
Am Fam Physician. 2007;75(1):85-90
A more recent article on arterial atherosclerosis is available.
Patient information: See related handout on vascular disease.
Author disclosure: Nothing to disclose.
Caring for patients with vascular illnesses has become increasingly more complex and has changed dramatically over the past 10 years, with a widening array of diagnostic and treatment options. Carotid artery stenting has the potential to become a viable alternative to open surgery in high-risk patients with carotid artery disease (i.e., patients older than 80 years and those with previous neck surgery or irradiation, contralateral carotid artery occlusion, contralateral laryngeal nerve injury, or angina). However, the effectiveness of carotid artery stenting as a therapy is still being evaluated in randomized trials. Endovascular aortic aneurysm repair is an option for patients who desire or require a less invasive modality and who have suitable aortic anatomy. Surgical reconstruction remains the standard treatment for ischemic rest pain and tissue loss (critical limb ischemia). Balloon angioplasty and stenting are treatment options for peripheral vascular disease, although treatment is dependent on the arterial segment or segments involved.
The care of patients with vascular conditions has changed dramatically during recent years with advances in treatment options for carotid artery disease, abdominal aortic aneurysms, and peripheral arterial disease. Open surgical procedures are being replaced, to a varying degree, by a combination of techniques employing catheters, balloons, and stents. Vascular illnesses often are associated with serious comorbidities, and minimally invasive diagnostic and therapeutic modalities can be beneficial.
Duplex ultrasonography and less invasive radiology (e.g., computed tomography [CT], angiography, magnetic resonance angiography) have improved the diagnosis and treatment of patients with vascular diseases, with less patient discomfort and decreased morbidity.
| Clinical recommendation | Evidence rating | References | Comments |
|---|---|---|---|
| Carotid endarterectomy is recommended for symptomatic patients with more than 50 percent carotid artery stenosis and for asymptomatic, low-risk patients with more than 60 percent carotid artery stenosis | A | 4–7 | Multicenter randomized controlled trials |
| Carotid artery stenting is an alternative to carotid endarterectomy for patients who have more than 50 percent carotid stenosis if symptomatic and more than 80 percent carotid stenosis if asymptomatic and are at high risk for surgery (i.e., patients older than 80 years and those with previous neck surgery or irradiation, contralateral carotid artery occlusion, contralateral laryngeal nerve injury, or angina). | B | 8 | One trial has shown carotid artery stenting to be not inferior to carotid endarterectomy; however, broad recommendations cannot be made, and stenting is still largely investigational. |
| Open repair is recommended for an aneurysm with a diameter greater than 5.5 cm in patients with at least a two-year life expectancy. | A | 15,16,18 | Consistent findings from randomized controlled trials15,16; evidence-based practice guideline18 |
| Endovascular repair is recommended in patients with an aneurysm greater than 5.5 cm in diameter who are at high risk for open repair. | B | 21–23 | There are minimal data directly comparing open and endovascular abdominal aortic aneurysm repair. However, endovascular repair appears to be associated with lower morbidity and mortality rates in the early perioperative period with equivalent long-term survival rates. |
| Surgical reconstruction is recommended for patients with ischemic rest pain and tissue loss (critical limb ischemia) | B | 27 | There are minimal modern data comparing observation with surgical reconstruction, although it is widely accepted that patients should undergo surgical intervention for critical limb ischemia. |
| Surgical reconstruction of the lower extremity is an option for patients with claudication. | B | 27 | Reconstruction surgery should be performed judiciously and cautiously in patients with claudication. A failed graft can convert claudication symptoms into critical ischemia. |
| Exercise is recommended to improve walking ability in patients with intermittent claudication. | A | 30 | — |
Carotid Artery Disease
Stroke causes an estimated 273,000 deaths annually in the United States, making it the third leading cause of death.1 Stroke also is the most common cause of disability; 15 to 30 percent of patients are permanently disabled, and 20 percent will require institutional care three months after onset.1 The 50 to 85 percent of strokes attributed to carotid artery disease are preventable. The most effective medical treatments to prevent stroke and transient ischemic attacks are antiplatelet therapy,2 blood pressure control, reduction of cholesterol levels, and smoking cessation.
