This is a corrected version of the article that appeared in print.
Am Fam Physician. 2012;85(1):25-32
Patient information: See related handout on caring for minor burns, written by the authors of this article.
Author disclosure: No relevant financial affiliations to disclose.
Most burn injuries can be managed on an outpatient basis by primary care physicians. Prevention efforts can significantly lower the incidence of burns, especially in children. Burns should be managed in the same manner as any other trauma, including a primary and secondary survey. Superficial burns can be treated with topical application of lotions, honey, aloe vera, or antibiotic ointment. Partial-thickness burns should be treated with a topical antimicrobial agent or an absorptive occlusive dressing to help reduce pain, promote healing, and prevent wound desiccation. Topical silver sulfadiazine is the standard treatment; however, newer occlusive dressings can provide faster healing and are often more cost-effective. Physicians must reevaluate patients frequently after a burn injury and be aware of the indications for referral to a burn specialist.
Each year more than 500,000 persons present to U.S. emergency departments with burns, and 40,000 are hospitalized.1,2 Consequently, most patients with burn wounds are treated in the outpatient setting, making primary care physicians the main treatment source for thousands of burn patients each year. This article discusses aspects of burn prevention to help minimize morbidity, current evaluation and management of minor burns in the outpatient setting, and indications for referral to specialty care or for transfer to a burn unit.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Burn prevention counseling should be included in well-child visits. | C | 3, 7–9 |
| If necessary, a tetanus shot should be given to patients with partial-thickness or full-thickness burns. | C | 17 |
| Minor thermal burns should be treated immediately with cool running water (not ice water). | C | 16, 19–21 |
