
A more recent article on chronic kidney disease is available.
Am Fam Physician. 2011;84(10):1138-1148
Author disclosure: No relevant financial affiliations to disclose.
Chronic kidney disease affects an estimated 27 million adults in the United States, and is associated with significantly increased risk of cardiovascular disease and stroke. Patients should be assessed annually to determine whether they are at increased risk of developing chronic kidney disease based on clinical and sociodemographic factors. Diabetes mellitus, hypertension, and older age are the primary risk factors that warrant screening. Other risk factors include cardiovascular disease, family history of chronic kidney disease, and ethnic and racial minority status. Serum creatinine levels can be used to estimate the glomerular filtration rate, and spot urine testing can detect proteinuria. After the diagnosis of chronic kidney disease is made, staging based on estimated glomerular filtration rate determines prognosis, evaluation, and management. Further evaluation should focus on the specific type of kidney disease and on identifying complications related to the disease stage. Patients should be assessed for risk factors leading to the further loss of kidney function and cardiovascular disease. Patients with estimated glomerular filtration rates less than 30 mL per minute per 1.73 m2, significant proteinuria, or rapid loss of kidney function should be referred to a nephrologist for further evaluation and management.
Chronic kidney disease (CKD) affects an estimated 27 million adults in the United States and is associated with increased mortality, morbidity, and health care costs.1,2 CKD is also associated with significantly increased risks of cardiovascular disease3 and stroke.4 The incidence and prevalence of CKD among U.S. adults have increased dramatically since 1991.5 More than 500,000 Americans were treated for end-stage renal disease in 2007.6 The increases are partly explained by the increasing prevalence of diabetes mellitus and hypertension, the leading risk factors for CKD. Awareness of CKD among patients has modestly increased in recent years, but remains low. According to the 2003–2004 National Health and Nutrition Examination Survey, less than 5 percent of patients with stage 1 or 2 CKD and less than 10 percent with stage 3 reported having been diagnosed with CKD; only 45 percent of patients with stage 4 were aware of their condition.7 Although clinical laboratories report estimated glomerular filtration rate (GFR) directly to physicians, CKD recognition remains low.8 In 2002, the National Kidney Foundation's Kidney Disease Outcomes Quality Initiative published practice guidelines to help primary care physicians identify patients with early CKD and improve health outcomes.9
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Physicians should screen at-risk populations for CKD using serum creatinine levels and random urine testing for albuminuria. | C | 9, 11, 12, 15 |
| Adults with cardiovascular disease should be screened for CKD. | C | 13 |
| The Chronic Kidney Disease Epidemiology Collaboration formula is more accurate than the Modification of Diet in Renal Disease equation or the Cockcroft-Gault equation, and should be used to estimate GFR. | C | 19, 21 |
| Acetaminophen is the analgesic of choice for short-term treatment of mild to moderate pain in patients with stage 3 to 5 CKD. | C | 33 |
| Nephrology consultation is indicated when the estimated GFR is less than 30 mL per minute per 1.73 m2. | C | 9 |
CKD is defined by the presence of structural or functional abnormalities of the kidney with or without an accompanying reduction in GFR. Persons with CKD may have one or more of the following: pathologic abnormalities, markers of kidney damage (i.e., imaging abnormalities and abnormalities in serum or urine, including proteinuria and abnormal urinary sediment), or GFR less than 60 mL per minute per 1.73 m2 for at least three months. If the duration of the abnormality is unknown, the possibility of acute kidney injury should be considered and appropriate evaluation performed for reversible causes. Most persons who have received kidney transplants are considered to have CKD.
Detection of CKD
INDICATIONS FOR SCREENING
Annual CKD screening is recommended by the American Diabetes Association,10 by the National Kidney Foundation for patients at risk,9,11 by the Joint National Committee on Hypertension12 for patients with diabetes and hypertension, and by the American Heart Association for patients with cardiovascular disease.13 The U.S. Preventive Services Task Force has not examined the evidence or made recommendations for screening. Cardiovascular disease, a family history of CKD, and ethnic or racial minority status do not significantly increase the risk of CKD in adults older than 60 who have diabetes and hypertension.14 Risk factors are listed in Table 1.9
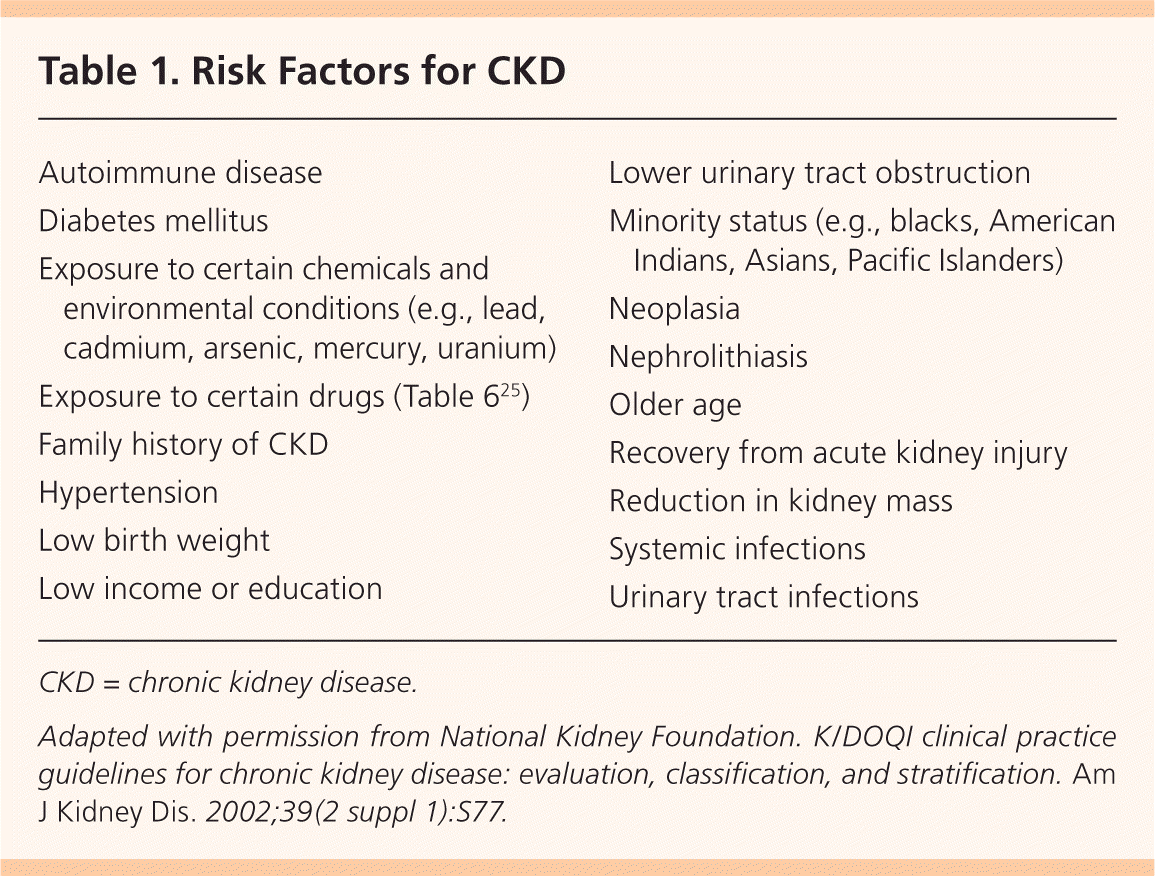
| Autoimmune disease |
| Diabetes mellitus |
| Exposure to certain chemicals and environmental conditions (e.g., lead, cadmium, arsenic, mercury, uranium) |
| Exposure to certain drugs (Table 625) |
| Family history of CKD |
| Hypertension |
| Low birth weight |
| Low income or education |
| Lower urinary tract obstruction |
| Minority status (e.g., blacks, American Indians, Asians, Pacific Islanders) |
| Neoplasia |
| Nephrolithiasis |
| Older age |
| Recovery from acute kidney injury |
| Reduction in kidney mass |
| Systemic infections |
| Urinary tract infections |
SCREENING TESTS
CKD is typically detected by measuring serum creatinine levels to calculate the GFR and by measuring the urinary albumin/creatinine ratio to detect proteinuria.15 Although the most common causes of CKD are diabetes and hypertension (Table 29,16 ), the disease can be caused by many other conditions. Urinalysis can detect signs of glomerulonephritis, tubulointerstitial disease, vasculitis, hereditary nephritis, and lupus nephritis; however, it is not routinely recommended in otherwise healthy patients.