DIAGNOSIS
The keys to treating patients with carotid artery disease are to differentiate between symptomatic and asymptomatic patients and to identify the severity of internal carotid stenosis. Typical symptoms are contralateral weakness, para-anesthesia, or anesthesia; ipsilateral blindness; and, if the dominant hemisphere is involved, dysphasia, aphasia, or speech apraxia. Asymptomatic patients generally are identified when a cervical bruit is heard on physical examination; however, the U.S. Preventive Services Task Force (USPSTF) found insufficient evidence to recommend for or against screening asymptomatic persons for carotid stenosis.3
Duplex ultrasonography of both carotid arteries should be performed when a bruit is heard or neurologic symptoms are identified. A symptomatic patient with more than 50 percent stenosis in the appropriate internal carotid artery should receive a referral for possible carotid artery reconstruction. An asymptomatic patient with more than 50 percent internal carotid stenosis should receive prompt referral for possible follow-up serial ultrasonography or intervention, depending on the degree of stenosis and the general condition of the patient.
TREATMENT
A Cochrane review showed that carotid endarterectomy was beneficial for stroke reduction in symptomatic patients with carotid stenosis greater than 50 percent.4 However, patients undergoing carotid endarterectomy should be good surgical candidates, and surgeons should have acceptably low complication rates (i.e., less than 6 percent).4,5
Clinical trials in the United States have established that carotid endarterectomy has a statistically significant benefit in patients with asymptomatic carotid artery disease,6 and a recent meta-analysis supports this finding.5 However, in an era of statin therapy and newer antihypertensive and antiplatelet agents (e.g., clopidogrel [Plavix]), there has been uncertainty about the clinical benefit of carotid endarterectomy for stroke reduction.
In 1993, the Asymptomatic Carotid Surgery Trial (ACST) began enrolling 3,120 patients7; this is about double the patient population of the Asymptomatic Carotid Atherosclerosis Study (ACAS), which with 1,662 participants had been the largest trial of patients with asymptomatic carotid artery disease. ACST has a 10-year follow-up and includes more modern medical management and surgical techniques than ACAS. Also, ACST better addresses the role of carotid endarterectomy in women, a population that had been underrepresented in prior studies.7 In 2004, the ACST collaborative group reported its five-year follow-up findings in asymptomatic patients with carotid stenosis of 60 percent or more.7 The five-year stroke risk was 12 percent for patients undergoing medical treatment and 6 percent for those undergoing carotid endarterectomy.7 Carotid endarterectomy was proved beneficial for asymptomatic patients (including women) with low surgical risk when performed by a surgeon who has had a low complication rate.7
The role of endovascular therapy for carotid artery disease is evolving. Initial endovascular approaches to carotid artery therapy employed balloon angioplasty without stents or embolic protection devices. More recently, distal protection devices (Figure 1) have been designed to prevent embolization during the deployment of carotid artery stents. Several large case series and registry trials have demonstrated the apparent safety and effectiveness of carotid artery stenting, but only one trial, the Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) trial has included a randomized comparison of carotid endarterectomy and stenting with distal protection.8

The SAPPHIRE trial evaluated patients considered to be at high risk for carotid endarterectomy (i.e., patients older than 80 years and those with previous neck surgery or irradiation, contralateral carotid artery occlusion, contralateral laryngeal nerve injury, or angina). The primary study end points included a composite of death, stroke, or myocardial infarction.8 The study showed that carotid artery stenting was not inferior to carotid end-arterectomy.8 Randomized trial data comparing the two treatments in average-risk patients are not yet available. The Carotid Revascularization Endarterectomy versus Stent Trial (CREST) is the only other major U.S. trial to include a randomized comparison of carotid artery stenting and carotid endarterectomy. The trial is currently underway.9 No study has compared carotid artery stenting with standard medical therapy (e.g., antiplatelet, anticoagulant, antihypertensive, or lipid agents).