| Superficial burns can be treated with topical application of lotion, honey, aloe vera, or antibiotic ointment. | B | 37–39 |
| Patients should be transferred to a burn center if they meet any of the American Burn Association referral criteria. | C | 52 |
Burn Prevention
Burn injuries are most common in children. Scalding accounts for 80 percent of burns in young children, often resulting from contact with hot objects or liquids after a child pulls a hot object off of the stove or countertop.2,3 Flame-related injuries are more common in patients older than six years.4,5 Children six to 16 years of age may experiment with lighters, lighter fluid, firecrackers, and gasoline. Older adults are another group at high risk of burn injury.4,6
Although burn prevention programs have not been evaluated in regard to their effect on mortality rates, the high prevalence of burn accidents makes prevention a worthwhile topic to include during well-child visits.7 Most burns are preventable, so it is crucial to educate families about potential household hazards (Table 1).3,8,9

| Always test bathwater3 |
| Check household smoke alarms regularly8 |
| Cook on the back burners of the stove when children are present3 |
| Do not leave a child unattended in the bathtub or near water faucets3 |
| Do not leave a child unattended near a fireplace9 |
| Keep matches, firecrackers, gasoline, and other explosives out of reach of children3 |
| Never hold a child when working with or around hot objects3 |
| Set household water heaters to less than 120°F (48.9°C)3 |
| Supervise children carefully while an exercise treadmill is in use3 |
Anatomy and Skin Function
The skin’s three anatomic layers (i.e., epidermis, dermis, and subcutaneous tissue) have functions that are lost after burn injuries. The epidermis is a barrier to bacteria and moisture loss. After a burn injury, local wound care and fluid management are required. The dermis provides elasticity and protection from mechanical trauma, and it contains blood vessels that supply all skin layers. When the skin is damaged, epidermal cells regenerate from cells deep within the dermal appendages, which is why deep dermal injury causes significant scarring and permanent skin damage.10,11