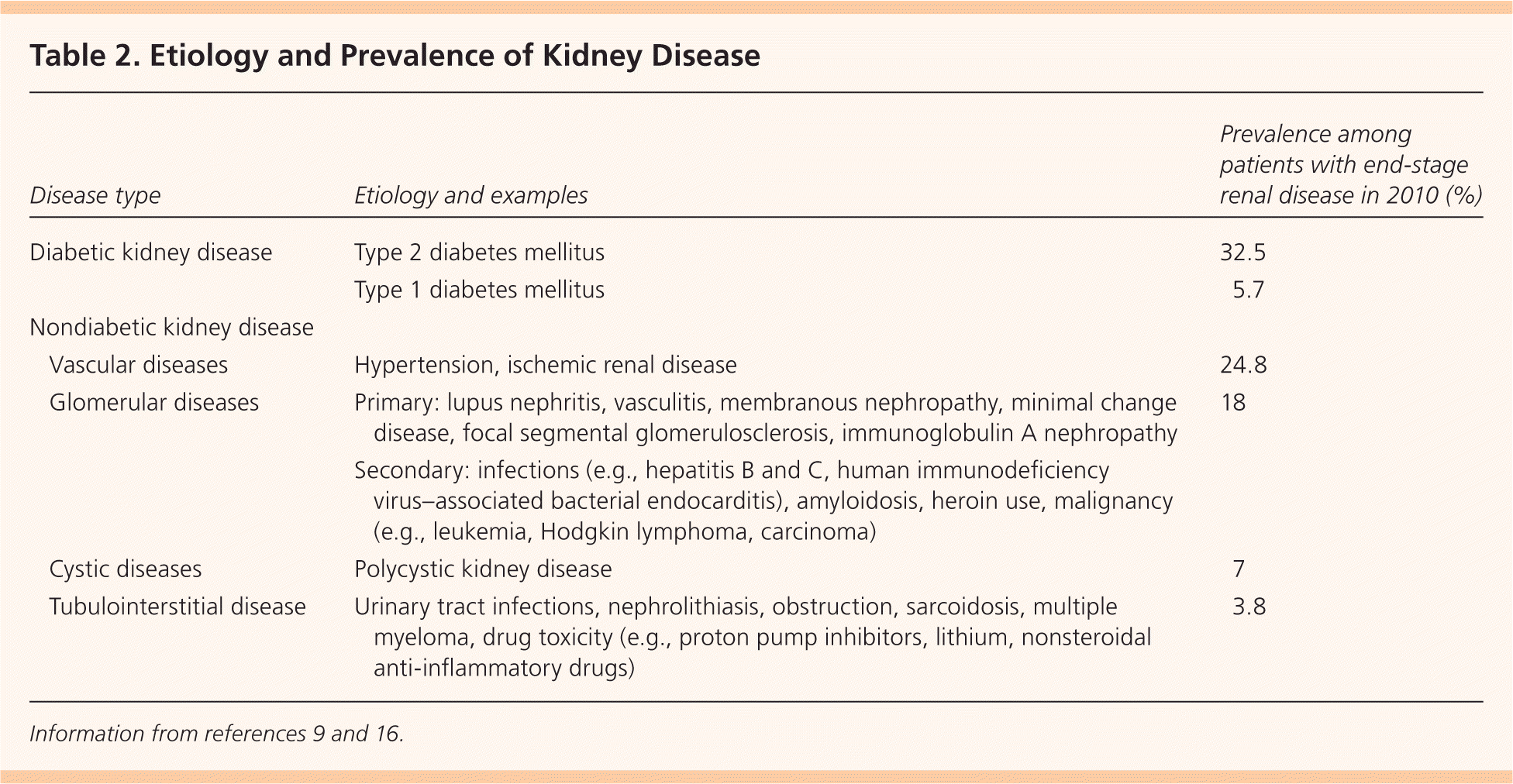
| Disease type | Etiology and examples | Prevalence among patients with end-stage renal disease in 2010 (%) | |
|---|---|---|---|
| Diabetic kidney disease | Type 2 diabetes mellitus | 32.5 | |
| Type 1 diabetes mellitus | 5.7 | ||
| Nondiabetic kidney disease | |||
| Vascular diseases | Hypertension, ischemic renal disease | 24.8 | |
| Glomerular diseases | Primary: lupus nephritis, vasculitis, membranous nephropathy, minimal change disease, focal segmental glomerulosclerosis, immunoglobulin A nephropathy | 18 | |
| Secondary: infections (e.g., hepatitis B and C, human immunodeficiency virus–associated bacterial endocarditis), amyloidosis, heroin use, malignancy (e.g., leukemia, Hodgkin lymphoma, carcinoma) | |||
| Cystic diseases | Polycystic kidney disease | 7 | |
| Tubulointerstitial disease | Urinary tract infections, nephrolithiasis, obstruction, sarcoidosis, multiple myeloma, drug toxicity (e.g., proton pump inhibitors, lithium, nonsteroidal anti-inflammatory drugs) | 3.8 | |
ESTIMATING GFR
The GFR is the best measure of kidney function. Normal GFR varies by age, sex, and body size. GFR is approximately 120 to 130 mL per minute per 1.73 m2 in young adults, and decreases by an average of 1 mL per minute per 1.73 m2 per year after 30 years of age.9 A GFR less than 60 mL per minute per 1.73 m2 represents a loss of at least one-half of normal kidney function; below this level, there is an increased prevalence of CKD complications.
Creatinine clearance is used to estimate the GFR. Because creatinine is filtered and secreted by the proximal tubules, the creatinine clearance exceeds the GFR. Generation of creatinine is determined by muscle mass and diet, whereas tubular secretion could be decreased by the use of medications such as trimethoprim and cimetidine (Tagamet).
The serum creatinine level is an insensitive marker of GFR early in the course of CKD. A 33 percent decrease in GFR may raise the creatinine level from 0.8 to only 1.2 mg per dL (70.72 to 106.08 μmol per L). If the prior creatinine level is not known, this decrease in GFR may go unrecognized. When estimated GFR is suspected to be inaccurate—for example, in patients with severe malnutrition or paraplegia—a 24-hour urine collection should be performed to evaluate creatinine clearance.
