
Am Fam Physician. 2012;85(9):883-889
A more recent article on diabetic kidney disease is available.
Patient information: A handout on this topic is available at https://familydoctor.org/familydoctor/en/diseases-conditions/diabetic-nephropathy.html.
Author disclosure: No relevant financial affiliations to disclose.
Nearly one-half of persons with chronic kidney disease have diabetes mellitus. Diabetes accounted for 44 percent of new cases of kidney failure in 2008. Diabetic nephropathy, also called diabetic kidney disease, is associated with significant macrovascular risk, and is the leading cause of kidney failure in the United States. Diabetic nephropathy usually manifests after 10 years' duration of type 1 diabetes, but may be present at diagnosis of type 2 diabetes. Screening for microalbuminuria should be initiated five years after diagnosis of type 1 diabetes and at diagnosis of type 2 diabetes. Screening for microalbuminuria with a spot urine albumin/creatinine ratio identifies the early stages of nephropathy. Positive results on two of three tests (30 to 300 mg of albumin per g of creatinine) in a six-month period meet the diagnostic criteria for diabetic nephropathy. Because diabetic nephropathy may also manifest as a decreased glomerular filtration rate or an increased serum creatinine level, these tests should be included in annual monitoring. Preventive measures include using an angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker in normotensive persons. Optimizing glycemic control and using an angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker to control blood pressure slow the progression of diabetic nephropathy, but implementing intensive glycemic and blood pressure control is associated with more adverse outcomes. Low-protein diets may also decrease adverse renal outcomes and mortality in persons with diabetic nephropathy.
Diabetic nephropathy (also called diabetic kidney disease) is the leading cause of kidney failure in the United States. According to the Centers for Disease Control and Prevention, in 2008, approximately 44 percent of all new cases of kidney failure were caused by diabetes mellitus; 48,374 persons with diabetes began treatment for end-stage renal disease (ESRD); and 202,290 persons with ESRD caused by diabetes were on long-term dialysis or had a renal transplant.1 In a 2010 U.S. Renal Data System report, 29.1 percent of persons with self-reported diabetes had stage 2 or 3 chronic kidney disease.2
Risk factors associated with development of microalbuminuria include higher blood pressure, higher blood glucose level, dyslipidemia, and smoking.3 Micro- and macroalbuminuria are associated with increased risk of major cardiovascular events and all-cause mortality.4 The overall prevalence of micro- and macroalbuminuria is up to 35 percent in both types of diabetes.1 However, persons with type 2 diabetes exhibit the highest prevalence, particularly Native American, Asian, Hispanic, and black persons, who are at higher risk of ESRD than non-Hispanic white persons.5 Diabetic nephropathy may progress from microalbuminuria to macroalbuminuria with progressive loss of glomerular filtration rate (GFR) until ESRD.6 After being diagnosed with diabetes, 2.0 percent of persons per year progress to microalbuminuria; 2.8 percent per year progress from microalbuminuria to macroalbuminuria; and 2.3 percent per year progress from macroalbuminuria to having an elevated plasma creatinine level or needing renal replacement therapy.7
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Persons with type 1 diabetes mellitus should be screened for microalbuminuria starting five years after diagnosis. | C | 6 |
| Persons with type 2 diabetes (without macroalbuminuria) should be screened for microalbuminuria at diagnosis and annually thereafter. | C | 6, 9 |
| Persons with type 1 or 2 diabetes and microalbuminuria should continue to be tested for albuminuria annually to monitor disease progression and response to therapy. | C | 6 |
| Normotensive persons with diabetes and microalbuminuria should be given an ACE inhibitor or angiotensin II receptor blocker to reduce progression to macroalbuminuria. | C | 10, 24, 25 |
| Combination therapy with ACE inhibitors and angiotensin II receptor blockers should be avoided in persons with diabetes, atherosclerosis, and evidence of end-organ damage. | C | 26 |
| ACE inhibitors should be discontinued if the patient's creatinine level increases more than 30 percent above baseline in the first two months of therapy (even in persons with an elevated baseline creatinine level of greater than 1.4 mg per dL [123.76 μmol per L]) or if hyperkalemia persists (serum potassium level of greater than 5.6 mEq per L [5.6 mmol per L]). | C | 29 |