Classification of Burns
Burn depth and size are important factors in determining whether a burn can be classified as minor, and are crucial in dictating the initial steps of burn assessment and management2 (Table 21,2,6,12 ). Superficial burns can often be managed on an outpatient basis, whereas full-thickness burns must be evaluated by a specialist for possible excision and grafting. Determination of burn depth can be complicated by the conversion of burns to a higher burn category within the first several days. Conversion occurs when the damaged skin continues to spread and burn depth increases because of thermal injury that did not fully present on initial assessment; therefore, frequent evaluation and reassessment are necessary for all categories of burns.13
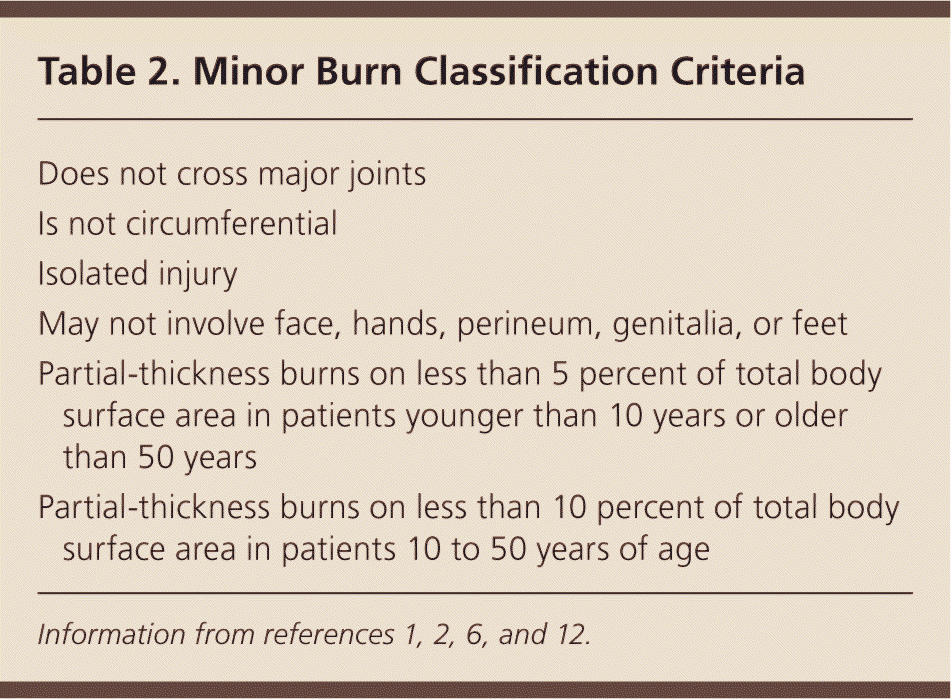
| Does not cross major joints |
| Is not circumferential |
| Isolated injury |
| May not involve face, hands, perineum, genitalia, or feet |
| Partial-thickness burns on less than 5 percent of total body surface area in patients younger than 10 years or older than 50 years |
| Partial-thickness burns on less than 10 percent of total body surface area in patients 10 to 50 years of age |
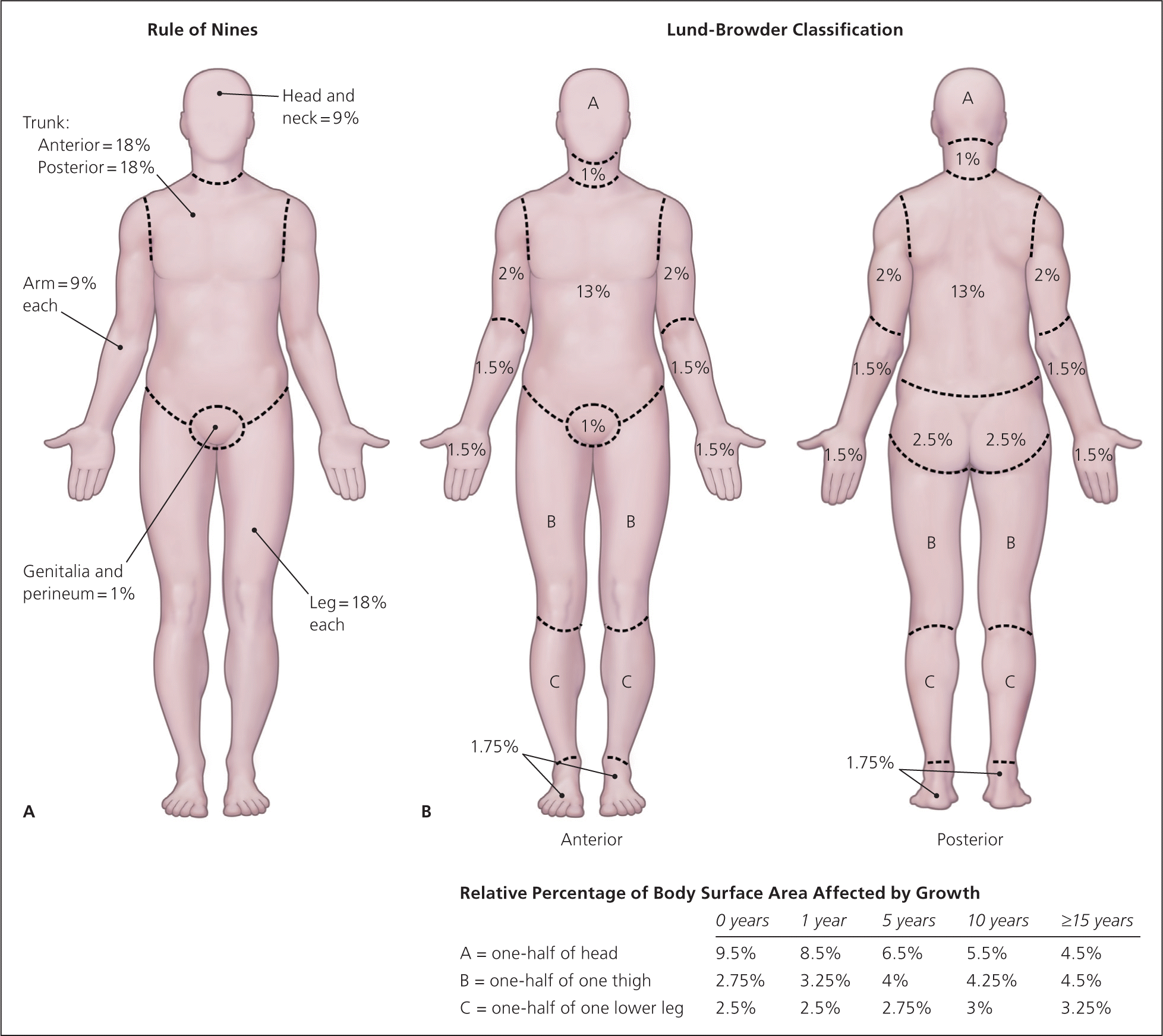
Burn size is determined by estimating the percentage of the patient’s body surface area that is covered by partial-thickness and full-thickness burns (Figure 1).14 First-degree burns are not incorporated into formal estimations of burn size. The Lund-Browder classification can be used for initial assessment of burn size in adults or children. The “rule of nines” diagram is helpful for rapid assessment of burn size, but this method is less accurate than the Lund-Browder classification, especially in children. The hand is often used to measure small burn areas; it correlates to 1 percent of total body surface area. Studies have shown that the adult hand is closer to 0.8 percent of total body surface area, and that a child’s hand is about 1 percent.10
