
A more recent article on pressure injuries is available.
Am Fam Physician. 2008;78(10):1186-1194
Patient information: See related handout on preventing bedsores, written by the authors of this article.
Author disclosure: Nothing to disclose.
A pressure ulcer is a localized injury to the skin or underlying tissue, usually over a bony prominence, as a result of unrelieved pressure. Predisposing factors are classified as intrinsic (e.g., limited mobility, poor nutrition, comorbidities, aging skin) or extrinsic (e.g., pressure, friction, shear, moisture). Prevention includes identifying at-risk persons and implementing specific prevention measures, such as following a patient repositioning schedule; keeping the head of the bed at the lowest safe elevation to prevent shear; using pressure-reducing surfaces; and assessing nutrition and providing supplementation, if needed. When an ulcer occurs, documentation of each ulcer (i.e., size, location, eschar and granulation tissue, exudate, odor, sinus tracts, undermining, and infection) and appropriate staging (I through IV) are essential to the wound assessment. Treatment involves management of local and distant infections, removal of necrotic tissue, maintenance of a moist environment for wound healing, and possibly surgery. Debridement is indicated when necrotic tissue is present. Urgent sharp debridement should be performed if advancing cellulitis or sepsis occurs. Mechanical, enzymatic, and autolytic debridement methods are nonurgent treatments. Wound cleansing, preferably with normal saline and appropriate dressings, is a mainstay of treatment for clean ulcers and after debridement. Bacterial load can be managed with cleansing. Topical antibiotics should be considered if there is no improvement in healing after 14 days. Systemic antibiotics are used in patients with advancing cellulitis, osteomyelitis, or systemic infection.
Pressure ulcers, also called decubitus ulcers, bedsores, or pressure sores, range in severity from reddening of the skin to severe, deep craters with exposed muscle or bone. Pressure ulcers significantly threaten the well-being of patients with limited mobility. Although 70 percent of ulcers occur in persons older than 65 years,1 younger patients with neurologic impairment or severe illness are also susceptible. Prevalence rates range from 4.7 to 32.1 percent in hospital settings2 and from 8.5 to 22 percent in nursing homes.3
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Compared with standard hospital mattresses, pressure-reducing devices decrease the incidence of pressure ulcers. | A | 10, 14 |
| There is no evidence to support the routine use of nutritional supplementation (vitamin C, zinc) and a high-protein diet to promote the healing of pressure ulcers. | C | 19 |
| Heel ulcers with stable, dry eschar do not need debridement if there is no edema, erythema, fluctuance, or drainage. | C | 8, 16 |
| Ulcer wounds should not be cleaned with skin cleansers or antiseptic agents (e.g., povidone-iodine [Betadine], hydrogen peroxide, acetic acid) because they destroy granulation tissue. | B | 8, 27, 28 |
Etiology
Pressure ulcers are caused by unrelieved pressure, applied with great force over a short period (or with less force over a longer period), that disrupts blood supply to the capillary network, impeding blood flow and depriving tissues of oxygen and nutrients. This external pressure must be greater than arterial capillary pressure to lead to inflow impairment and resultant local ischemia and tissue damage. The most common sites for pressure ulcers are the sacrum, heels, ischial tuberosities, greater trochanters, and lateral malleoli.
Prevention
RISK ASSESSMENT
Risk assessment begins by identifying risk factors and inspecting the skin. Risk factors for pressure ulcers are classified as intrinsic or extrinsic (Table 1).4 Caregivers should be educated about risk assessment and prevention and should inspect patients often to prevent pressure ulcers or identify them at early stages. Risk assessment scales may further heighten awareness, but have limited predictive ability and no proven effect on pressure ulcer prevention.5 The Braden Scale (Online Figure A) is the most commonly used tool for predicting pressure ulcer risk6 (http://www.bradenscale.com/bradenscale.htm).