| Adding hydrochlorothiazide to an ACE inhibitor in persons with diabetes, microalbuminuria, and hypertension is recommended to increase the likelihood of normalized albuminuria. | C | 30 |
| The American Diabetes Association recommends limiting protein intake in persons with diabetes to 0.8 to 1 g per kg per day in earlier stages of chronic kidney disease and to 0.8 g per kg per day in later stages to improve urine albumin excretion and estimated GFR. | C | 6 |
| Supplemental folic acid (2.5 mg), vitamin B6 (pyridoxine, 25 mg), and cyanocobalamin (1 mg) should be avoided in persons with type 1 or 2 diabetes and macroalbuminuria because of a higher risk of decline in GFR, and increased risk of myocardial infarction, stroke, and all-cause mortality (number needed to harm = 11). | B | 36 |
Screening
Several studies have analyzed the effectiveness and cost-effectiveness of diabetic nephropathy screening. Diabetic nephropathy typically manifests after 10 years' duration of type 1 diabetes, whereas approximately 3 percent of persons with newly diagnosed type 2 diabetes have overt nephropathy.8 Screening for microalbuminuria should begin five years after diagnosis of type 1 diabetes.6 Given the poor reliability of estimating length of disease at diagnosis of type 2 diabetes, consensus guidelines recommend that these persons be screened for microalbuminuria starting at diagnosis.6,9 However, patient-oriented evidence to support this recommendation (i.e., less ESRD in screened persons) is lacking.6,10 Persons with type 1 or 2 diabetes and microalbuminuria should continue to be tested for albuminuria annually to monitor disease progression and response to therapy.6
The diagnostic reference standard for defining microalbuminuria is detection of 30 to 300 mg of albumin in a 24-hour urine sample.10 A spot urine albumin/creatinine ratio, preferably in a first-morning void, correlates well with a 24-hour urine albumin excretion rate and accurately predicts renal events.11,12 Because this test is easily administered, it is the first-line annual screening test for most persons with diabetes. Microalbuminuria is defined as an albumin/creatinine ratio of 30 to 300 mg of albumin per g of creatinine.10
Macroalbuminuria, or overt nephropathy, is defined as an albumin/creatinine ratio of more than 300 mg of albumin per g of creatinine.10 At this level, protein is easily detected on routine urinalysis or urine dipstick. Several conditions, including marked hyperglycemia and hypertension, may cause transient elevations of urinary albumin (Table 1).9,11,13 Because up to 36 percent of persons with type 2 diabetes have renal insufficiency without micro- or macroalbuminuria, annual screening for diabetic nephropathy should also include measurement of serum creatinine and estimation of GFR.6,9,14
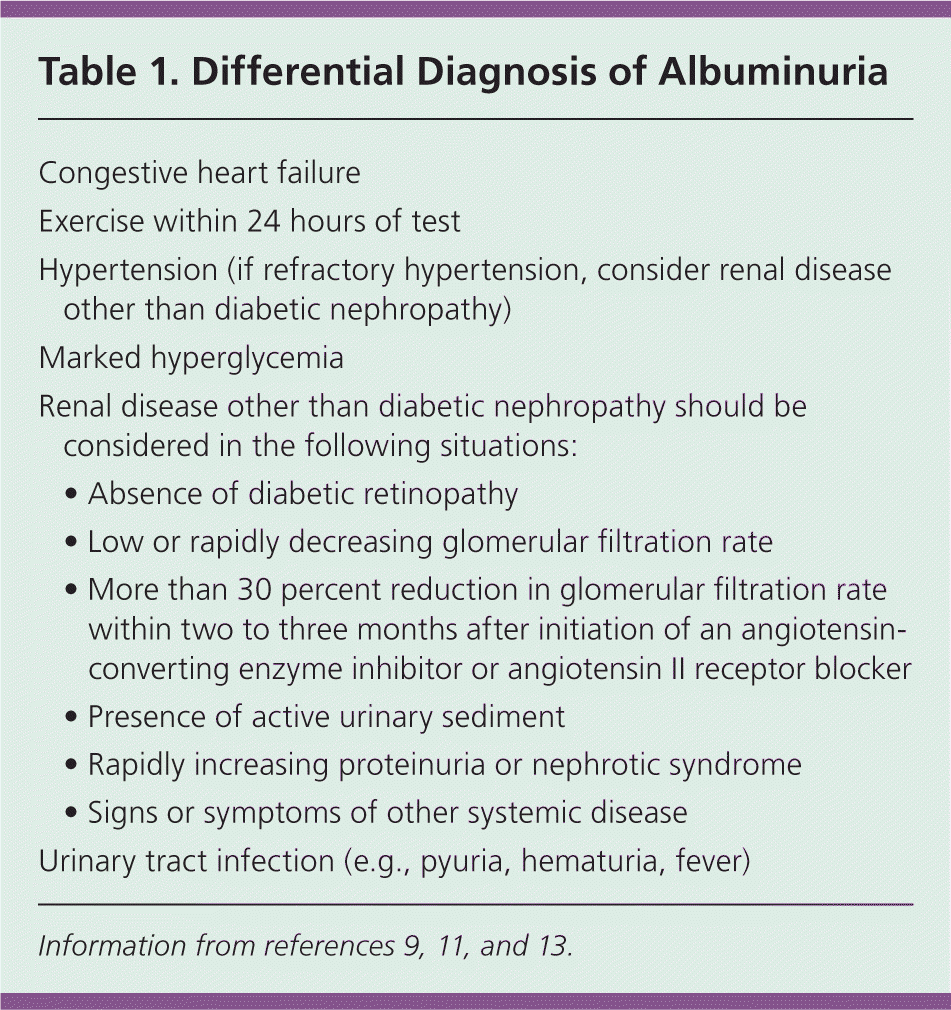
| Congestive heart failure | |
| Exercise within 24 hours of test | |
| Hypertension (if refractory hypertension, consider renal disease other than diabetic nephropathy) | |
| Marked hyperglycemia | |
| Renal disease other than diabetic nephropathy should be considered in the following situations: | |
| • Absence of diabetic retinopathy | |
| • Low or rapidly decreasing glomerular filtration rate | |
| • More than 30 percent reduction in glomerular filtration rate within two to three months after initiation of an angiotensinconverting enzyme inhibitor or angiotensin II receptor blocker | |
| • Presence of active urinary sediment | |
| • Rapidly increasing proteinuria or nephrotic syndrome | |
| • Signs or symptoms of other systemic disease | |
| Urinary tract infection (e.g., pyuria, hematuria, fever) | |
Diagnosis
Laboratory evaluation of diabetic nephropathy is summarized in Table 2.9,10 Persistent microalbuminuria is the earliest sign of diabetic nephropathy. Once a screening test detects microalbuminuria, it should be confirmed with additional spot urine tests over the next three to six months. Two out of three samples falling within the microalbuminuria (30 to 300 mg of albumin per g of creatinine) or macroalbuminuria (more than 300 mg of albumin per g of creatinine) range confirm the classification.10 The National Kidney Foundation recommends that all persons with chronic kidney disease undergo renal ultrasonography to distinguish potentially reversible causes of kidney disease.15 Several conditions, including albuminuria in the absence of retinopathy, should prompt consideration of renal biopsy to identify nondiabetic causes of renal disease10 (Table 19,11,13 ).