SUPERFICIAL (FIRST-DEGREE) BURNS
SUPERFICIAL PARTIAL-THICKNESS (SUPERFICIAL SECOND-DEGREE) BURNS
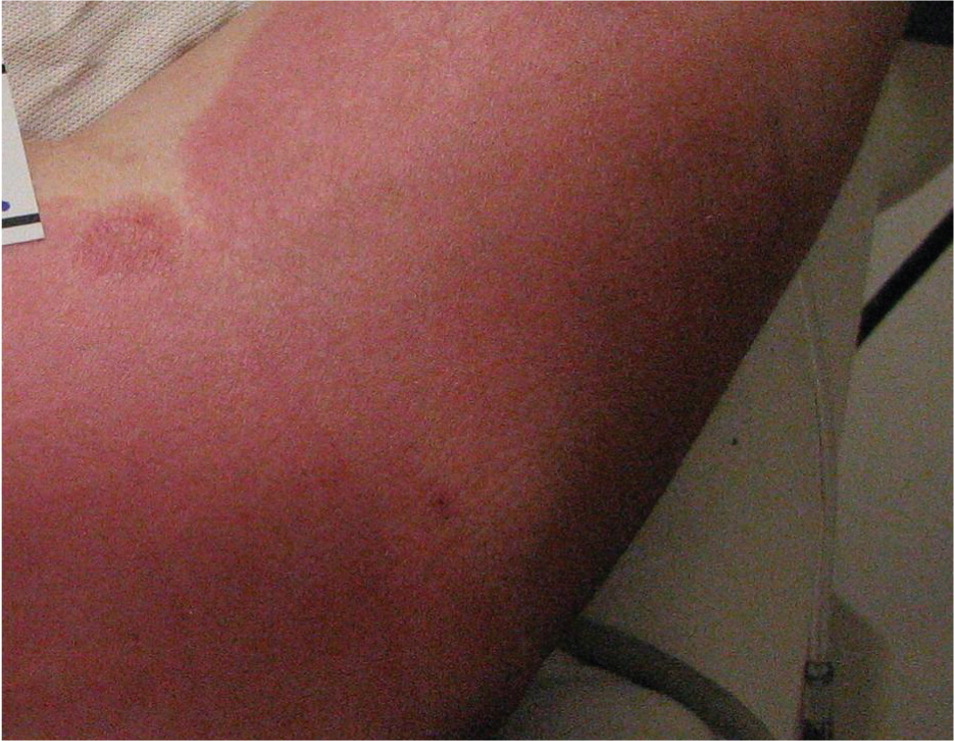
Second-degree burns involve all of the epidermis and part of the underlying dermis. Superficial partial-thickness burns damage the upper layers of the papillary dermis; they are identified by clear blisters and weeping, wet, erythematous skin, and they blanch painfully when touched (Figures 3 and 4). These burns heal within two weeks and generally do not cause scarring; however, scarring and pigment changes are possible.2,10
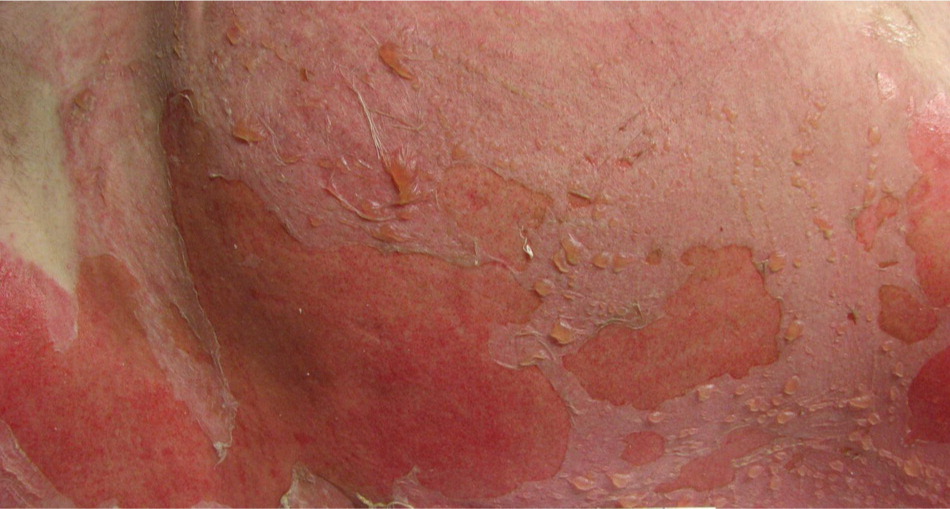
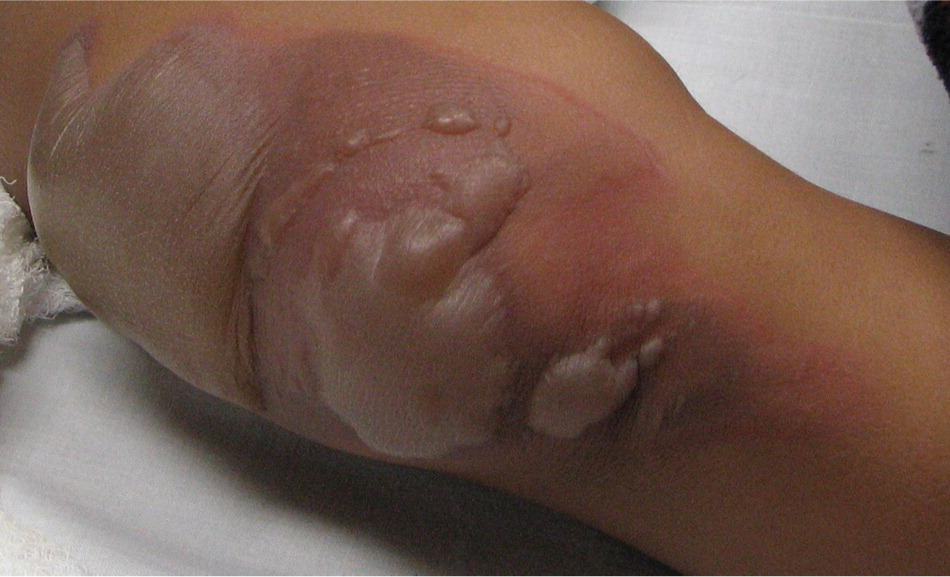
DEEP PARTIAL-THICKNESS (DEEP SECOND-DEGREE) BURNS
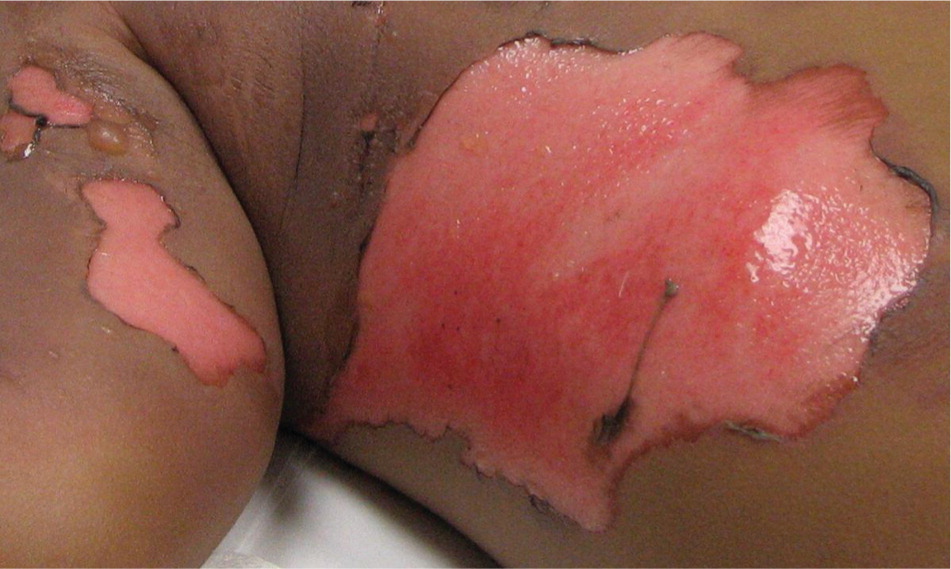
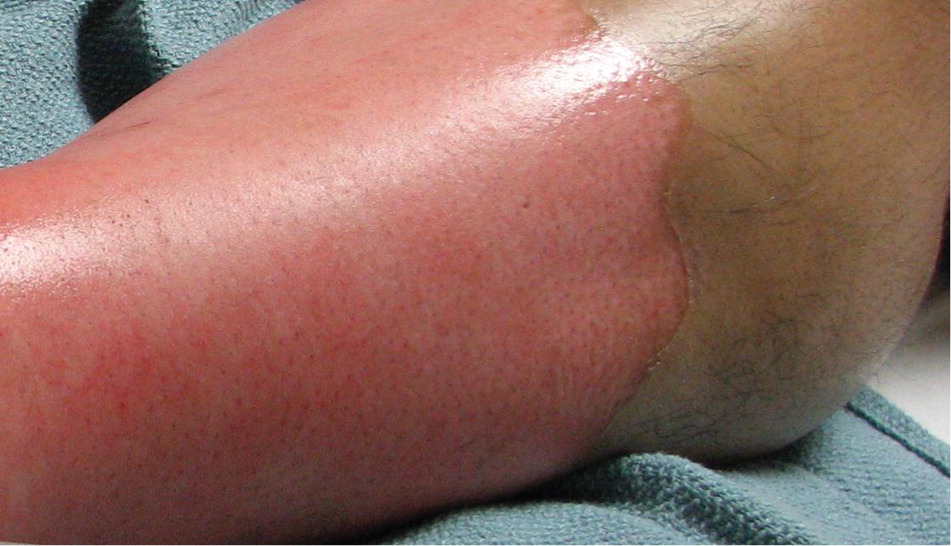
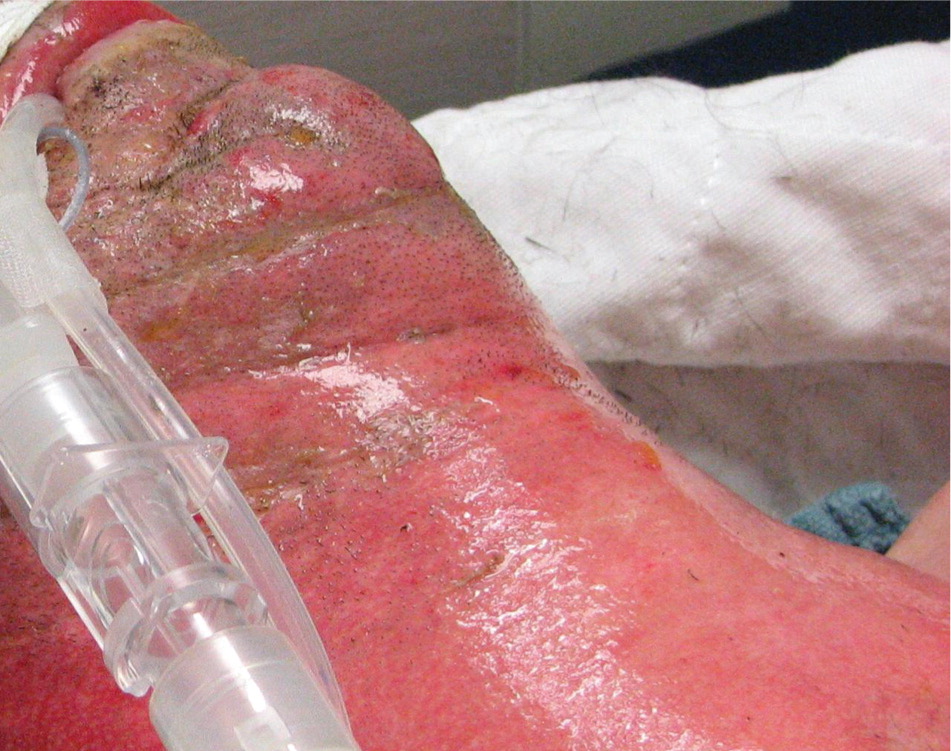
FULL-THICKNESS (THIRD-DEGREE) BURNS
FOURTH-DEGREE BURNS
Fourth-degree burns destroy all skin layers and extend into muscle, tendon, or bone.15
Initial Management of Burns
More than 95 percent of burn wounds can be successfully managed in the outpatient setting.16 Excellent results can be achieved by primary care physicians with knowledge of basic concepts of burn care. Close monitoring and follow-up are important aspects of outpatient management because of the dynamic and fragile progression of burn injuries.16 Goals of burn management include rapid healing, pain control, return of full function to the injured area, and good aesthetic results.1
All burns are considered trauma; therefore, the initial evaluation should include a primary survey, ensuring that body surface areas are covered after inspection because damage to the epidermis can result in temperature regulation problems. Because of the risk of airway edema and possible inhalation injury, burns to the face or neck should always prompt evaluation of the patient’s airway, regardless of the burn size. The secondary survey should include