Three equations are typically used to estimate GFR: the Cockcroft-Gault equation,17 the Modification of Diet in Renal Disease (MDRD) equation,18 and the more accurate Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI)19 formula (Table 3).17–19 The Cockcroft-Gault equation systematically overestimates GFR. The MDRD is reasonably accurate in patients with CKD, but it underestimates GFR when it is greater than 60 mL per minute per 1.73 m2, and it may misidentify persons with normal kidney function as having CKD. The MDRD can also be affected by fluctuations in creatinine production and fluid balance; it gives falsely elevated estimated GFRs in malnourished and overhydrated patients and falsely decreased GFRs due to increased serum creatinine levels in patients taking trimethoprim and cimetidine.20 The CKD-EPI formula provides better GFR estimation in patients with reduced and normal kidney function.19 In a recent study, the CKD-EPI was found to be the most accurate formula in estimating GFR.21
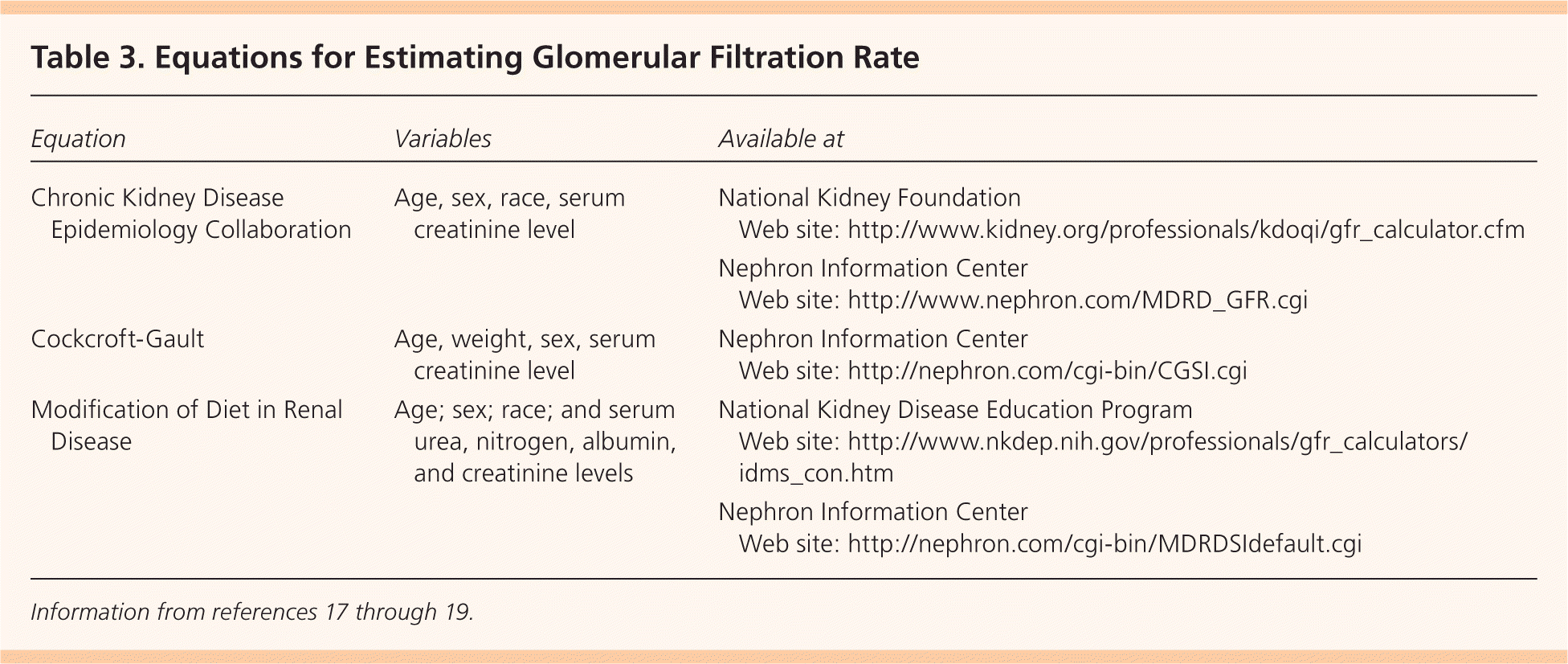
| Equation | Variables | Available at | |
|---|---|---|---|
| Chronic Kidney Disease Epidemiology Collaboration | Age, sex, race, serum creatinine level | National Kidney Foundation | |
| Web site: http://www.kidney.org/professionals/kdoqi/gfr_calculator.cfm | |||
| Nephron Information Center | |||
| Web site: http://www.nephron.com/MDRD_GFR.cgi | |||
| Cockcroft-Gault | Age, weight, sex, serum creatinine level | Nephron Information Center | |
| Web site: http://nephron.com/cgi-bin/CGSI.cgi | |||
| Modification of Diet in Renal Disease | Age; sex; race; and serum urea, nitrogen, albumin, and creatinine levels | National Kidney Disease Education Program | |
| Web site: http://www.nkdep.nih.gov/professionals/gfr_calculators/idms_con.htm | |||
| Nephron Information Center | |||
| Web site: http://nephron.com/cgi-bin/MDRDSIdefault.cgi | |||
Markers of Kidney Damage
PROTEINURIA
Proteinuria refers to increased excretion of any urinary protein, including albumin and other serum proteins (tubular proteins). A normal urinary protein/creatinine ratio is less than 200 mg per g; proteinuria is a predictor of total mortality and CKD progression, and can help determine the type of CKD. A normal urinary albumin/creatinine ratio is less than 30 mg per g. Patients with albumin/creatinine ratios of 30 to 300 mg per g are classified as having microalbuminuria, and those with ratios greater than 300 mg per g are classified as having macroalbuminuria.10,11
Urine dipstick testing is insensitive for the measurement of small amounts of albumin and is not recommended for CKD screening in patients at risk. This test is positive when a large amount (greater than 500 to 1,000 mg per day) of protein is excreted. Patients with positive urine dipstick results should repeat the test in the absence of urinary tract infection, ketosis, and hypovolemia. If the second result is positive, the urinary protein/creatinine ratio should be obtained within three months. Persistent proteinuria can be diagnosed by two positive protein/creatinine ratios one to two weeks apart.9 Diabetes, the leading cause of nephrotic syndrome in the United States, is diagnosed when the protein/creatinine ratio is greater than 3,000 mg (3.0 g) per g.
ALBUMINURIA
Albumin is a sensitive marker of CKD caused by diabetes, hypertension, and glomerular diseases. Microalbuminuria was the most common abnormality associated with the diagnosis of stages 1 and 2 CKD in the National Health and Nutrition Examination Survey.5 Microalbuminuria is an inherent part of the diabetes disease process but also can be present with nonrenal conditions (e.g., obesity, inflammation, cancer).22 Microalbuminuria may indicate increased vascular permeability rather than kidney injury.23
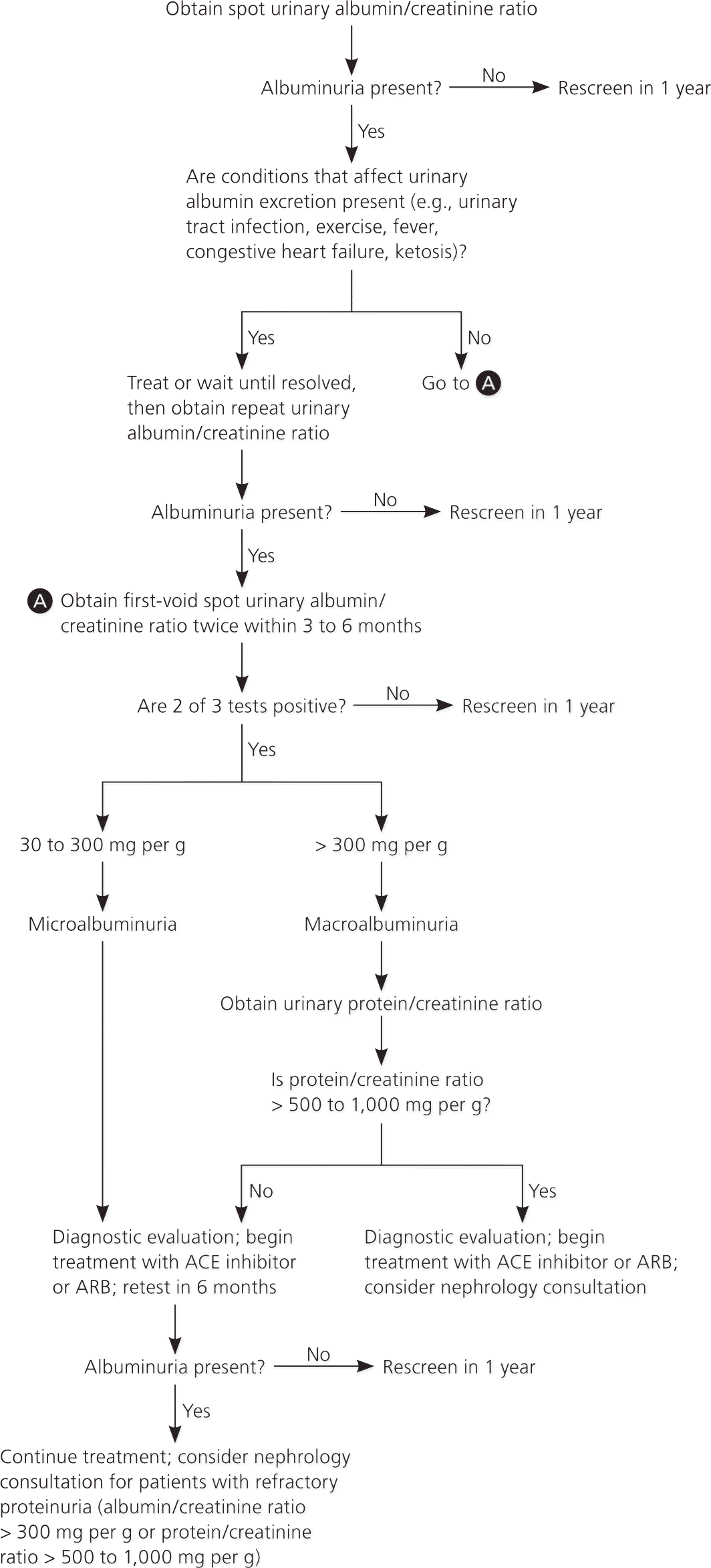
Patients with diabetes and microalbuminuria who progress to macroalbuminuria are more likely to progress to end-stage renal disease. Diabetic kidney disease can be diagnosed based on the urinary albumin/creatinine ratio, duration of diabetes, and presence of diabetic retinopathy (Table 4).11 When albuminuria reaches the range of macroalbuminuria, albumin becomes the dominant urinary protein, and the advantage of measuring albuminuria over proteinuria is generally lost.9 Two out of three abnormal readings are required to confirm persistent albuminuria (Figure 1).9,15,24

| Screening initiation |
| At the time of diagnosis of type 2 diabetes mellitus |
| Five years after diagnosis of type 1 diabetes mellitus |
| Screening frequency |
| Annually |
| Diagnostic criteria |
| Macroalbuminuria |
| Microalbuminuria in patients who have had type 1 diabetes for more than 10 years |
| Microalbuminuria in the presence of diabetic retinopathy |
| Clinical findings that should prompt consideration of other causes |
| Absence of albuminuria in patients with stage 3 to 5 chronic kidney disease |
| Absence of diabetic retinopathy |
| Active urinary sediment (cells or casts) |
| Low GFR at the time of diagnosis |
| More than 30 percent reduction in GFR within two to three months after initiation of an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker |
| Rapidly decreasing GFR (more than 4 mL per minute per 1.73 m2 per year) |
| Rapidly increasing proteinuria or nephrotic syndrome |
| Refractory hypertension |
| Signs or symptoms of other systemic disease |
OTHER MARKERS
Other markers of kidney damage include hematuria, cellular casts, serum markers, and imaging abnormalities. Clinical judgment based on the assessment of CKD risk factors should be used to determine if measurement of other markers of kidney damage is indicated.