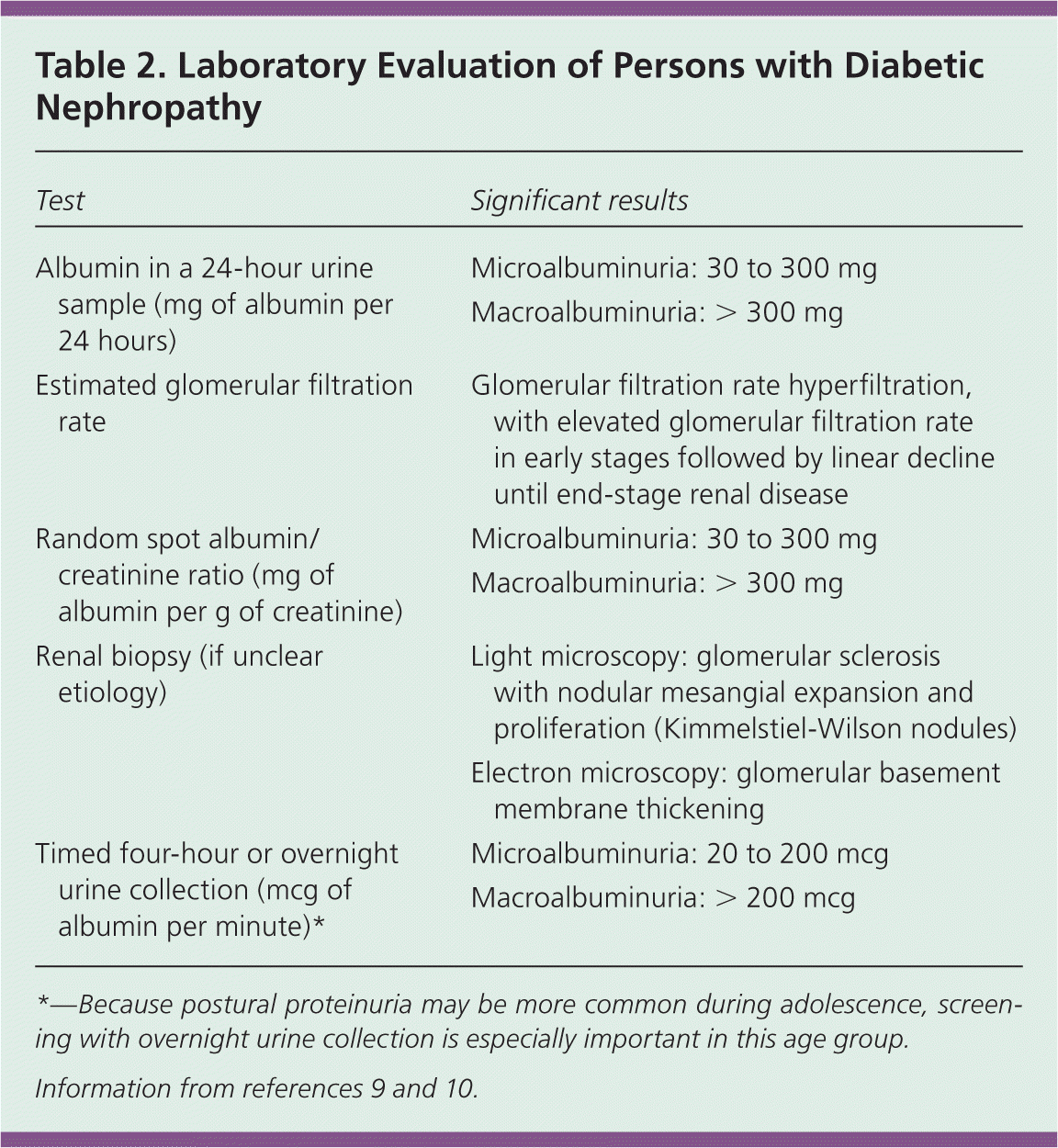
| Test | Significant results |
|---|---|
| Albumin in a 24-hour urine sample (mg of albumin per 24 hours) | Microalbuminuria: 30 to 300 mg |
| Macroalbuminuria: > 300 mg | |
| Estimated glomerular filtration rate | Glomerular filtration rate hyperfiltration, with elevated glomerular filtration rate in early stages followed by linear decline until end-stage renal disease |
| Random spot albumin/creatinine ratio (mg of albumin per g of creatinine) | Microalbuminuria: 30 to 300 mg |
| Macroalbuminuria: > 300 mg | |
| Renal biopsy (if unclear etiology) | Light microscopy: glomerular sclerosis with nodular mesangial expansion and proliferation (Kimmelstiel-Wilson nodules) |
| Electron microscopy: glomerular basement membrane thickening | |
| Timed four-hour or overnight urine collection (mcg of albumin per minute)* | Microalbuminuria: 20 to 200 mcg |
| Macroalbuminuria: > 200 mcg |
Preventing Progression
One in eight persons with type 1 diabetes and microalbuminuria reverts to normal urine albumin excretion without treatment.10 The mainstays of treatment to prevent progression of diabetic nephropathy have traditionally included achieving adequate glycemic control and lowering blood pressure with an angiotensin-converting enzyme (ACE) inhibitor or angiotensin II receptor blocker (Table 3).6,8,10,16–19 Weight loss, smoking cessation, and decreased dietary protein have also been recommended.20 The primary treatment goal is prevention of ESRD, because micro- and macroalbuminuria alone are asymptomatic conditions.
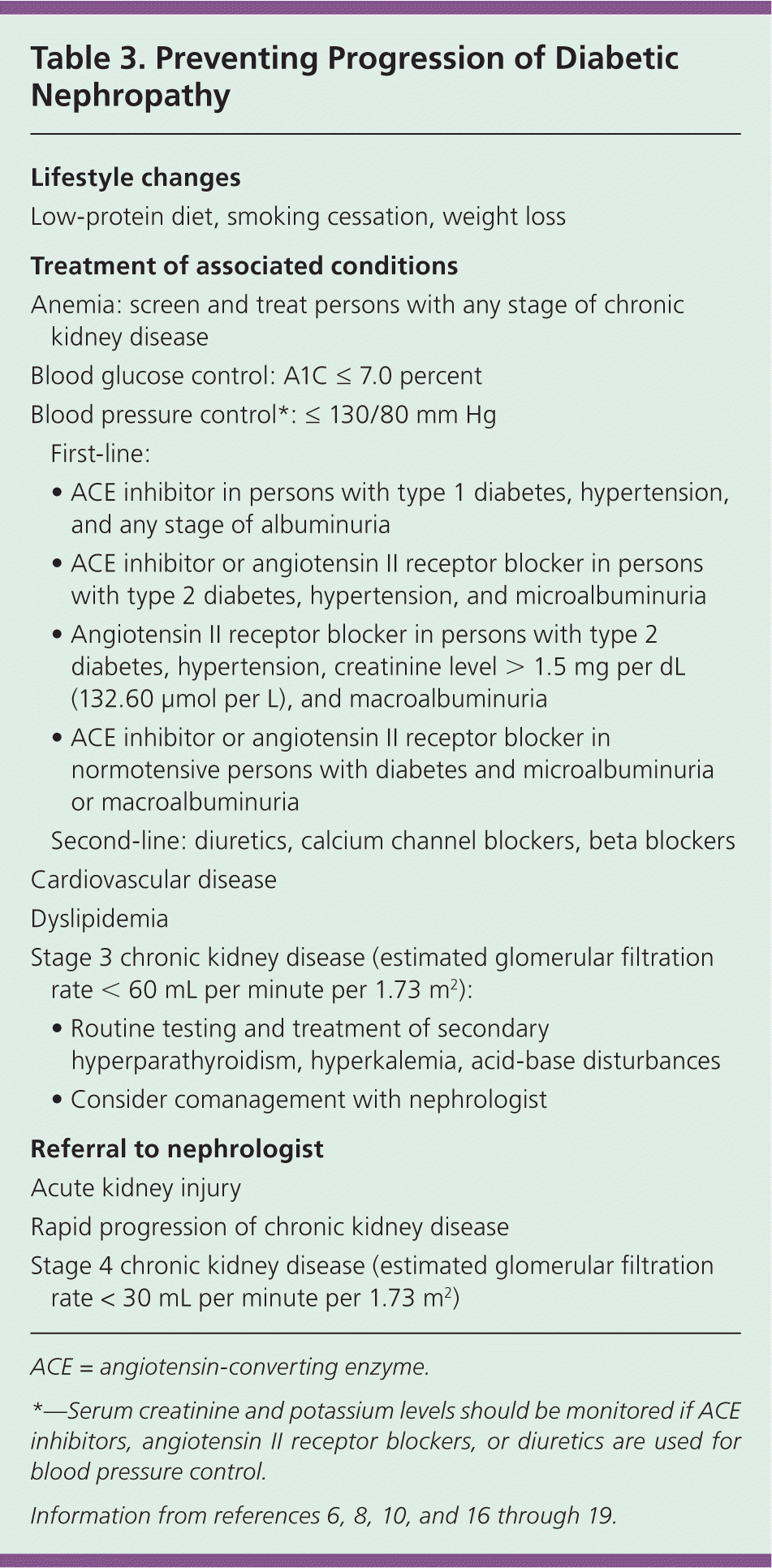
| Lifestyle changes | |
| Low-protein diet, smoking cessation, weight loss | |
| Treatment of associated conditions | |
| Anemia: screen and treat persons with any stage of chronic kidney disease | |
| Blood glucose control: A1C ≤ 7.0 percent | |
| Blood pressure control*: ≤ 130/80 mm Hg | |
| First-line: | |
| • ACE inhibitor in persons with type 1 diabetes, hypertension, and any stage of albuminuria | |
| • ACE inhibitor or angiotensin II receptor blocker in persons with type 2 diabetes, hypertension, and microalbuminuria | |
| • Angiotensin II receptor blocker in persons with type 2 diabetes, hypertension, creatinine level > 1.5 mg per dL (132.60 μmol per L), and macroalbuminuria | |
| • ACE inhibitor or angiotensin II receptor blocker in normotensive persons with diabetes and microalbuminuria or macroalbuminuria | |
| Second-line: diuretics, calcium channel blockers, beta blockers | |
| Cardiovascular disease | |
| Dyslipidemia | |