a careful evaluation of the burned area and consideration of abuse. The size, depth, and circumference of the burn should be evaluated. These initial evaluations will be used in decisions about inpatient versus outpatient management. A tetanus shot should be given to all patients with more than a first-degree burn.12,17,18
Immediate treatment of minor thermal burns with cool running water is controversial but often recommended. Animal studies have shown that exposing the burned area to cool running water for 20 minutes reduces the depth of injury, increases reepithelialization, and improves cosmetic outcomes; however, human studies are limited and show that the benefits last for only one hour.19–24 Although cool water is an acceptable home treatment for minor burns, ice water immersion is not because it can lead to further injury and hypothermia.16,22 Any materials that could cause further injury should be removed. Immediate attention should be given to pain control. Because burns can take weeks to heal, judicious use of narcotic analgesics is recommended. Adequate analgesia should be obtained before cleaning the wound or applying dressings.16
After evaluation and pain control, the wound must be cleaned. Scrubbing the wound with povidone/iodine solution (Betadine), chlorhexidine (Peridex), or other cleaning agents is not recommended.16 Cleaning the wound with sterile water is generally adequate to remove debris. Management of blisters in patients with partial-thickness burns is controversial, but overwhelming evidence has shown that small blisters (less than 6 mm) should be left intact.18 Large blisters with thin walls should be debrided; they will likely rupture on their own, and it is beneficial from a pressure and infection standpoint to apply dressings directly to the wound bed. Blisters that prevent proper movement of a joint or that are likely to rupture should be debrided.18
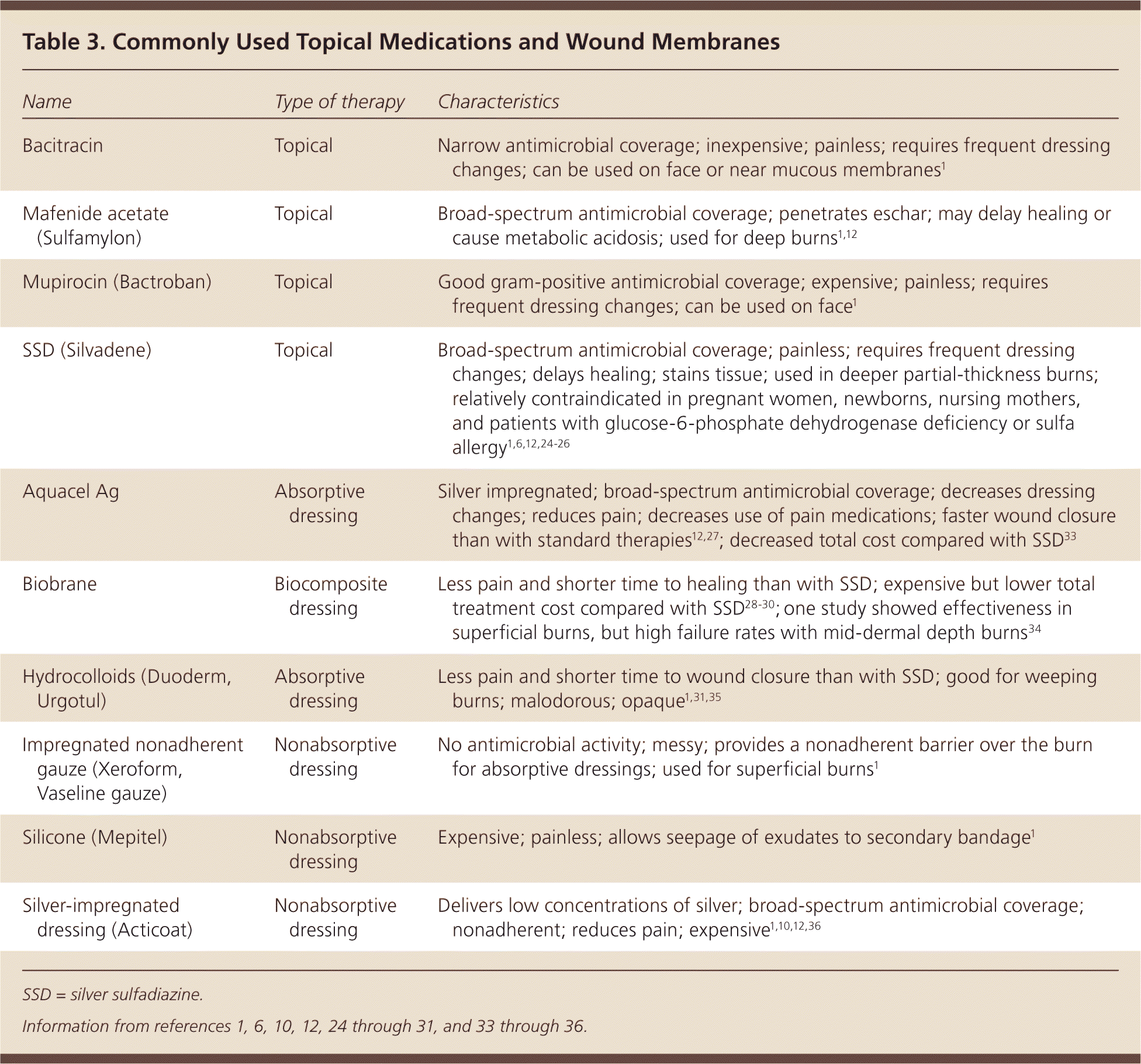