CKD Staging
Staging is an important step for determining the prognosis, evaluation, and management of CKD. Staging is based on the level of estimated GFR, irrespective of diagnosis (Table 5).9 Markers of kidney damage are required for stages 1 and 2. Using the current classification system, nearly 50 percent of U.S. adults older than 70 years have stage 3 CKD, and most have no evidence of albuminuria.5 Older patients with stage 3 CKD without proteinuria and stable creatinine levels on repeat testing at three to six months are unlikely to progress to end-stage renal disease and do not have increased mortality risk. Physicians may choose to defer further evaluation in such patients, avoid the use of nephrotoxic medications (Table 625 ), and adjust the dosages of those that are excreted by the kidneys20 (Table 79,26 ).
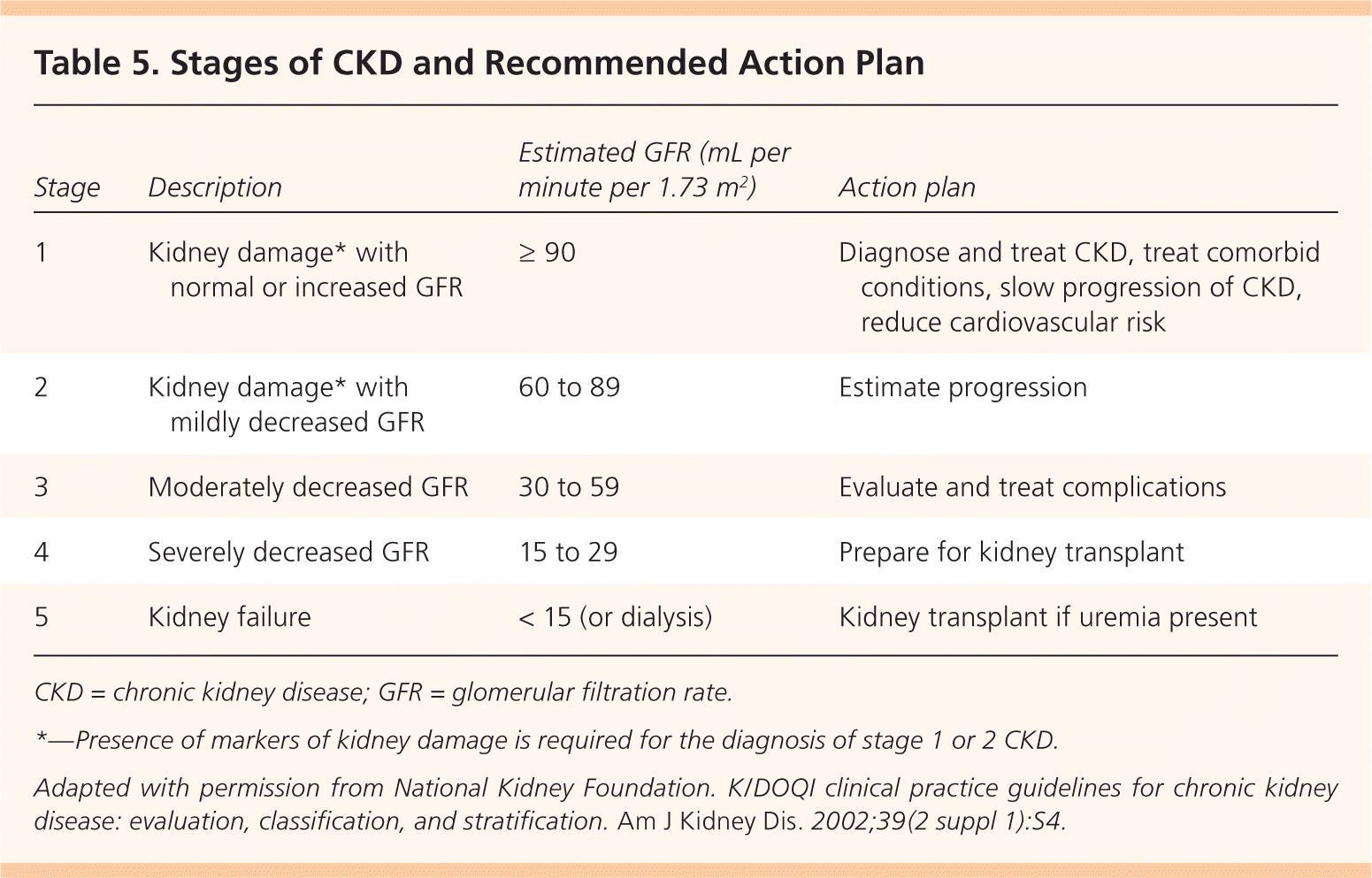
| Stage | Description | Estimated GFR (mL per minute per 1.73 m2 ) | Action plan |
|---|---|---|---|
| 1 | Kidney damage* with normal or increased GFR | ≥ 90 | Diagnose and treat CKD, treat comorbid conditions, slow progression of CKD, reduce cardiovascular risk |
| 2 | Kidney damage* with mildly decreased GFR | 60 to 89 | Estimate progression |
| 3 | Moderately decreased GFR | 30 to 59 | Evaluate and treat complications |
| 4 | Severely decreased GFR | 15 to 29 | Prepare for kidney transplant |
| 5 | Kidney failure | < 15 (or dialysis) | Kidney transplant if uremia present |
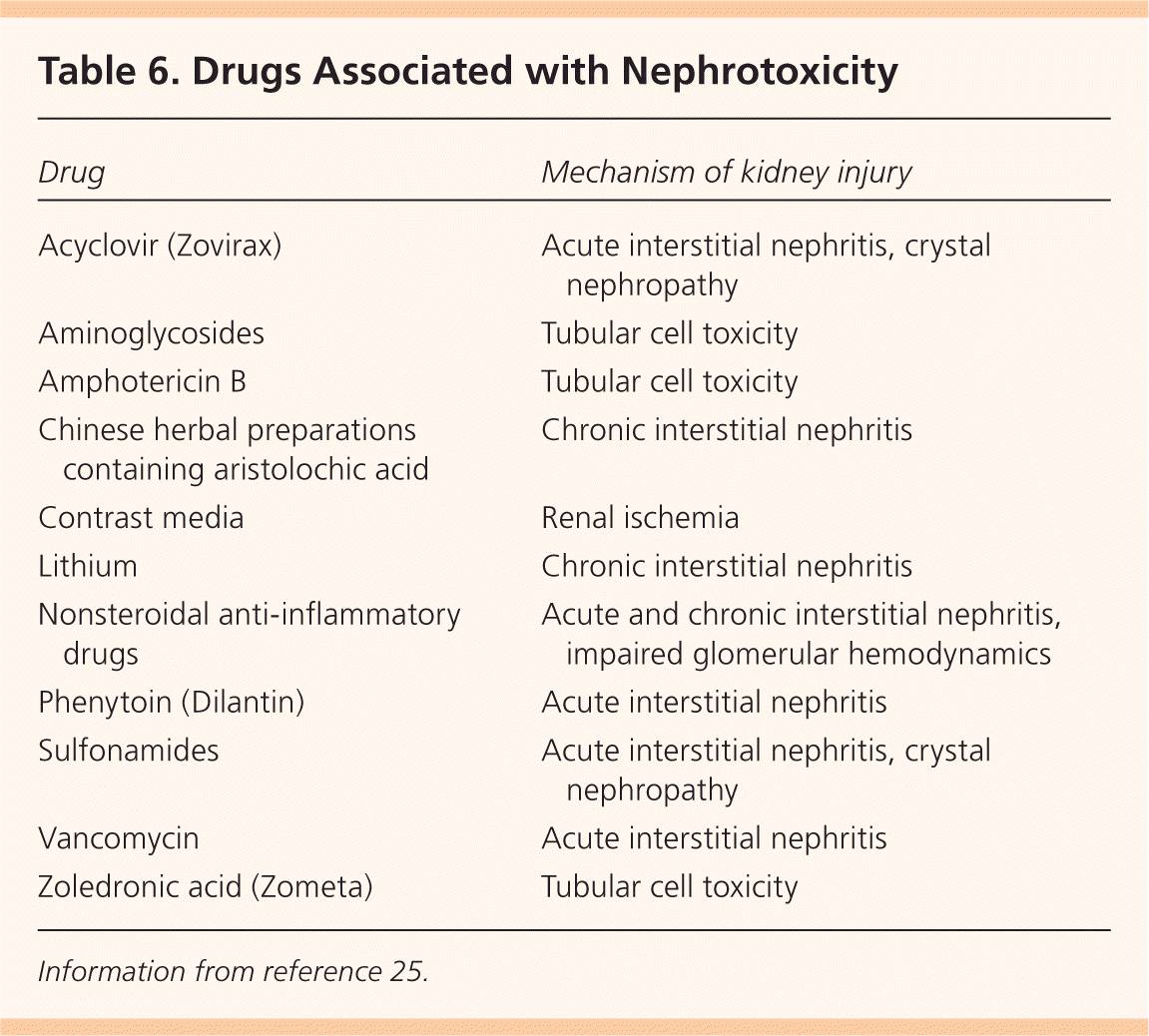
| Drug | Mechanism of kidney injury |
|---|---|
| Acyclovir (Zovirax) | Acute interstitial nephritis, crystal nephropathy |
| Aminoglycosides | Tubular cell toxicity |
| Amphotericin B | Tubular cell toxicity |
| Chinese herbal preparations containing aristolochic acid | Chronic interstitial nephritis |
| Contrast media | Renal ischemia |
| Lithium | Chronic interstitial nephritis |
| Nonsteroidal anti-inflammatory drugs | Acute and chronic interstitial nephritis, impaired glomerular hemodynamics |
| Phenytoin (Dilantin) | Acute interstitial nephritis |
| Sulfonamides | Acute interstitial nephritis, crystal nephropathy |
| Vancomycin | Acute interstitial nephritis |
| Zoledronic acid (Zometa) | Tubular cell toxicity |
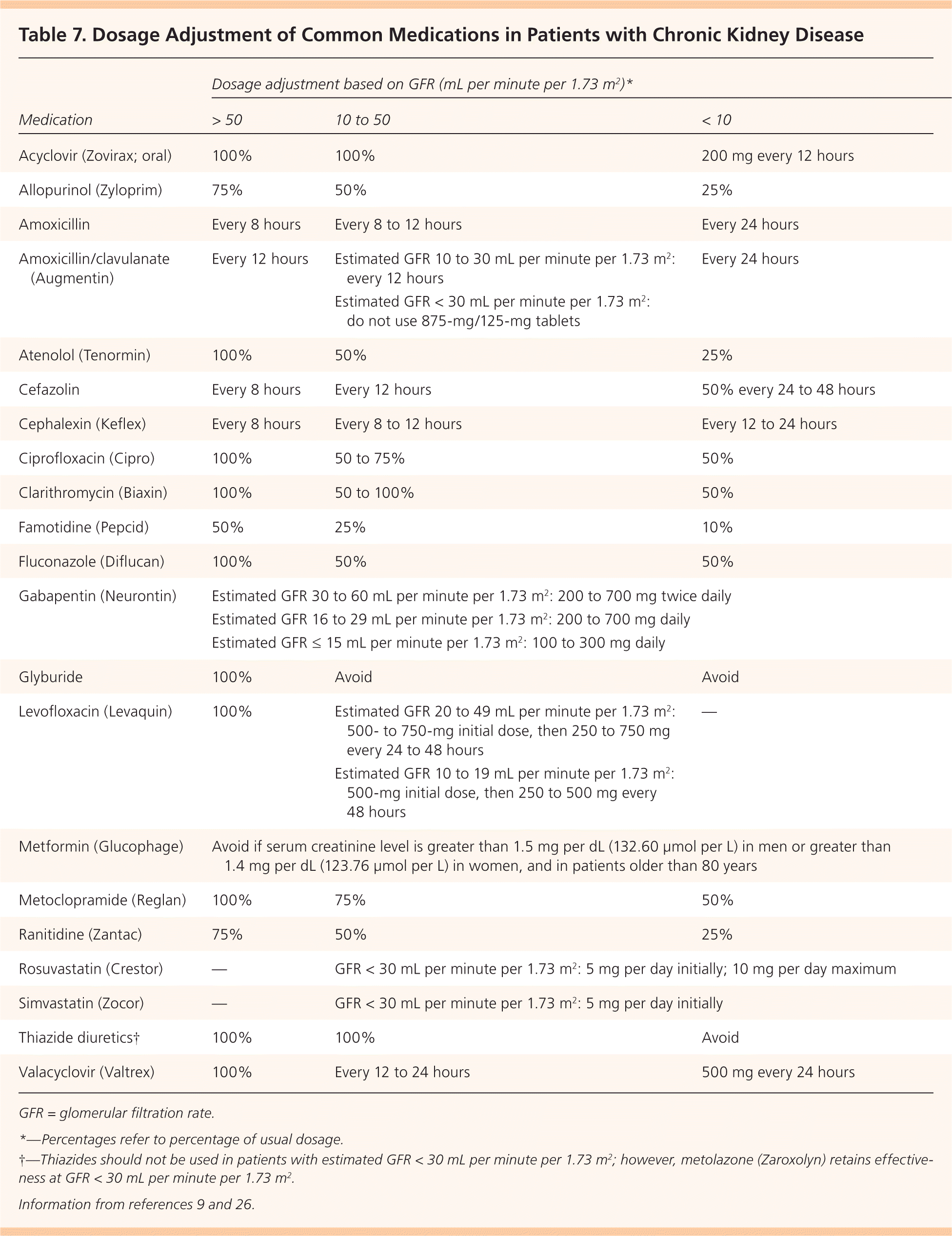
| Medication | Dosage adjustment based on GFR (mL per minute per 1.73 m2 )* | ||
