
A more recent article on chronic kidney disease is available.
Am Fam Physician. 2012;86(8):749-754
Author disclosure: No relevant financial affiliations to disclose.
Chronic kidney disease is common and associated with significant morbidity. Given the high risk of cardiovascular morbidity and mortality in patients with chronic kidney disease, it is important to identify and treat related risk factors. However, there is growing uncertainty about the benefits of some recommended treatment targets. The National Kidney Foundation's Kidney Disease Outcomes Quality Initiative guidelines recommend an A1C level of less than 7 percent in patients with diabetes mellitus, although there is no evidence that treatment to this goal reduces cardiovascular events or progression to end-stage renal disease. Optimal blood pressure goals are controversial, and further study is needed to determine these goals in relation to amount of proteinuria. Concurrent use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers leads to worsening kidney function and is not recommended. Lipid-lowering therapy has been shown to reduce the risk of cardiovascular events and mortality, but not progression of chronic kidney disease. The treatment of anemia in patients with chronic kidney disease, particularly the use of erythropoiesis-stimulating agents and optimal hemoglobin goals, is also controversial. Studies have shown increased morbidity and mortality with use of erythropoiesis-stimulating agents aimed at normalizing hemoglobin levels. Patients with chronic kidney disease are at high risk of morbidity and mortality from the use of intravenous contrast agents. Isotonic intravenous hydration with sodium bicarbonate or saline has been shown to prevent contrast-induced nephropathy. Gadolinium-based contrast agents should be avoided if the glomerular filtration rate is less than 30 mL per minute per 1.73 m2 because of the risk of nephrogenic systemic fibrosis.
