-2, and 5.6 percent to HSV-1 and -2 coinfection.27 These results ref lect the seroprevalence of HSV-1 and -2 in the United States.28 However, most patients with HSV infection do not develop erythema multiforme. In addition, patients with herpes-associated erythema multiforme may have clinically apparent HSV reactivation without an episode of erythema multiforme or erythema multiforme without clinically apparent HSV infection.10,29
The pathogenesis of herpes-associated erythema multiforme has been well studied and is consistent with a delayed-type hypersensitivity reaction.3,30 The disease begins with the transport of viral DNA fragments to distant skin sites by peripheral blood mononuclear cells. HSV genes within DNA fragments are expressed on keratinocytes, leading to the recruitment of HSV-specific CD4+ TH1 cells (helper T cells involved in cell-mediated immunity). The CD4+ cells respond to viral antigens with production of interferon-γ, initiating an inflammatory cascade.3 Drug-associated erythema multiforme lesions test positive for tumor necrosis factor α and not interferon-γ as in herpes-associated erythema multiforme lesions, suggesting a varying mechanism.30
Clinical Presentation
Erythema multiforme is a self-limited eruption that usually has mild or no prodromal symptoms.31 Patients may experience itching and burning at the site of the eruption.6 The individual lesions begin acutely as numerous sharply demarcated red or pink macules that then become papular (Figure 1).8,31 The papules may enlarge gradually into plaques several centimeters in diameter. The central portion of the papules or plaques gradually becomes darker red, brown, dusky, or purpuric. Crusting or blistering sometimes occurs in the center of the lesions. The characteristic “target” or “iris” lesion (Figures 2 through 4) has a regular round shape and three concentric zones: a central dusky or darker red area, a paler pink or edematous zone, and a peripheral red ring. Some target lesions have only two zones, the dusky or darker red center and a pink or lighter red border.1,31 Target lesions may not be apparent until several days after the onset, when lesions of various clinical morphology usually are present, hence the name erythema “multiforme.”10
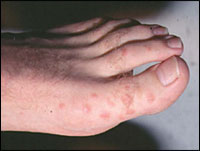
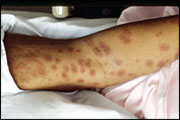
The skin lesions of erythema multiforme usually appear symmetrically on the distal extremities and progress proximally.2 Lesions on the dorsal surfaces of the hands and extensor aspects of the extremities are most characteristic.8 Palms and soles also may be involved.6 Mucosal lesions may occur but usually are limited to the oral cavity.1 Erythema multiforme resolves spontaneously in three to five weeks without sequelae, but it may recur.3 Patients in whom it recurs may have multiple episodes per year. In a study involving 65 patients with recurrent erythema multiforme, the mean number of attacks per year was six, with a range of two to 24; the mean duration of the disease was 9.5 years.29
Diagnosis
Erythema multiforme is diagnosed clinically. In patients who have target lesions with a preceding or coexisting HSV infection, the diagnosis can be made easily.31 Skin biopsy is not necessary when the clinical picture is clear because biopsy findings are not specific for erythema multiforme. In unclear cases, such as an atypical presentation or recurrent erythema multiforme without documented HSV infection, biopsy may help rule out other diagnoses. Laboratory tests (e.g., HSV-1 and -2, immunoglobulin M and G) may confirm a suspected history of HSV infection, but they are not required.
Skin biopsy results vary depending on the clinical morphology and the duration of the lesions’ existence as well as the area of the lesion from which the specimen is obtained (i.e., the center portion or the outer zone).31 The early stage of the red macules and papules shows a perivascular mononuclear cell infiltrate.3 Biopsy of the edematous zone of the target lesion may show pronounced dermal edema histologically; necrotic keratinocytes or epidermal changes usually occur in the central portion of the target lesion.31,32
The differential diagnosis (Table 330,32) of early erythema multiforme includes drug eruption, polymorphic light eruption, urticaria, urticarial vasculitis, viral exanthems, and other hypersensitivity reactions.32 Because erythema multiforme often resembles urticaria at the onset of the eruption, it is important to distinguish the clinical features. The individual lesions of erythema multiforme in typical cases are present and fixed for at least one week, and some evolve into target lesions.33 In contrast, the individual lesions of urticaria exist at the same site for less than 24 hours,8 and the centers of the lesions appear normal or as red as the borders.6 Target lesions with dusky or purpuric centers may resemble pityriasis rosea, lupus erythematosus, vasculitis, or figurate erythema.32 When bullous lesions are present, erythema multiforme must be distinguished from the autoimmune bullous diseases.31
| Autoimmune bullous diseases |
| Drug eruption |
| Figurate erythema |
| Lupus erythematosus |
| Pityriasis rosea |
| Polymorphic light eruption |
| Stevens-Johnson syndrome |
| Toxic epidermal necrolysis |
| Urticaria |
| Urticarial vasculitis |
| Vasculitis |
| Viral exanthems |
| Other hypersensitivity reactions |
Treatment
Management of erythema multiforme involves determining the etiology when possible. The first step is to treat the suspected infectious disease or to discontinue the causal drug.
Mild cases of erythema multiforme do not require treatment.6 Oral antihistamines and topical steroids may be used to provide symptom relief.8 In patients with coexisting or recent HSV infection, early treatment with oral acyclovir (Zovirax) may lessen the number and duration of cutaneous lesions.32,34 Topical acyclovir applied to preceding HSV lesions does not prevent herpes-associated erythema multiforme.10 Prednisone may be used in patients with many lesions at dosages of 40 to 80 mg per day for one to two weeks then tapered rapidly.6 However, its use is controversial.2 There have been no controlled studies of prednisone’s effectiveness, and its use in patients with herpes-associated erythema multiforme may lower the patient’s resistance to HSV and promote recurrent HSV infection followed by recurrent erythema multiforme.10
Recurrent erythema multiforme may be treated with continuous oral acyclovir (400 mg two times per day) even if HSV is not an obvious precipitating factor.6 Oral acyclovir has been shown to be effective in the suppression of recurrent erythema multiforme in a double-blind placebo-controlled trial.35 Valacyclovir (Valtrex; 500 to 1,000 mg per day) and famciclovir (Famvir; 125 to 250 mg per day) have greater oral bioavailability than acyclovir and may be tried in patients who have no response to acyclovir.4,36 The dosage of the antiviral may be reduced once the patient is recurrence free for four months, and eventually the drug may be discontinued. The dosage must be adjusted for patients with renal insufficiency.
Patients with recurrent erythema multiforme despite the use of suppressive antiviral therapy should be referred to a dermatologist for further treatment. In such patients, dapsone (100 to 150 mg per day) has resulted in partial or complete suppression of erythema multiforme.29 Antimalarials (mepacrine [Atabrine; unavailable in the United States] and hydroxychloroquine [Plaquenil]) also have been tried.6 Azathioprine (Imuran; 100 to 150 mg per day) has been used successfully in patients for whom other treatments have failed.29 Recurrent erythema multiforme also has been treated with cyclosporine (Sandimmune),37 and two patients with persistent erythema multiforme have been treated with thalidomide (Thalomid).38 Although these medications have shown benefit in some patients, the evidence to support their use for erythema multiforme is limited.