|---|---|---|---|
| > 50 | 10 to 50 | < 10 | |
| Acyclovir (Zovirax; oral) | 100% | 100% | 200 mg every 12 hours |
| Allopurinol (Zyloprim) | 75% | 50% | 25% |
| Amoxicillin | Every 8 hours | Every 8 to 12 hours | Every 24 hours |
| Amoxicillin/clavulanate (Augmentin) | Every 12 hours | Estimated GFR 10 to 30 mL per minute per 1.73 m2: every 12 hours | Every 24 hours |
| Estimated GFR < 30 mL per minute per 1.73 m2: do not use 875-mg/125-mg tablets | |||
| Atenolol (Tenormin) | 100% | 50% | 25% |
| Cefazolin | Every 8 hours | Every 12 hours | 50% every 24 to 48 hours |
| Cephalexin (Keflex) | Every 8 hours | Every 8 to 12 hours | Every 12 to 24 hours |
| Ciprofloxacin (Cipro) | 100% | 50 to 75% | 50% |
| Clarithromycin (Biaxin) | 100% | 50 to 100% | 50% |
| Famotidine (Pepcid) | 50% | 25% | 10% |
| Fluconazole (Diflucan) | 100% | 50% | 50% |
| Gabapentin (Neurontin) | Estimated GFR 30 to 60 mL per minute per 1.73 m2: 200 to 700 mg twice daily | ||
| Estimated GFR 16 to 29 mL per minute per 1.73 m2: 200 to 700 mg daily | |||
| Estimated GFR ≤ 15 mL per minute per 1.73 m2: 100 to 300 mg daily | |||
| Glyburide | 100% | Avoid | Avoid |
| Levofloxacin (Levaquin) | 100% | Estimated GFR 20 to 49 mL per minute per 1.73 m2: 500- to 750-mg initial dose, then 250 to 750 mg every 24 to 48 hours | — |
| Estimated GFR 10 to 19 mL per minute per 1.73 m2: 500-mg initial dose, then 250 to 500 mg every 48 hours | |||
| Metformin (Glucophage) | Avoid if serum creatinine level is greater than 1.5 mg per dL (132.60 μmol per L) in men or greater than 1.4 mg per dL (123.76 μmol per L) in women, and in patients older than 80 years | ||
| Metoclopramide (Reglan) | 100% | 75% | 50% |
| Ranitidine (Zantac) | 75% | 50% | 25% |
| Rosuvastatin (Crestor) | — | GFR < 30 mL per minute per 1.73 m2: 5 mg per day initially; 10 mg per day maximum | |
| Simvastatin (Zocor) | — | GFR < 30 mL per minute per 1.73 m2: 5 mg per day initially | |
| Thiazide diuretics† | 100% | 100% | Avoid |
| Valacyclovir (Valtrex) | 100% | Every 12 to 24 hours | 500 mg every 24 hours |
CKD staging does not include risk-modifying parameters such as degree of albuminuria, age, sex, and cardiovascular risk factors. Revised guidelines, currently under development, will address those issues.