| Intrinsic | |
| Limited mobility | |
| Spinal cord injury | |
| Cerebrovascular accident | |
| Progressive neurologic disorders (Parkinson disease, Alzheimer disease, multiple sclerosis) | |
| Pain | |
| Fractures | |
| Postsurgical procedures | |
| Coma or sedation | |
| Arthropathies | |
| Poor nutrition | |
| Anorexia | |
| Dehydration | |
| Poor dentition | |
| Dietary restriction | |
| Weak sense of smell or taste | |
| Poverty or lack of access to food | |
| Comorbidities | |
| Diabetes mellitus | |
| Depression or psychosis | |
| Vasculitis or other collagen vascular disorders | |
| Peripheral vascular disease | |
| Decreased pain sensation | |
| Immunodeficiency or use of corticosteroid therapy | |
| Congestive heart failure | |
| Malignancies | |
| End-stage renal disease | |
| Chronic obstructive pulmonary disease | |
| Dementia | |
| Aging skin | |
| Loss of elasticity | |
| Decreased cutaneous blood flow | |
| Changes in dermal pH | |
| Flattening of rete ridges | |
| Loss of subcutaneous fat | |
| Decreased dermal-epidermal blood flow | |
| Extrinsic | |
| Pressure from any hard surface (e.g., bed, wheelchair, stretcher) | |
| Friction from patient's inability to move well in bed | |
| Shear from involuntary muscle movements | |
| Moisture | |
| Bowel or bladder incontinence | |
| Excessive perspiration | |
| Wound drainage | |
INTERVENTIONS
Preventive measures should be used in at-risk patients. Pressure reduction to preserve microcirculation is a mainstay of preventive therapy. There is no evidence to determine an optimal patient repositioning schedule, and schedules may need to be determined empirically.7 According to recommendations from the Agency for Health Care Policy and Research, patients who are bedridden should be repositioned every two hours.8 To minimize shear, the head of the bed should not be elevated more than 30 degrees and should be maintained at the lowest degree of elevation needed to prevent other medical complications, such as aspiration and worsening congestive heart failure symptoms.7 Some patients can reduce pressure by repositioning themselves using manual aids, such as a trapeze bar.
Pressure-reducing devices can reduce pressure or relieve pressure (i.e., lower tissue pressure to less than the capillary closing pressure of 32 mm Hg) and are classified as static (stationary) or dynamic.9 Static devices include foam, water, gel, and air mattresses or mattress overlays. Dynamic devices, such as alternating pressure devices and low–air-loss and air-fluidized surfaces, use a power source to redistribute localized pressure. Dynamic devices are generally noisy and more expensive than static devices. Pressure-reducing surfaces lower ulcer incidence by 60 percent compared with standard hospital mattresses, although there is no clear difference among pressure-reducing devices.10,11 The benefit of dynamic versus static surfaces is unclear. Dynamic surfaces should be considered if a patient cannot reposition him- or herself independently or if the patient has a poorly healing ulcer.7 If there is less than 1 inch of material between the bed and pressure ulcer when feeling beneath the static surface, the device may not be effective and an alternative should be considered.7 Other pressure-reducing devices include chair cushions and pillows, foam wedges, and materials that are placed between the knees or used to relieve heel pressure. Ring cushions can cause pressure points and should not be used.
Other preventive interventions include nutritional and skin care assessments. Although poor nutrition is associated with pressure ulcers, a causal relationship has not been established.12 One large trial has shown that oral nutritional supplementation reduces risk, but several other trials have not.13 A Cochrane review concluded that there is insufficient evidence on the relationship between nutrition and pressure ulcer prevention.14 A more recent meta-analysis concluded that dietitian consultation and the use of skin moisturizers are reasonable preventive measures.11 However, the role of bactericidal and growth factor preparations is unclear. Continence care programs have not proved successful.15 Despite proper risk assessment and preventive interventions, some pressure ulcers are unavoidable.
Assessment
Assessment of an established pressure ulcer involves a complete medical evaluation of the patient. A comprehensive history includes the onset and duration of ulcers, previous wound care, risk factors, and a list of health problems and medications. Other factors such as psychological health, behavioral and cognitive status, social and financial resources, and access to caregivers are critical in the initial assessment and may influence treatment plans. The presence of a pressure ulcer may indicate that the patient does not have access to adequate services or support. The patient may need more intensive support services, or care-givers may need more training, respite, or assistance with lifting and turning the patient. Patients with communication or sensory disorders are particularly vulnerable to pressure ulcers because they may not feel discomfort or may express discomfort in atypical ways.