Abdominal Aortic Aneurysms
An estimated 30,000 persons die each year from a ruptured abdominal aortic aneurysm.10 Because ruptured aneurysms generally are associated with high morbidity and mortality rates (up to 90 percent), elective repair usually is recommended. A main characteristic of the condition is that patients usually are asymptomatic until the aneurysm ruptures. Primary care physicians should evaluate for a pulsatile abdominal mass as a routine part of the physical examination in patients older than 60 years.11
DIAGNOSIS
Screening recommendations for abdominal aortic aneurysms vary.12,13 A consensus statement from the Society for Vascular Surgery (Table 110 ) recommends ultrasonography of the abdominal aorta in men 60 to 85 years of age, women 60 to 85 years of age with cardiovascular risk factors, and men and women older than 50 years with a family history of abdominal aortic aneurysms; patients who are not candidates for intervention should not be screened.10 After ultrasonography, patients with aortic diameters less than 3.0 cm require no further testing, those with aneurysms 3.0 to 4.5 cm in diameter should have annual ultrasound examinations, and those with aneurysms greater than 4.5 cm in diameter should be referred to a vascular sub-specialist or surgeon. Patients whose aneurysms expand by more than 0.5 cm in six months or by more than 1.0 cm in one year should be referred.10
The USPSTF recommends screening only in men 65 to 75 years of age who have smoked more than 100 cigarettes and suggests that women do not benefit from screening.12 In 2007, the recently passed Screening Abdominal Aortic Aneurysms Very Efficiently (SAAAVE) Act will allow Medicare reimbursements for screening in men with a history of smoking and in men and women 65 to 74 years of age with a family history of abdominal aortic aneurysms.14
Further imaging studies (e.g., abdominal and pelvic CT, magnetic resonance imaging) are not needed prior to vascular referral because subspecialists often must repeat the imaging studies, which increases expense, radiation exposure, and contrast load.
Randomized studies have shown that the annual rupture rate for aneurysms less than 5.5 cm in diameter is 0.6 to 3.2 percent in carefully followed patients.15,16 However, the annual risk of rupture is higher for aneurysms with larger diameters: 9.4 percent for 5.5 to 5.9 cm, 10.2 percent for 6.0 to 6.9 cm, and 32.5 percent for greater than 7.0 cm.17
The point at which the annual rupture risk exceeds the operative mortality rate of 2.7 to 5.8 percent (the mortality rates from two multicenter randomized trials) occurs when the aneurism is between 5.0 and 5.4 cm in diameter.15,16 Therefore, many vascular subspecialists recommend repair for an aneurysm with a diameter greater than 5.5 cm in patients with an expected survival of at least two years.18 Endovascular repair could be considered in high-risk patients with less than a two-year expected survival. Controversy exists about whether endovascular or open repair is more effective for treating abdominal aortic aneurysms.
TREATMENT
Endovascular repair was introduced in 1991 as an alternative for high-risk patients with large abdominal aortic aneurysms.19 Over the past decade, endovascular repair has proved to be a safe and effective treatment. During the procedure, the bilateral femoral artery is exposed via groin incisions. An endograft (modular, bifurcated fabric graft supported by metallic stents) is placed through the femoral artery using fluoroscopic control.
Endovascular repair is minimally invasive, and patients return to baseline function more quickly than with open repair. However, patients undergoing endovascular repair will require lifetime surveillance with abdominal CT (one, six, and 12 months after the procedure and then annually for life) to evaluate for blood leakage around the endograft into the aneurysm sac (Figure 2). Endograft migration, stent fracture, and loss of integrity of the stent graft can occur. If the endograft fails to exclude the aneurysm from the blood flow, the patient will require secondary interventions, which usually include endovascular procedures; occasionally an open repair or removal of the endograft is required. However, with current endograft technology, these adverse events occur relatively infrequently (in 10 to 15 percent of appropriately selected patients).20
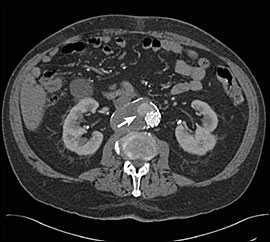
Endovascular repair of abdominal aortic aneurysms is a relatively new treatment, and long-term outcomes have yet to be demonstrated. A meta-analysis and systematic review of short-term results showed that endovascular repair was associated with reduced blood loss, shorter hospitalization, and reduced perioperative morbidity, compared with open repair.21,22 The Dutch Randomized Endovascular Aneurysm Management (DREAM) trial, an ongoing multicenter prospective study, confirmed that endovascular repair of abdominal aortic aneurysms reduced perioperative mortality rates by 1.2 percent, compared with 4.6 percent with open repair.23 However, the long-term results of this trial have shown that the perioperative survival advantage is not sustained after the first perioperative year.24
Peripheral arterial Disease
Peripheral arterial disease affects 3 to 6 percent of persons approximately 60 years of age, and the prevalence increases with age.25 Patients may need interventions to reduce the risk of coronary ischemia and vascular referral for treatment.