| Stage 3 chronic kidney disease (estimated glomerular filtration rate < 60 mL per minute per 1.73 m2): | |
| • Routine testing and treatment of secondary hyperparathyroidism, hyperkalemia, acid-base disturbances | |
| • Consider comanagement with nephrologist | |
| Referral to nephrologist | |
| Acute kidney injury | |
| Rapid progression of chronic kidney disease | |
| Stage 4 chronic kidney disease (estimated glomerular filtration rate < 30 mL per minute per 1.73 m2) | |
GLYCEMIC CONTROL
Because approximately one-half of persons with stage 3 or 4 chronic kidney disease have A1C levels higher than 7.0 percent,2 guidelines from the American Diabetes Association and others have recommended aggressive blood glucose control with the goal of preventing diabetic nephropathy.6 However, three large, well-designed, randomized controlled trials comparing intensive glycemic control with standard control found a reduction in progression to macroalbuminuria with intensive control, but increased hypoglycemic episodes, and no difference in doubling of creatinine or eventual need for dialysis (Table 4).13,21,22 The final A1C was 6.4 to 6.9 percent in the intensive-control groups, and 7.3 to 8.4 percent in the standard-control groups.13,21,22 The ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial had to be halted early because of increased all-cause mortality in the intensive-control group.21,23
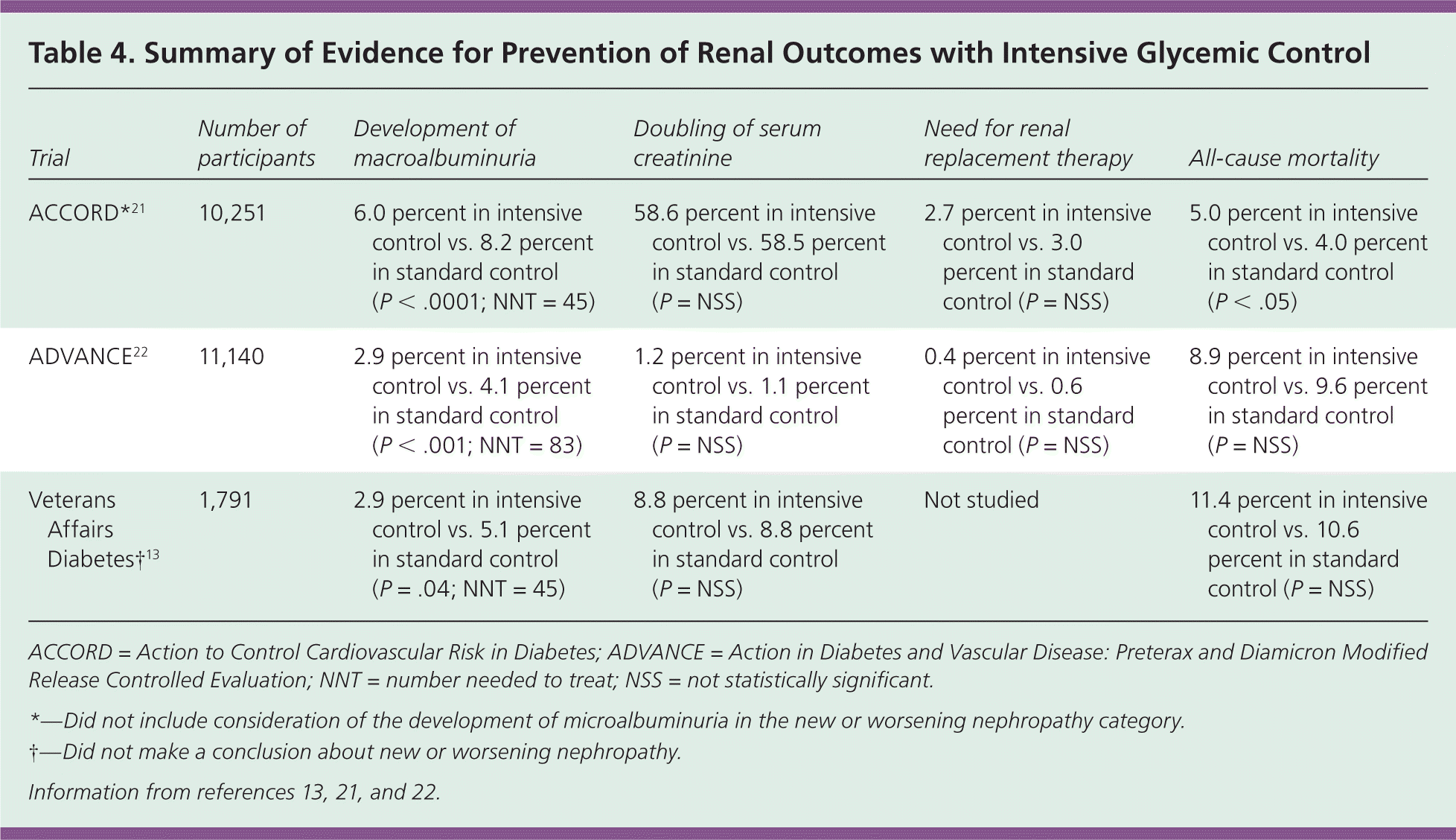
| Trial | Number of participants | Development of macroalbuminuria | Doubling of serum creatinine | Need for renal replacement therapy | All-cause mortality |
|---|---|---|---|---|---|
| ACCORD*21 | 10,251 | 6.0 percent in intensive control vs. 8.2 percent in standard control (P < .0001; NNT = 45) | 58.6 percent in intensive control vs. 58.5 percent in standard control (P = NSS) | 2.7 percent in intensive control vs. 3.0 percent in standard control (P = NSS) | 5.0 percent in intensive control vs. 4.0 percent in standard control (P < .05) |
| ADVANCE22 | 11,140 | 2.9 percent in intensive control vs. 4.1 percent in standard control (P < .001; NNT = 83) | 1.2 percent in intensive control vs. 1.1 percent in standard control (P = NSS) | 0.4 percent in intensive control vs. 0.6 percent in standard control (P = NSS) | 8.9 percent in intensive control vs. 9.6 percent in standard control (P = NSS) |
| Veterans Affairs Diabetes†13 | 1,791 | 2.9 percent in intensive control vs. 5.1 percent in standard control (P = .04; NNT = 45) | 8.8 percent in intensive control vs. 8.8 percent in standard control (P = NSS) | Not studied | 11.4 percent in intensive control vs. 10.6 percent in standard control (P = NSS) |