The physician should note the number, location, and size (length, width, and depth) of ulcers and assess for the presence of exudate, odor, sinus tracts, necrosis or eschar formation, tunneling, undermining, infection, healing (granulation and epithelialization), and wound margins. Most importantly, the physician should determine the stage of each ulcer (Figures 1 through 4).
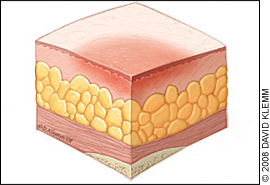
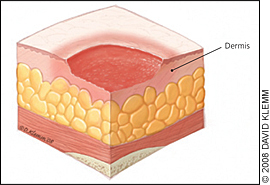
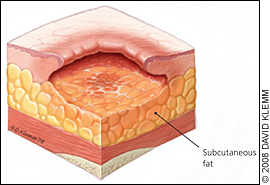
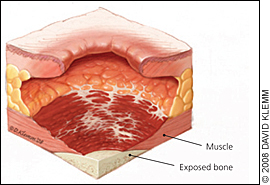
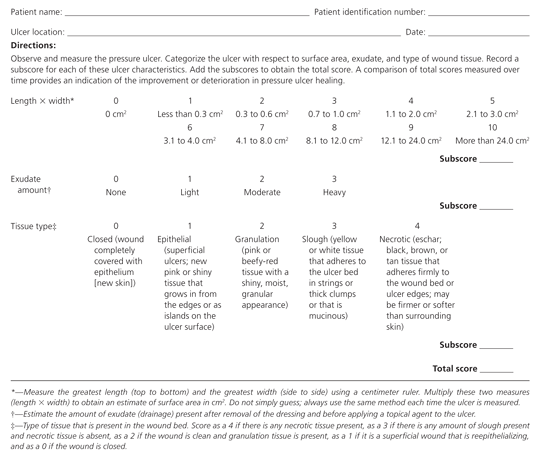
Table 2 presents the National Pressure Ulcer Advisory Panel's staging system for pressure ulcers.16 In a person with dark skin pigmentation, a stage I ulcer may appear as a persistent red, blue, or purple discoloration. The stage of an ulcer cannot be determined until enough slough or eschar is removed to expose the base of the wound. Ulcers do not progress through stages in formation or healing. The Pressure Ulcer Scale for Healing tool (Figure 5) can be used to monitor healing progress.17
| Stage | Description |
|---|---|
| Suspected deep-tissue injury | Purple or maroon localized area of discolored, intact skin or blood-filled blister caused by damage to underlying soft tissue from pressure or shear; the discoloration may be preceded by tissue that is painful, firm, mushy, boggy, or warmer or cooler compared with adjacent tissue |
| I | Intact skin with nonblanchable redness of a localized area, usually over a bony prominence; dark pigmented skin may not have visible blanching, and the affected area may differ from the surrounding area; the affected tissue may be painful, firm, soft, or warmer or cooler compared with adjacent tissue |
| II | Partial-thickness loss of dermis appearing as a shallow, open ulcer with a red-pink wound bed, without slough; may also appear as an intact or open/ruptured serum-filled blister; this stage should not be used to describe skin tears, tape burns, perineal dermatitis, macerations, or excoriations |
| III | Full-thickness tissue loss; subcutaneous fat may be visible, but bone, tendon, or muscle is not exposed; slough may be present, but does not obscure the depth of tissue loss; may include undermining and tunneling* |
| IV | Full-thickness tissue loss with exposed bone, tendon, or muscle; slough or eschar may be present on some parts of the wound bed; often includes undermining and tunneling* |
| Unstageable | Full-thickness tissue loss with the base of the ulcer covered by slough (yellow, tan, gray, green, or brown) or eschar (tan, brown, or black) in the wound bed |
Nutritional Evaluation
Despite the consensus that adequate nutrition is important in wound healing, documentation of its effect on ulcer healing is limited; recommendations are based on observational evidence and expert opinion. Nutritional screening is part of the general evaluation of patients with pressure ulcers. Table 3 presents markers for identifying protein-calorie malnutrition.18 In patients who are malnourished, dietary consultation is recommended and a swallowing evaluation should be considered. Intervention should include encouraging adequate dietary intake using the patient's favorite foods, mealtime assistance, and snacks throughout the day. High-calorie foods and supplements should be used to prevent malnutrition. If oral dietary intake is inadequate or impractical, enteral or parenteral feeding should be considered, if compatible with the patient's wishes, to achieve positive nitrogen balance (approximately 30 to 35 calories per kg per day and 1.25 to 1.5 g of protein per kg per day). Protein, vitamin C, and zinc supplements should be considered if intake is insufficient and deficiency is present, although data supporting their effectiveness in accelerating healing have been inconsistent.19