Topical burn care is the topic of many studies and discussions. Burn wounds heal best in moist—not wet—environments that promote reepithelialization and prevent cellular dehydration. This environment is best created by applying a topical agent or occlusive dressing to reduce fluid loss1 (Table 31,6,10,12,24–31, 33–36 ). [ corrected] Topical agents provide pain control, promote healing, and prevent wound infection and desiccation.12
| Name | Type of therapy | Characteristics |
|---|---|---|
| Bacitracin | Topical | Narrow antimicrobial coverage; inexpensive; painless; requires frequent dressing changes; can be used on face or near mucous membranes1 |
| Mafenide acetate (Sulfamylon) | Topical | Broad-spectrum antimicrobial coverage; penetrates eschar; may delay healing or cause metabolic acidosis; used for deep burns1,12 |
| Mupirocin (Bactroban) | Topical | Good gram-positive antimicrobial coverage; expensive; painless; requires frequent dressing changes; can be used on face1 |
| SSD (Silvadene) | Topical | Broad-spectrum antimicrobial coverage; painless; requires frequent dressing changes; delays healing; stains tissue; used in deeper partial-thickness burns; relatively contraindicated in pregnant women, newborns, nursing mothers, and patients with glucose-6-phosphate dehydrogenase deficiency or sulfa allergy1,6,12,24–26 |
| Aquacel Ag | Absorptive dressing | Silver impregnated; broad-spectrum antimicrobial coverage; decreases dressing changes; reduces pain; decreases use of pain medications; faster wound closure than with standard therapies12,27; decreased total cost compared with SSD33 |
| Biobrane | Biocomposite dressing | Less pain and shorter time to healing than with SSD; expensive but lower total treatment cost compared with SSD28–30; one study showed effectiveness in superficial burns, but high failure rates with mid-dermal depth burns34 |
| Hydrocolloids (Duoderm, Urgotul) | Absorptive dressing | Less pain and shorter time to wound closure than with SSD; good for weeping burns; malodorous; opaque1,31,35 |