The American Diabetes Association continues to recommend an A1C goal of 7.0 percent, and suggests lower targets if they can be achieved without significant hypoglycemic or adverse events. Less stringent adherence to the 7.0 percent guideline is also acceptable for persons with extensive comorbidities, micro- or macrovascular complications, history of severe hypoglycemia, limited life expectancy, or long-standing diabetes refractory to appropriate management.6
BLOOD PRESSURE CONTROL
In addition to glycemic control with an A1C goal of 7.0 percent or less, lowering blood pressure to 130/80 mm Hg or less has been shown to slow the progression of nephropathy.24 More than two-thirds of adults with diabetes have a blood pressure of 140/90 mm Hg or greater, or use prescription medications for hypertension.1 Observational studies have shown that estimated GFR typically declines at rates of greater than 10 mL per minute per 1.73 m2 per year in persons with diabetes, poorly controlled hypertension, and macroalbuminuria, but much more slowly (1 to 4 mL per minute per 1.73 m2 per year) in those with effective blood pressure control.10
In a systematic review, ACE inhibitors reduced the risk of microalbuminuria in persons with diabetes and both with and without hypertension compared with placebo and calcium channel blockers.25 In a recent large randomized controlled trial, 4,733 participants were assigned to either intensive control (systolic blood pressure goal of less than 120 mm Hg) or standard control (systolic blood pressure goal of less than 140 mm Hg). The intensive-control group had a lower incidence of macroalbuminuria but significantly higher rates of serious adverse events such as hypotension, bradycardia, elevated serum creatinine level, and declining estimated GFR. There were no differences in the rates of nonfatal myocardial infarction, nonfatal stroke, death from cardiovascular causes, or ESRD, or in the need for dialysis. This study did not support a systolic blood pressure goal of less than 120 mm Hg for persons with diabetes.16
Treatment with ACE inhibitors or angiotensin II receptor blockers for hypertension is more effective in reducing the decline in kidney function than treatment with other blood pressure–lowering drugs. Persons with diabetes, microalbuminuria, and with or without hypertension should be given an ACE inhibitor or angiotensin II receptor blocker to reduce progression to macroalbuminuria.10,24,25