| Unintentional weight loss of 5 percent or more in the previous 30 days or of 10 percent or more in the previous 180 days |
| Weight less than 80 percent of ideal |
| Serum albumin level less than 3.5 g per dL (35 g per L)* |
| Prealbumin level less than 15 mg per dL (150 mg per L)* |
| Transferrin level less than 200 mg per dL (2 g per L) |
| Total lymphocyte count less than 1,500 per mm3 (1.50 × 109 per L) |
Management
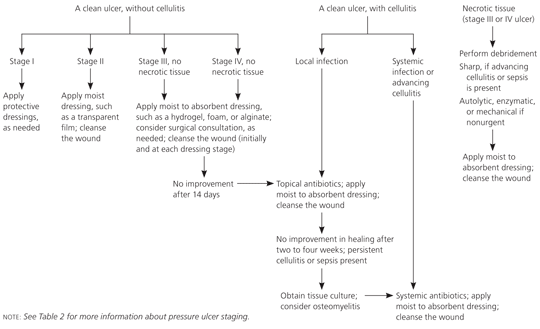
The management of pressure ulcers is interdisciplinary, including primary care physicians, dermatologists, infectious disease consultants, social workers, psychologists, dietitians, podiatrists, home and wound-care nurses, rehabilitation professionals, and surgeons. The basic components of pressure ulcer management are reducing or relieving pressure on the skin, debriding necrotic tissue, cleansing the wound, managing bacterial load and colonization, and selecting a wound dressing. Figure 6 is a brief overview of these key components.18
The pressure-reducing devices used in preventive care also apply to treatment. Static devices are useful in a patient who can change positions independently. A low–air-loss or air-fluidized bed may be necessary for patients with multiple large ulcers or a nonhealing ulcer, after flap surgeries, or when static devices are not effective. No one device is preferred.
Pain assessment should be completed, especially during repositioning, dressing changes, and debridement. Patients at the highest risk of pressure ulcers may not have full sensation or may require alternate pain assessment tools to aid in communication. The goal is to eliminate pain by covering the wound, adjusting pressure-reducing surfaces, repositioning the patient, and providing topical or systemic analgesia. Small randomized controlled trials show that topical opioid (diamorphine gel; not available in the United States) and nonopioid (lidocaine/prilocaine [EMLA]) preparations reduce pain during dressing changes and debridement.20,21
Necrotic tissue promotes bacterial growth and impairs wound healing, and it should be debrided until eschar is removed and granulation tissue is present. Debridement, however, is not recommended for heel ulcers that have stable, dry eschar without edema, erythema, fluctuance, or drainage.8,16 Debridement methods include sharp, mechanical, enzymatic, and autolytic. Sharp debridement using a sterile scalpel or scissors may be performed at bedside, although more extensive debridement should be performed in the operating room. Sharp debridement is needed if infection occurs or to remove thick and extensive eschar. Healing after sharp debridement requires adequate vascularization; thus, vascular assessment for lower extremity ulcers is recommended.22 Anticoagulation is a relative contraindication for sharp debridement.