The National Kidney Foundation defines chronic kidney disease (CKD) as a glomerular filtration rate (GFR) of less than 60 mL per minute per 1.73 m2, or evidence of kidney damage with or without a decreased GFR, for three or more months.1 Approximately 13 percent of adult Americans and 44 percent of persons 65 years and older meet this definition.2,3 CKD is classified in stages based on decreasing levels of GFR (Table 1).1
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Patients with nondiabetic kidney disease and a random urine total protein-to-creatinine ratio greater than 200 mg per g, and those with diabetic kidney disease, should be treated with an ACE inhibitor or an angiotensin II receptor blocker. | A | 15 |
| Concurrent use of ACE inhibitors and angiotensin II receptor blockers should be avoided because of symptomatic hypotension and worsening kidney function. | A | 24 |
| Hemoglobin goals should not exceed 11 g per dL (110 g per L) in patients receiving erythropoiesis-stimulating agents due to the risk of major cardiovascular events. | A | 39 |
| Gadolinium should be avoided in patients with a glomerular filtration rate less than 30 mL per minute per 1.73 m2, or with acute kidney injury caused by hepatorenal syndrome or in the perioperative liver transplantation period. | B | 49 |
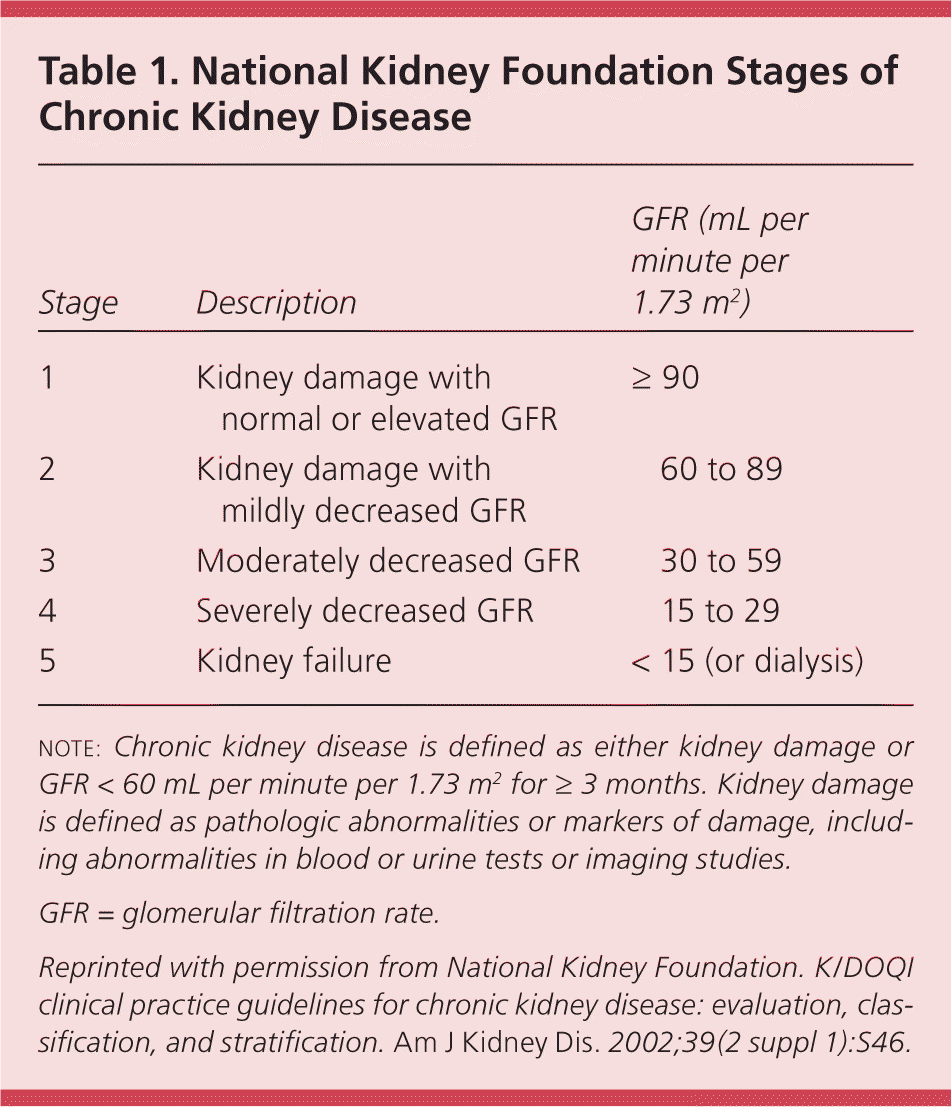
| Stage | Description | GFR (mL per minute per 1.73 m2 ) |
|---|---|---|
| 1 | Kidney damage with normal or elevated GFR | ≥ 90 |
| 2 | Kidney damage with mildly decreased GFR | 60 to 89 |
| 3 | Moderately decreased GFR | 30 to 59 |
| 4 | Severely decreased GFR | 15 to 29 |
| 5 | Kidney failure | < 15 (or dialysis) |
Patients with CKD are more likely to die of cardiovascular disease than to require dialysis.4 Therefore, reduction of morbidity and mortality in patients with CKD requires management of cardiovascular disease risk factors. This review summarizes recent evidence for the management of risk factors that affect cardiovascular disease and the progression of CKD, as well as controversies regarding some elements of recommended practice. Complications arising from use of intravenous contrast agents are also discussed.