A large randomized trial showed that in persons with diabetes, atherosclerosis, and end-organ damage, ACE inhibitors and angiotensin II receptor blockers were equally effective in preventing progression of diabetic nephropathy. However, the combination of an ACE inhibitor and an angiotensin II receptor blocker is not recommended because it provided no additional benefit and actually led to higher serum creatinine levels and an increased rate of dialysis.26
In a meta-analysis of 4,008 persons with diabetic nephropathy from 36 trials comparing ACE inhibitors with placebo; 3,331 persons from four trials comparing angiotensin II receptor blockers with placebo; and 206 persons from three trials comparing ACE inhibitors with angiotensin II receptor blockers, both agents had similar effects on renal outcomes (i.e., ESRD, doubling of creatinine, progression of microalbuminuria to macroalbuminuria, and remission from microalbuminuria to normoalbuminuria). ACE inhibitors significantly reduced all-cause mortality at the maximal tolerated dose compared with lower doses, but angiotensin II receptor blockers did not.27 Similar results were noted in a Cochrane review of 50 trials involving 13,215 persons.28 ACE inhibitors should be discontinued if the creatinine level increases by 30 percent above baseline in the first two months of therapy (even in persons with an elevated baseline creatinine level of greater than 1.4 mg per dL [123.76 μmol per L]) or if hyperkalemia persists (serum potassium level of greater than 5.6 mEq per L [5.6 mmol per L]).29
In a randomized double-blind controlled trial, 332 persons who had hypertension, type 2 diabetes, and albuminuria were treated with benazepril (Lotensin) combined with either amlodipine (Norvasc) or hydrochlorothiazide. Both medication combinations significantly reduced the urine albumin/creatinine ratio and blood pressure, and resulted in similar delayed progression to overt proteinuria. In the same study, persons who had microalbuminuria and hypertension were more likely to revert to normoalbuminuria when taking the hydrochlorothiazide/ACE inhibitor combination, suggesting that initial treatment with this combination might be most beneficial in persons with microalbuminuria.30
Several other medication combinations also have been evaluated. Based on the ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation) trial, perindopril (Aceon) and indapamide reduce new-onset albuminuria, progression of nephropathy, and mortality, with no difference in rates of ESRD.22,31 Smaller studies show successful decreases in albumin/creatinine ratio by adding spironolactone (Aldactone) to lisinopril (Zestril) in