Mechanical debridement includes wet-to-dry dressings, hydrotherapy, wound irrigation, and whirlpool bath debridement.23 Wet-to-dry dressings adhere to devitalized tissue, which is removed with dressing changes (dry dressings should not be moistened before removal). However, viable tissue may also be removed and the process may be painful.24 Hydrotherapy via whirlpool bath debridement or irrigation may loosen debris. Enzymatic debridement is useful in the long-term care of patients who cannot tolerate sharp debridement; however, it takes longer to be effective and should not be used when infection is present.25,26
Wounds should be cleansed initially and with each dressing change. Use of a 35-mL syringe and 19-gauge angiocatheter provides a degree of force that is effective yet safe; use of normal saline is preferred. Wound cleansing with antiseptic agents (e.g., povidone-iodine [Betadine], hydrogen peroxide, acetic acid) should be avoided because they destroy granulation tissue.27
Dressings that maintain a moist wound environment facilitate healing and can be used for autolytic debridement.28 Synthetic dressings (Table 4) reduce caregiver time, cause less discomfort, and potentially provide more consistent moisture.18 These dressings include transparent films, hydrogels, alginates, foams, and hydrocolloids. Transparent films effectively retain moisture, and may be used alone for partial-thickness ulcers or combined with hydrogels or hydrocolloids for full-thickness wounds. Hydrogels can be used for deep wounds with light exudate. Alginates and foams are highly absorbent and are useful for wounds with moderate to heavy exudate. Hydrocolloids retain moisture and are useful for promoting autolytic debridement. Dressing selection is dictated by clinical judgment and wound characteristics; no moist dressing (including saline-moistened gauze) is superior.29 A wet-to-dry dressing should only be used for debridement and is not a substitute for a wound dressing. Because there are numerous dressing options, physicians should be familiar with one or two products in each category or should obtain recommendations from a wound care consultant.
| Dressing type | Description | Indication | Advantages | Disadvantages | Example (brand names) |
|---|---|---|---|---|---|
| Transparent film | Adhesive, semipermeable, polyurethane membrane that allows water to vaporize and cross the barrier | Management of stage I and II pressure ulcers with light or no exudates May be used with hydrogel or hydrocolloid dressings for full-thickness wounds | Retains moisture Impermeable to bacteria and other contaminants Facilitates autolytic debridement Allows for wound observation Does not require secondary dressing (e.g., tape, wrap) | Not recommended for infected wounds or wounds with drainage Requires border of intact skin for adhesion May dislodge in high-friction areas Not recommended on fragile skin | Bioclusive, Carrafilm, Dermaview, Mefilm, Opsite, Polyskin, Suresite, 3M Tegaderm, Uniflex |
| Hydrogel | Water- or glycerin-based amorphous gels, impregnated gauze, or sheet dressings Amorphous and impregnated gauze fill the dead space tissue and can be used for deep wounds | Management of stages II, III, and IV ulcers; deep wounds; and wounds with necrosis or slough | Soothing, reduces pain Rehydrates wound bed Facilitates autolytic debridement Fills dead tissue space Easy to apply and remove Can be used in infected wounds or to pack deep wounds | Not recommended for wounds with heavy exudate Dehydrates easily if not covered Difficult to secure (amorphous and impregnated gauze need secondary dressing) May cause maceration | Acryderm, Aquaflo, Aquagauze, Carradres, Carraguaze, Carrasmart, Carrasyn, Dermagauze, Dermasyn, Felxigel, SAF-Gel, Solosite, 3M Tegagel, Transigel |
| Alginate | Derived from brown seaweed; composed of soft, nonwoven fibers shaped into ropes or pads | May be used as primary dressing for stages III and IV ulcers, wounds with moderate to heavy exudate or tunneling, and infected or noninfected wounds | Absorbs up to 20 times its weight Forms a gel within the wound Conforms to the shape of the wound Facilitates autolytic debridement Fills in dead tissue space Easy to apply and remove | Not recommended with light exudate or dry scarring or for superficial wounds May dehydrate the wound bed Requires secondary dressing | Algicell, Algisite M, Carboflex, Carraginate, Dermaginate, Kalginate, Kaltostat, Melgisorb, Restore Calcicare, Sorbsan, 3M Tegagen |