Evaluation of CKD
A thorough initial investigation includes determining the etiology and type of CKD and evaluating for comorbidities. The patient and family histories, physical examination, and blood pressure and weight measurements are the most valuable parts of the CKD evaluation (Table 8).9,27,28 Laboratory tests should include measurement of serum electrolytes and glucose, and a fasting lipid panel. Urinalysis should be performed to evaluate urinary sediment and the urinary albumin/creatinine or protein/creatinine ratio9 (Table 99,11,29 ). Additional testing may be required to identify rare causes of CKD. Renal ultrasonography is recommended to evaluate kidney size and assess for possible structural abnormalities.9
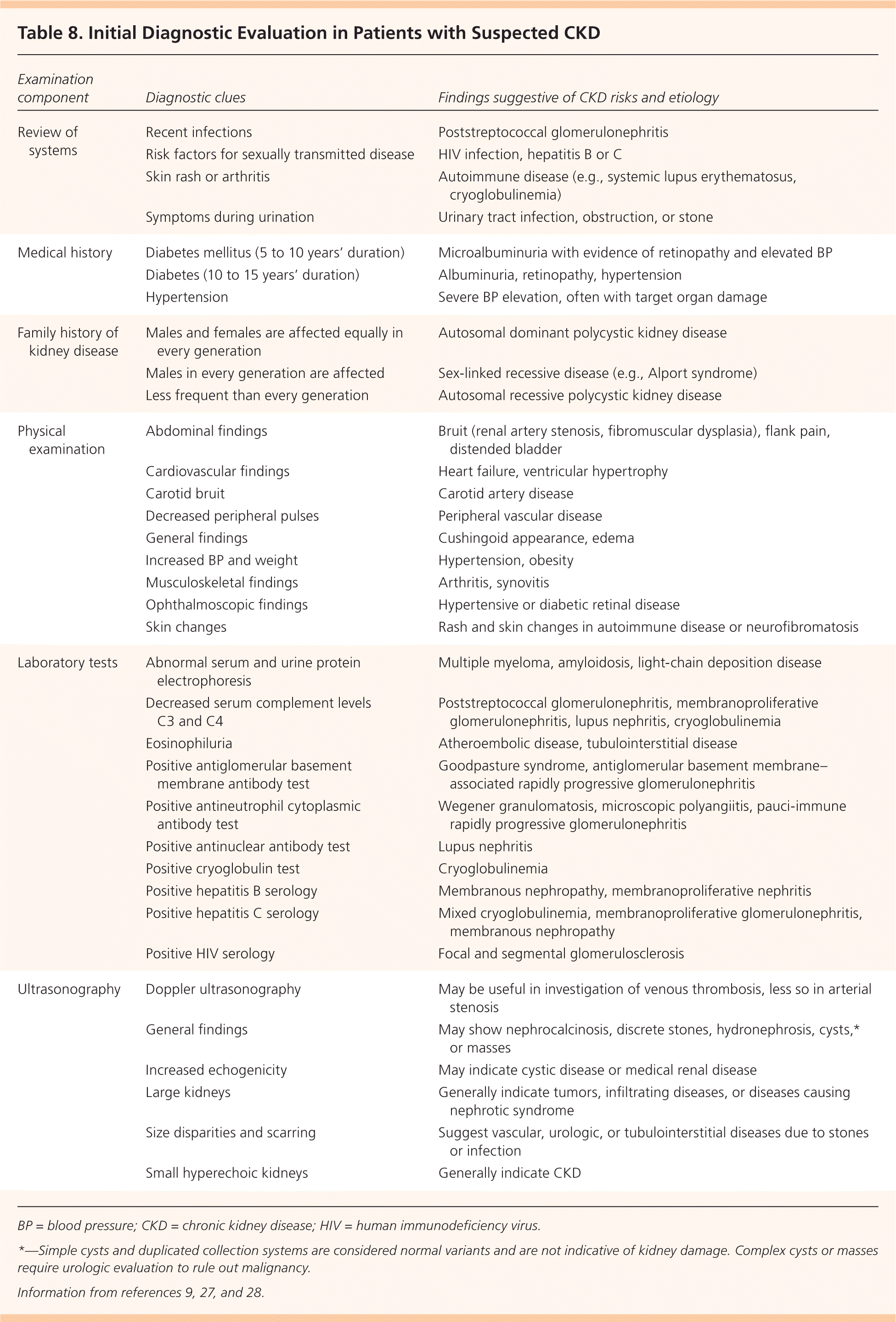
| Examination component | Diagnostic clues | Findings suggestive of CKD risks and etiology |
|---|---|---|
| Review of systems | Recent infections | Poststreptococcal glomerulonephritis |
| Risk factors for sexually transmitted disease | HIV infection, hepatitis B or C | |
| Skin rash or arthritis | Autoimmune disease (e.g., systemic lupus erythematosus, cryoglobulinemia) | |
| Symptoms during urination | Urinary tract infection, obstruction, or stone | |
| Medical history | Diabetes mellitus (5 to 10 years' duration) | Microalbuminuria with evidence of retinopathy and elevated BP |
| Diabetes (10 to 15 years' duration) | Albuminuria, retinopathy, hypertension | |
| Hypertension | Severe BP elevation, often with target organ damage | |
| Family history of kidney disease | Males and females are affected equally in every generation | Autosomal dominant polycystic kidney disease |
| Males in every generation are affected | Sex-linked recessive disease (e.g., Alport syndrome) | |
| Less frequent than every generation | Autosomal recessive polycystic kidney disease | |
| Physical examination | Abdominal findings | Bruit (renal artery stenosis, fibromuscular dysplasia), flank pain, distended bladder |
| Cardiovascular findings | Heart failure, ventricular hypertrophy | |
| Carotid bruit | Carotid artery disease | |
| Decreased peripheral pulses | Peripheral vascular disease | |
| General findings | Cushingoid appearance, edema | |
| Increased BP and weight | Hypertension, obesity | |
| Musculoskeletal findings | Arthritis, synovitis | |
| Ophthalmoscopic findings | Hypertensive or diabetic retinal disease | |
| Skin changes | Rash and skin changes in autoimmune disease or neurofibromatosis | |
| Laboratory tests | Abnormal serum and urine protein electrophoresis | Multiple myeloma, amyloidosis, light-chain deposition disease |
| Decreased serum complement levels C3 and C4 | Poststreptococcal glomerulonephritis, membranoproliferative glomerulonephritis, lupus nephritis, cryoglobulinemia | |