Diabetes Mellitus
Diabetes mellitus is the leading cause of kidney failure in the United States and is a major risk factor for cardiovascular disease.5 The combination of diabetes and CKD is one of the most potent predictors of adverse cardiovascular events and death.6 Expert consensus and the Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines on diabetes and CKD recommend a goal A1C level of less than 7 percent in patients with diabetes, which corresponds to a preprandial plasma glucose level of 70 to 130 mg per dL (3.9 to 7.2 mmol per L) and postprandial plasma glucose level of less than 180 mg per dL (10 mmol per L).6–8
A few small randomized controlled trials have shown that an A1C level of approximately 7 percent preserves GFR, except in those with proteinuria.6 Large randomized controlled trials in high-risk patients with long-standing diabetes have not demonstrated a reduction in cardiovascular events, the need for dialysis, or death caused by renal disease at this goal.9,10 Additionally, a higher rate of complications, including severe hypoglycemia and death, was observed with more intensive blood glucose control. Goals pertaining to A1C levels should be individually determined based on a patient's comorbidities, functional status, and other vascular risk factors. Higher goals may be more appropriate for older adults and those with limited life expectancy in whom the risks of intense glycemic control outweigh the benefits.11,12
Proteinuria and Hypertension
Treatment of proteinuria and hypertension with antihypertensive medications reduces the risk of cardiovascular disease and slows progression of CKD. Proteinuria is a marker of kidney damage, and a risk factor for accelerated progression of kidney disease. It is increasingly recognized as an independent risk factor for all-cause and cardiovascular mortality.13–15 The K/DOQI guidelines recommend that patients with nondiabetic kidney disease and a random (spot) urine total protein-to-creatinine ratio greater than 200 mg per g, and those with diabetic kidney disease, should be treated with an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin II receptor blocker (ARB), regardless of the presence of hypertension.15 ACE inhibitors and ARBs have similar ability to reduce proteinuria and have achieved an absolute risk reduction for requiring dialysis from 3.5 to 6 percent over two to three years in patients with diabetic and nondiabetic kidney disease.16–19
There is less evidence to support the effectiveness of ACE inhibitors and ARBs in slowing the progression of CKD in patients without proteinuria. The benefit of these medications in older adults with CKD is uncertain, because most of these patients do not have proteinuria, and most trials did not enroll patients older than 70 years.20 There is often concern about the benefits and risks of ACE inhibitors and ARBs in patients with advanced CKD, but evidence suggests a reduction of adverse renal outcomes even in advanced stages.21,22 Adverse effects of ACE inhibitor or ARB therapy include hyperkalemia and a decline in GFR, but these agents generally may be continued if the GFR decline over four months is less than 30 percent from baseline value and serum potassium is 5.5 mEq per L (5.5 mmol per L) or less.15
The recommendation for concurrent use of ACE inhibitors and ARBs has recently been reevaluated. Because their combined use more effectively reduces proteinuria compared with monotherapy, some guidelines have adopted this approach.15,16,23 A randomized controlled trial comparing patients given an ACE inhibitor and an ARB with those given either drug alone found no difference with respect to a composite outcome of cardiovascular death, myocardial infarction, stroke, or hospitalization because of heart failure. Notably, adverse effects, including worsening renal function, occurred more frequently with combination therapy.24 The majority of current evidence recommends against the combined use of ACE inhibitors and ARBs.
Optimal blood pressure goals in patients with CKD are a source of controversy. Based primarily on observational data, the K/DOQI guidelines and the seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure recommend a blood pressure goal of less than 130/80 mm Hg for patients with diabetic and nondiabetic CKD.15,25 In examining the benefits of lower blood pressure goals, a 2009 Cochrane review of patients with CKD found no reduction in cardiovascular events, stroke, end-stage renal disease, or total mortality in those with lower (135/85 mm Hg or less) versus standard (140 to 160/90 to 100 mm Hg or less) blood pressure goals.26 Similarly, a systematic review found that lower versus higher blood pressure goals in nondiabetic patients with CKD did not reduce the risk of adverse outcomes, including kidney failure, cardiovascular events, and death, but suggested that lower targets might benefit patients with proteinuria greater than 300 mg per day.27 These findings challenge current recommendations for lower than usual blood pressure goals to reduce progression of kidney disease or cardiovascular events. Additional randomized controlled trials are needed to clarify optimal blood pressure targets in the context of CKD and in relation to degree of proteinuria.