| Foam | Provides a moist environment and thermal insulation; available as pads, sheets, and pillow dressings | May be used as primary dressing (to provide absorption and Insulation) or as secondary dressing (for wounds with packing) for stages II to IV ulcers with variable drainage | Nonadherent, although some have adherent borders Repels contaminants Easy to apply and remove Absorbs light to heavy exudate May be used under compression Recommended for fragile skin | Not effective for wounds with dry eschar May require a secondary dressing | Allevyn, Biatain, Carrasmart, Curafoam, Dermalevin, Epigard, Hydrocell, Lyofoam, Mepilex, Optifoam, Polyderm, Polymem, SOF-foam, Tielle, Vigifoam |
| Hydrocolloid | Occlusive or semiocclusive dressings composed of materials such as gelatin and pectin; available in various forms (e.g., wafers, pastes, powders) | May be used as primary or secondary dressing for stages II to IV ulcers, wounds with slough and necrosis, or wounds with light to moderate exudates Some may be used for stage I ulcers | Impermeable to bacteria and other contaminants Facilitates autolytic debridement Self-adherent, molds well Allows observation, if transparent May be used under compression products (compression stockings, wraps, Unna boot) May be applied over alginate dressing to control drainage | Not recommended for wounds with heavy exudate, sinus tracts, or infection May curl at edges May injure fragile skin upon removal Contraindicated for wounds with packing | Carrasmart, Combiderm, Comfeel, Dermafilm, Duoderm, Exuderm, Hyperion, MPM Excel, Nuderm, Primacol, RepliCare, Restore, Sorbex, 3M Tegaderm, Ultec |
| Moistened gauze | 2 × 2- or 4 × 4-inch square of gauze soaked in saline for packing | May be used for stages III and IV ulcers and for deep wounds, especially those with tunneling or undermining | Accessible | Must be remoistened often Time-consuming to apply | Fluffed Kerlix, Plain Nugauze |
Urinary catheters or rectal tubes may be needed to prevent bacterial infection from feces or urine. Pressure ulcers are invariably colonized with bacteria; however, wound cleansing and debridement minimize bacterial load. A trial of topical antibiotics, such as silver sulfadiazine cream (Silvadene), should be used for up to two weeks for clean ulcers that are not healing properly after two to four weeks of optimal wound care. Quantitative bacteria tissue cultures should be performed for nonhealing ulcers after a trial of topical antibiotics or if there are signs of infection (e.g., increased drainage, odor, surrounding erythema, pain, warmth). A superficial swab specimen may be used; however, a needle aspiration or ulcer biopsy (preferred) is more clinically significant.30 Systemic antibiotics are not recommended unless there is evidence of advancing cellulitis, osteomyelitis, and bacteremia.
Ulcers are difficult to resolve. Although more than 70 percent of stage II ulcers heal after six months of appropriate treatment, only 50 percent of stage III ulcers and 30 percent of stage IV ulcers heal within this period. Surgical consultation should be obtained for patients with clean stage III or IV ulcers that do not respond to optimal patient care or when quality of life would be improved with rapid wound closure. Surgical approaches include direct closure; skin grafts; and skin, musculocutaneous, and free flaps. However, randomized controlled trials of surgical repair are lacking and recurrence rates are high.
Complications
Although noninfectious complications of pressure ulcers occur, systemic infections are the most prevalent. Noninfectious complications include amyloidosis, heterotopic bone formation, perinealurethral fistula, pseudoaneurysm, Marjolin ulcer, and systemic complications of topical treatment. Infectious complications include bacteremia and sepsis, cellulitis, endocarditis, meningitis, osteomyelitis, septic arthritis, and sinus tracts or abscesses.8 Osteomyelitis has been reported in 17 to 32 percent of infected ulcers and may lead to nonhealing ulcers with or without systemic manifestations.37 Plain radiographs and bone scans are often unreliable. Magnetic resonance imaging has a 98 percent sensitivity and 89 percent specificity for osteomyelitis in patients with pressure ulcers38; however, needle biopsy of the bone (via orthopedic consultation) is recommended and can guide antibiotic therapy. Bacteremia may occur with or without osteomyelitis, causing unexplained fever, tachycardia, hypotension, or altered mental status.39 Overall mortality is high with both conditions,40 and empirical antibiotics pending culture results should cover methicillin-resistant Staphylococcus aureus, anaerobes, enterococci, and gram-negative organisms, such as Pseudomonas, Proteus, and Providencia species.41