| Eosinophiluria | Atheroembolic disease, tubulointerstitial disease | |
| Positive antiglomerular basement membrane antibody test | Goodpasture syndrome, antiglomerular basement membrane–associated rapidly progressive glomerulonephritis | |
| Positive antineutrophil cytoplasmic antibody test | Wegener granulomatosis, microscopic polyangiitis, pauci-immune rapidly progressive glomerulonephritis | |
| Positive antinuclear antibody test | Lupus nephritis | |
| Positive cryoglobulin test | Cryoglobulinemia | |
| Positive hepatitis B serology | Membranous nephropathy, membranoproliferative nephritis | |
| Positive hepatitis C serology | Mixed cryoglobulinemia, membranoproliferative glomerulonephritis, membranous nephropathy | |
| Positive HIV serology | Focal and segmental glomerulosclerosis | |
| Ultrasonography | Doppler ultrasonography | May be useful in investigation of venous thrombosis, less so in arterial stenosis |
| General findings | May show nephrocalcinosis, discrete stones, hydronephrosis, cysts,* or masses | |
| Increased echogenicity | May indicate cystic disease or medical renal disease | |
| Large kidneys | Generally indicate tumors, infiltrating diseases, or diseases causing nephrotic syndrome | |
| Size disparities and scarring | Suggest vascular, urologic, or tubulointerstitial diseases due to stones or infection | |
| Small hyperechoic kidneys | Generally indicate CKD |
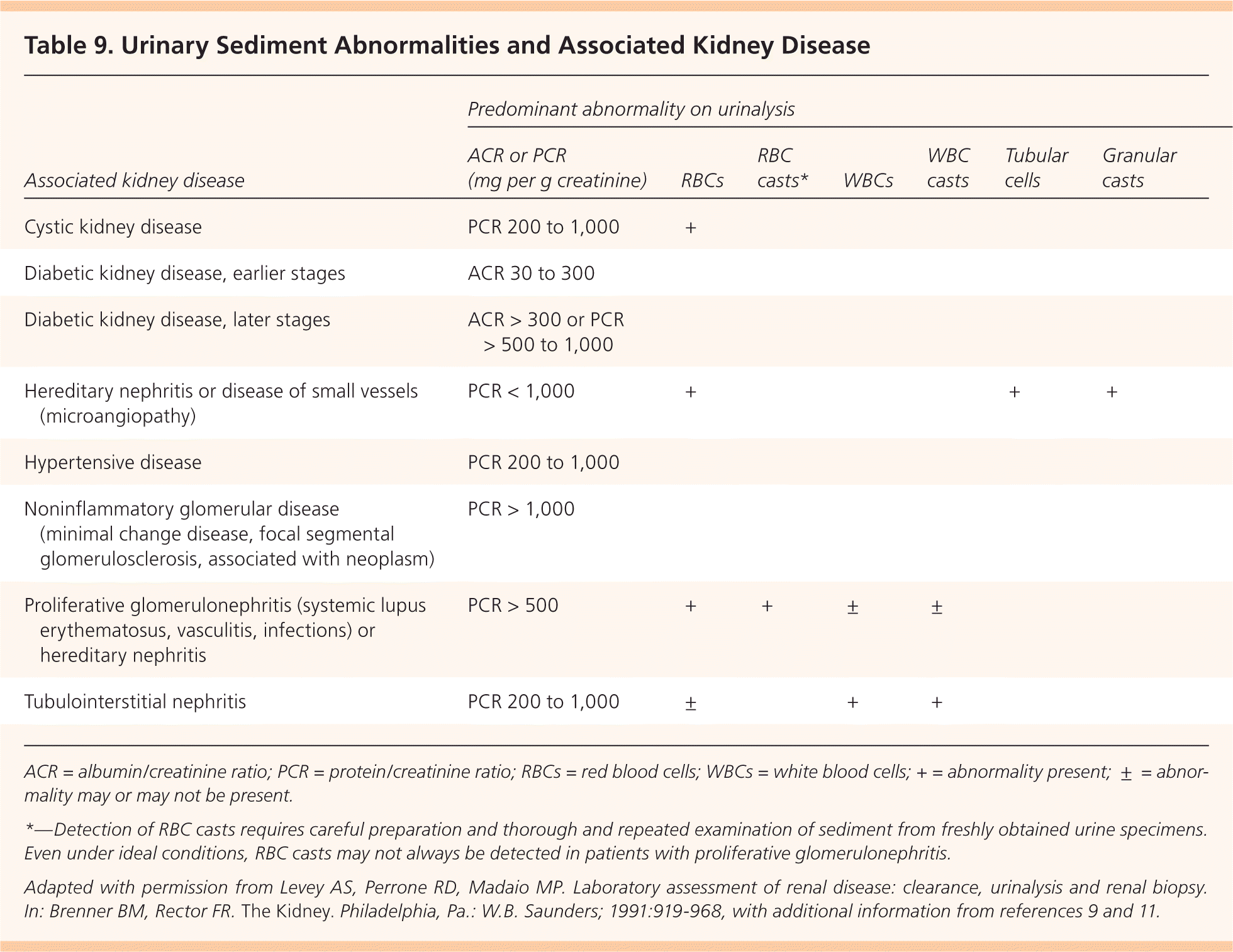
| Associated kidney disease | Predominant abnormality on urinalysis | ||||||
|---|---|---|---|---|---|---|---|
| ACR or PCR (mg per g creatinine) | RBCs | RBC casts* | WBCs | WBC casts | Tubular cells | Granular casts | |
| Cystic kidney disease | PCR 200 to 1,000 | + | |||||
| Diabetic kidney disease, earlier stages | ACR 30 to 300 | ||||||
| Diabetic kidney disease, later stages | ACR > 300 or PCR > 500 to 1,000 | ||||||
| Hereditary nephritis or disease of small vessels (microangiopathy) | PCR < 1,000 | + | + | + | |||
| Hypertensive disease | PCR 200 to 1,000 | ||||||
| Noninflammatory glomerular disease (minimal change disease, focal segmental glomerulosclerosis, associated with neoplasm) | PCR > 1,000 | ||||||
| Proliferative glomerulonephritis (systemic lupus erythematosus, vasculitis, infections) or hereditary nephritis | PCR > 500 | + | + | ± | ± | ||
| Tubulointerstitial nephritis | PCR 200 to 1,000 | ± | + | + | |||
Cardiovascular disease risk assessment—especially smoking status and lipid levels—is important because death is more likely than progression to dialysis in any stage of CKD.9 Electrocardiography is recommended to identify left ventricular hypertrophy.