persons with type 2 diabetes and microalbuminuria; adding avosentan (not available in the United States) to an ACE inhibitor or angiotensin II receptor blocker in persons with type 2 diabetes and macroalbuminuria; and adding aliskiren (Tekturna) to losartan (Cozaar) in persons with type 2 diabetes and macroalbuminuria. However, specific patient-oriented evidence is lacking.32–34 Table 3 summarizes recommendations for drug therapy in persons with diabetic nephropathy.6,8,10,16–19
DIET
Dietary restrictions, including low-protein and low-iron diets, also may have a beneficial effect in slowing the progression of diabetic nephropathy, but evidence is limited for translating disease-oriented markers, such as urine albumin excretion rate or GFR, into patient-oriented outcomes, such as progression to ESRD or mortality. The American Diabetes Association recommends limiting protein intake in persons with diabetes to 0.8 to 1 g per kg per day in earlier stages of chronic kidney disease and to 0.8 g per kg per day in later stages to improve urine albumin excretion and estimated GFR.6
In a small randomized controlled trial, a low-protein diet (0.6 g per kg per day) slowed progression to ESRD or death compared with a normal-protein diet (0.89 g per kg per day).17 Improvements in proteinuria also were demonstrated in a systematic review of eight randomized trials evaluating low- versus normal-protein diets in 519 persons with type 1 or 2 diabetes and nephropathy, and in a randomized trial comparing a soy protein diet (35 percent animal, 35 percent textured soy, and 30 percent vegetable proteins) with a control diet (70 percent animal and 30 percent vegetable proteins).18,19
A cohort study of 191 persons with diabetes referred to a nephrologist for advanced renal disease showed that a 50 percent carbohydrate-restricted, low-iron, polyphenolenriched diet was superior to a conventional protein-restricted diet for avoiding renal replacement therapy independent of blood pressure, average A1C level, initial renal dysfunction, or use of ACE inhibitors.35 In a randomized double-blind placebo-controlled trial of 238 persons with type 1 or 2 diabetes and macroalbuminuria, persons receiving supplemental folic acid (2.5 mg), vitamin B6 (pyridoxine, 25 mg), and cyanocobalamin (1 mg) had a significantly greater decline in GFR, and increased risk of myocardial infarction, stroke, and all-cause mortality compared with persons receiving placebo (number needed to harm = 11).36 Table 5 summarizes patient- and disease-oriented evidence regarding development of diabetic nephropathy.