| Impregnated nonadherent gauze (Xeroform, Vaseline gauze) | Nonabsorptive dressing | No antimicrobial activity; messy; provides a nonadherent barrier over the burn for absorptive dressings; used for superficial burns1 |
| Silicone (Mepitel) | Nonabsorptive dressing | Expensive; painless; allows seepage of exudates to secondary bandage1 |
| Silver-impregnated dressing (Acticoat) | Nonabsorptive dressing | Delivers low concentrations of silver; broad-spectrum antimicrobial coverage; nonadherent; reduces pain; expensive1,10,12,36 |
Superficial burns can be treated successfully with topical application of lotion, honey, aloe vera, or antibiotic ointment.37 The lipid component of these treatments accelerates the repair of damaged skin and reduces drying.38,39 Although there are no medication requirements for patients with superficial burns, evidence has shown that topical nonsteroidal anti-inflammatory drugs and aloe vera reduce pain.1,39 Topical corticosteroids have not been shown to reduce the inflammatory reaction; therefore, they should not be used to treat superficial thermal burns or sunburns.40 Partial-thickness burns should be treated with a topical antimicrobial agent or an absorptive occlusive dressing to reduce pain, promote healing, and prevent wound desiccation. Topical silver sulfadiazine (Silvadene) is the standard antimicrobial treatment for partial-thickness burns; however, it is relatively contraindicated in patients with sulfa allergy, pregnant and lactating women, and newborns.20,25,26
Numerous small studies have compared newer occlusive dressings with silver sulfadiazine.28–30,32,41,42 However, a 2008 Cochrane review found only minimal evidence to guide physicians because the included studies were flawed.43 The authors concluded that the use of newer occlusive dressings should be considered instead of silver sulfadiazine because they resulted in faster healing, decreased pain, fewer dressing changes, and improved patient satisfaction. Some newer occlusive dressings are more cost-effective than silver sulfadiazine.41 Physicians must educate patients on the proper method for changing dressings at home.