Patients with an estimated GFR of less than 60 mL per minute per 1.73 m2 require further evaluation to assess for complications. Evaluation for anemia is recommended in women with hemoglobin levels less than 12 g per dL (120 g per L) and in men with levels less than 13.5 g per dL (135 g per L), in addition to nutritional assessment and evaluation for bone disease (Table 10).9,30,31
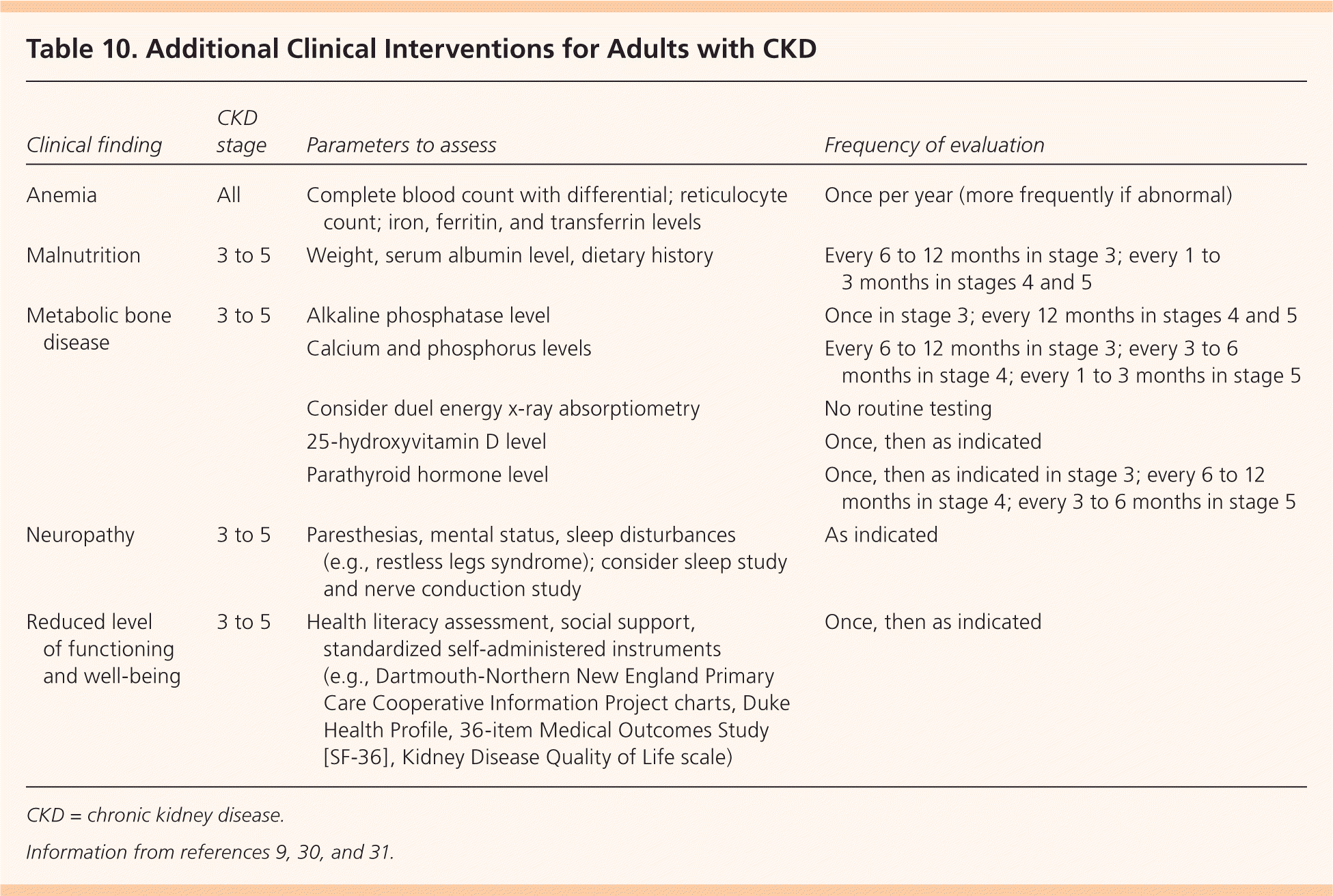
| Clinical finding | CKD stage | Parameters to assess | Frequency of evaluation |
|---|---|---|---|
| Anemia | All | Complete blood count with differential; reticulocyte count; iron, ferritin, and transferrin levels | Once per year (more frequently if abnormal) |
| Malnutrition | 3 to 5 | Weight, serum albumin level, dietary history | Every 6 to 12 months in stage 3; every 1 to 3 months in stages 4 and 5 |
| Metabolic bone disease | 3 to 5 | Alkaline phosphatase level | Once in stage 3; every 12 months in stages 4 and 5 |
| Calcium and phosphorus levels | Every 6 to 12 months in stage 3; every 3 to 6 months in stage 4; every 1 to 3 months in stage 5 | ||
| Consider duel energy x-ray absorptiometry | No routine testing | ||
| 25-hydroxyvitamin D level | Once, then as indicated | ||
| Parathyroid hormone level | Once, then as indicated in stage 3; every 6 to 12 months in stage 4; every 3 to 6 months in stage 5 | ||
| Neuropathy | 3 to 5 | Paresthesias, mental status, sleep disturbances (e.g., restless legs syndrome); consider sleep study and nerve conduction study | As indicated |
| Reduced level of functioning and well-being | 3 to 5 | Health literacy assessment, social support, standardized self-administered instruments (e.g., Dartmouth-Northern New England Primary Care Cooperative Information Project charts, Duke Health Profile, 36-item Medical Outcomes Study [SF-36], Kidney Disease Quality of Life scale) | Once, then as indicated |
Short-term risks of GFR reduction (e.g., volume depletion, urinary tract obstruction, use of nephrotoxic agents) require immediate recognition to prevent irreversible deterioration of renal function. High cumulative exposure to nonsteroidal anti-inflammatory drugs is associated with rapid progression of CKD.32 Acetaminophen is the analgesic of choice for short-term treatment of mild to moderate pain in patients with stage 3 to 5 CKD.33 Nonsteroidal anti-inflammatory drugs may be used in patients with stage 3 CKD for short-term therapy with regular renal function monitoring.33
Indications for Nephrology Referral
Nephrology consultation is indicated when the estimated GFR is less than 30 mL per minute per 1.73 m2, or earlier if necessary (Table 11).9,15 Partnership between primary care physicians and nephrologists is key to successful CKD management. The National Kidney Foundation's suggested multidisciplinary clinical action plan for CKD is available at http://www.kidney.org/professionals/KDOQI/cap.cfm.
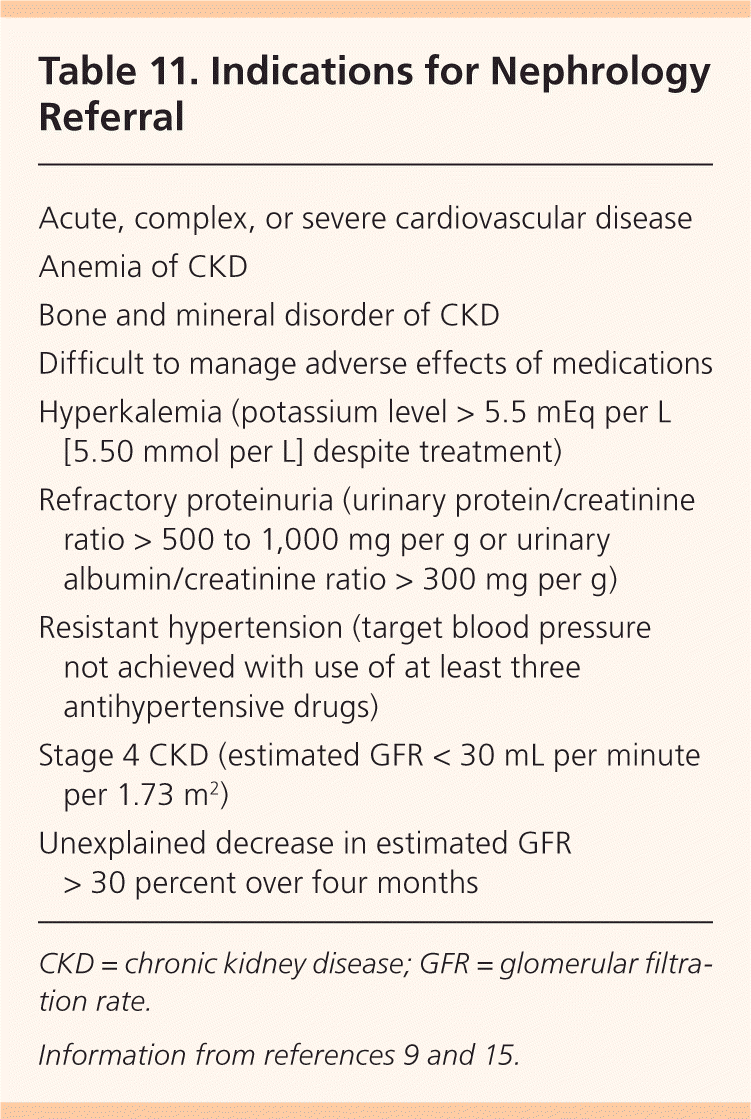
| Acute, complex, or severe cardiovascular disease |
| Anemia of CKD |
| Bone and mineral disorder of CKD |
| Difficult to manage adverse effects of medications |
| Hyperkalemia (potassium level > 5.5 mEq per L [5.50 mmol per L] despite treatment) |
| Refractory proteinuria (urinary protein/creatinine ratio > 500 to 1,000 mg per g or urinary albumin/creatinine ratio > 300 mg per g) |
| Resistant hypertension (target blood pressure not achieved with use of at least three antihypertensive drugs) |
| Stage 4 CKD (estimated GFR < 30 mL per minute per 1.73 m2) |
| Unexplained decrease in estimated GFR > 30 percent over four months |