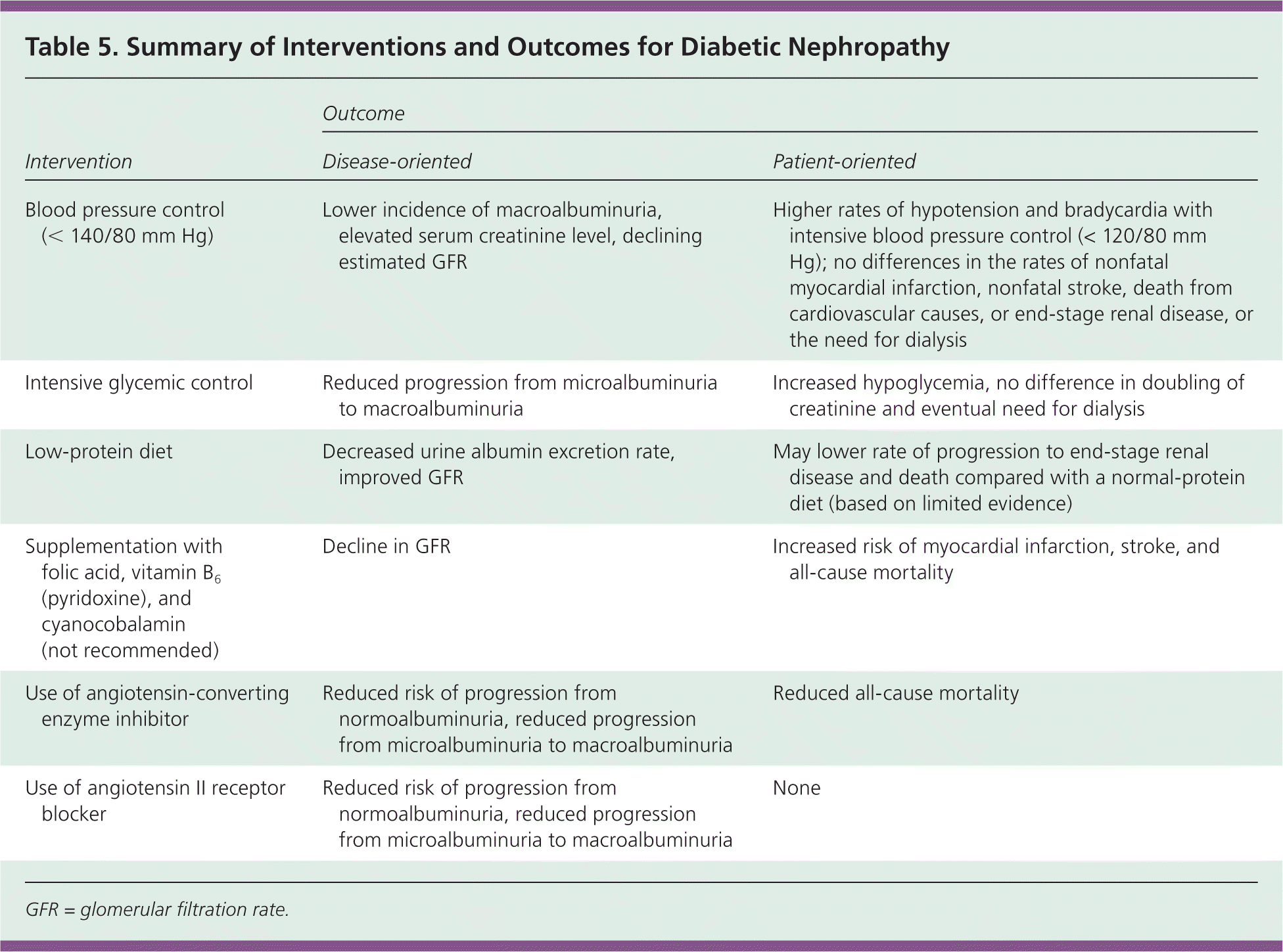
| Intervention | Outcome | |
|---|---|---|
| Disease-oriented | Patient-oriented | |
| Blood pressure control (< 140/80 mm Hg) | Lower incidence of macroalbuminuria, elevated serum creatinine level, declining estimated GFR | Higher rates of hypotension and bradycardia with intensive blood pressure control (< 120/80 mm Hg); no differences in the rates of nonfatal myocardial infarction, nonfatal stroke, death from cardiovascular causes, or end-stage renal disease, or the need for dialysis |
| Intensive glycemic control | Reduced progression from microalbuminuria to macroalbuminuria | Increased hypoglycemia, no difference in doubling of creatinine and eventual need for dialysis |
| Low-protein diet | Decreased urine albumin excretion rate, improved GFR | May lower rate of progression to end-stage renal disease and death compared with a normal-protein diet (based on limited evidence) |
| Supplementation with folic acid, vitamin B6 (pyridoxine), and cyanocobalamin (not recommended) | Decline in GFR | Increased risk of myocardial infarction, stroke, and all-cause mortality |
| Use of angiotensin-converting enzyme inhibitor | Reduced risk of progression from normoalbuminuria, reduced progression from microalbuminuria to macroalbuminuria | Reduced all-cause mortality |
| Use of angiotensin II receptor blocker | Reduced risk of progression from normoalbuminuria, reduced progression from microalbuminuria to macroalbuminuria | None |
Data Sources: A PubMed search was conducted for diabetic nephropathy–related topics, including clinical reviews, randomized controlled trials, and meta-analyses. Search terms included diabetic nephropathy, microalbuminuria, macroalbuminuria, diabetic kidney disease, renal replacement therapy, diabetes, diet, and glycemic and blood pressure control. Relevant publications from the Cochrane database, Essential Evidence, National Guideline Clearinghouse, U.S. Preventive Services Task Force, the Centers for Disease Control and Prevention, and the American Diabetes Association also were reviewed. Search date: January 31, 2011.