A systematic review showed that prophylactic systemic antibiotics administered in the hospital setting did not improve mortality 44; therefore, they generally are not recommended for burns.
Long-term Management and Referral
Although cellulitis is not common in burns, it can cause the skin to become severely erythematous, exudative, painful, and swollen. This is a difficult process to assess because wounds generally are erythematous, painful, and swollen as they heal. Infections can progress rapidly; some of the most common pathogens found in burn wounds include Staphylococcus aureus, Streptococcus pyogenes, Pseudomonas aeruginosa, and Acinetobacter and Klebsiella species.12,45 Antibiotic treatment depends on local drug resistance and should be tailored toward broad coverage of gram-negative and gram-positive bacteria.
Pruritus and neuropathic pain are common postburn complications. Histamine H1 receptor antagonists such as cetirizine (Zyrtec) are the safest pharmacologic treatment for postburn pruritus.46 Topical doxepin, a tricyclic antidepressant with potent antihistamine properties, reduces postburn pruritus and erythema.47,48 Small studies have shown that pulsed dye laser treatment is effective for postburn pruritus46,49; however, more research is necessary. A recent retrospective review found that pregabalin (Lyrica) reduced postburn neuropathic pain in 69 percent of patients.50
The American Burn Association has established criteria to help physicians determine when to refer patients to burn centers51,52 (Table 451 ). One-half of patients treated at non-burn centers meet these criteria for transfer, and a higher percentage of patients treated at non-burn centers are discharged to a nursing home, resulting in a higher burden on the health care system when these criteria are not followed.

| Any patient with burns and concomitant trauma (e.g., fractures) in whom the burn injury poses the greatest risk of morbidity or death |
| Burns in children at hospitals without qualified personnel or equipment for the care of children |
| Burns in patients who will require special social, emotional, or rehabilitative intervention |
| Burns in patients with preexisting medical disorders that could complicate management, prolong recovery, or affect mortality |
| Burns that involve the face, hands, feet, genitalia, perineum, or major joints |
| Chemical burns |
| Electrical burns, including lightning injury |
| Inhalation injury |
| Partial-thickness burns on more than 10 percent of the total body surface area |
| Third-degree (full-thickness) burns in any age group |
Patients with burns that extend over a joint should be referred for occupational and physical therapy while the wound is healing if loss of function or range of motion is anticipated. Because of the pain associated with burns, patients often restrict their activity, which results in stiffness and weakness of the surrounding joints.3 Referral to a burn specialist is indicated in patients with full-thickness burns; burns to the hands, feet, perineum, or genital areas (because of the anatomy and function of these areas)10,16; and circumferential burns (because of the risk of compartment syndrome). Patients with burns to the face also should be referred, because these burns can result in significant psychological trauma and identity issues.
Referral to a surgeon or burn specialist should be considered for patients with wounds that worsen over the first 72 hours or that begin to cause significant scarring or any degree of contracture.10 If there is uncertainty about burn management at any time during outpatient treatment, a consultation should be obtained.
Data Sources: PubMed, Ovid, the Cochrane database, the Centers for Disease Control and Prevention Web site, and Essential Evidence Plus were searched using the key words outpatient burns, partial thickness treatments, burn management, burn prevention, topical burn treatments, and sunburn management. Search dates: April and June 2010, and October 2011.