DIAGNOSIS
The clinical manifestation of peripheral arterial disease is ischemic rest pain or ischemic tissue loss (nonhealing ulcers or gangrene) or intermittent claudication. Peripheral arterial disease may be diagnosed in patients with an ankle-arm index less than 0.9 (or a toe-arm index less than 0.6 in patients with calcified arteries).26 Patients with ischemic rest pain or tissue loss require prompt referral to a vascular subspecialist. Patients with lifestyle-limiting claudication with diminished ankle-arm indices at rest or after exercise also may need referral.
TREATMENT
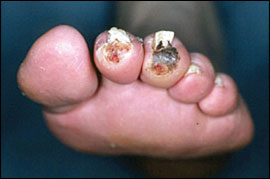
Ischemic rest pain and tissue loss are termed critical limb ischemia because without arterial reconstruction, 80 to 90 percent of patients will experience limb loss six to 12 months after the initial diagnosis. Therefore, critical limb ischemia is an absolute indication for arterial reconstruction. A Cochrane review showed that reconstruction surgery resulted in lower amputation rates and better restoration of blood flow.27 Figure 3 shows a foot wound caused by critical limb ischemia.
Surgical reconstruction is an option for claudication27; however, medical management and risk factor modification should be the primary mode of therapy. Without intervention, the risk of limb loss in patients presenting with claudication is only 5 percent at five years.28 However, claudication significantly reduces quality of life; patients with intermittent claudication rate their state of health only slightly better than patients with congestive heart failure and slightly worse than patients with chronic obstructive pulmonary disease.29
Medical therapy can modestly improve claudication symptoms. The use of cilostazol (Pletal; 100 mg twice a day), in conjunction with a dedicated walking program, can improve comfortable walking distance in about one half of patients.30 A major contraindication to cilostazol is a patient history of congestive heart failure. Pentoxifylline (Trental) is probably not as effective.31
Patients with peripheral arterial disease have a coronary ischemia risk equivalent to that of patients with overt coronary disease (e.g., those with angina or prior myocardial infarction).32 Therefore, patients need aggressive risk factor modification (e.g., smoking cessation, cholesterol-lowering therapy, beta-blocker therapy with or without angiotensin-converting enzyme inhibitors, tight glycemic control, antiplatelet therapy).30
Peripheral arterial disease secondary to atherosclerosis can affect the aortoiliac, femoral popliteal, or infrapopliteal arterial segments or any combination of these segments. Treatment options vary according to the arterial segment or segments involved.33 Balloon angioplasty or stenting is a better option for focal lesions in the iliac arteries than for those in the arteries below the inguinal ligament. Long-segment stenosis, total occlusions, and lesions below the inguinal ligament are better treated with surgical reconstruction because it provides superior long-term patency compared with endovascular therapies (Figure 4).
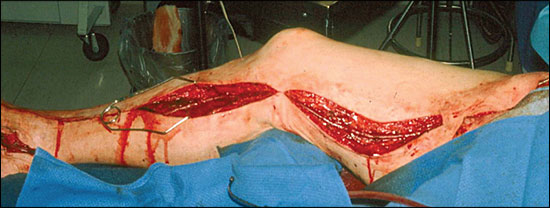
Increased perioperative mortality, morbidity, and disability rates occur in sick and older patients undergoing reconstruction surgery. Therefore, despite the previously mentioned principles, endovascular modalities are increasingly applied to less favorable arterial lesions in patients who are poor candidates for major surgery.