Dyslipidemia
There is no prospective evidence that treating dyslipidemia prevents the progression of CKD or diabetic nephropathy, but evidence does support treatment to prevent cardiovascular events. A 2009 Cochrane meta-analysis found that statins, when compared with placebo, significantly decreased the risk of all-cause and cardiovascular mortality in patients with CKD.28 A large randomized controlled trial in patients with moderate to advanced CKD demonstrated that simvastatin/ezetimibe (Vytorin) reduced major atherosclerotic events by 17 percent, but not progression to dialysis or transplantation.29
Based on evidence of the benefits of lipid lowering in the general population, the K/DOQI dyslipidemia guidelines concur with the National Cholesterol Education Program Adult Treatment Panel III guidelines and recommend that all adults with CKD have a complete fasting lipid profile, with treatment goals of low-density lipoprotein cholesterol levels less than 100 mg per dL (2.59 mmol per L) and triglyceride levels less than 150 mg per dL (1.69 mmol per L).30 Statins are a first-line therapy, and are generally well tolerated by patients with CKD. As in the general population, it is important to consider life expectancy and competing health concerns when deciding on lipid-lowering therapy. In patients 65 to 75 years of age with cardiovascular disease, statin use reduced the risk of cardiovascular events and all-cause mortality.31 In adults older than 80 years, however, lipid-lowering therapy has not affected all-cause mortality, nor is there clear evidence as to whether these patients should be started or continued on lipid-lowering agents.32
Anemia
There has been extensive research into the benefits and risks of erythropoiesis-stimulating agents and optimal hemoglobin goals in patients with CKD. Higher hemoglobin goals were recommended because of an association with improved quality of life and survival in observational studies.33,34 However, randomized controlled trials have not confirmed these associations. A Cochrane meta-analysis of 22 trials found no difference or a higher risk of all-cause mortality and worse cardiovascular outcomes in higher (greater than 13.3 g per dL [133 g per L]) versus lower (less than 12 g per dL [120 g per L]) hemoglobin target groups.35
Three randomized controlled trials have greatly affected practice in this area. Two trials randomized CKD patients to higher versus lower hemoglobin targets, which were achieved with erythropoiesis-stimulating agents.36,37 The higher hemoglobin target groups had higher rates of death, adverse cardiovascular events, and dialysis. A third trial randomized 4,038 patients with type 2 diabetes, CKD, and anemia to darbepoetin alfa (Aranesp) or placebo.38 The darbepoetin alfa group had a higher risk of stroke, and no improvement in the primary composite outcomes of death and nonfatal cardiovascular events or end-stage renal disease. In these three trials, quality-of-life results were mixed. Taken together, the evidence demonstrates inconsistent quality-of-life benefit, and increases in mortality, cardiovascular events, and adverse renal outcomes with higher hemoglobin goals.
Given evidence of harm associated with higher hemoglobin levels, the K/DOQI anemia guidelines were updated in 2007 to reflect a hemoglobin target range of 11 to 12 g per dL (110 to 120 g per L) in patients with CKD who receive erythropoiesis-stimulating agents.34 The guidelines do not recommend a specific hemoglobin level at which to initiate these agents. The U.S. Food and Drug Administration (FDA) recommends this treatment only when a patient's hemoglobin level is less than 10 g per dL (100 g per L), when the rate of hemoglobin decline suggests a need for a blood transfusion, and when the reduction of transfusion-related risks, such as alloimmunization, is a goal.39 Additionally, new FDA labels now warn against erythropoiesis-stimulating agents to achieve hemoglobin levels greater than 11 g per dL because of the risk of death and major cardiovascular events.
Contrast-Induced Nephropathy
Contrast-induced nephropathy is an increase in serum creatinine greater than 25 percent from baseline or an absolute increase greater than 0.5 mg per dL (44.2 μmol per L) within the first few days after receipt of intravenous contrast.40 Contrast-induced nephropathy can precipitate the need for immediate dialysis, and has been associated with an increased risk for major cardiovascular events, increased length of hospital stay, and mortality.41 A validated risk score based on eight variables predicts the risk of contrast-induced nephropathy, in-hospital dialysis, and one-year mortality in patients undergoing a percutaneous coronary intervention (Table 2).42 Patients with CKD and a GFR less than 60 mL per minute per 1.73 m2 are at high risk of contrast-induced nephropathy, and preventive measures should be considered.43 Choose alternative imaging without contrast if the perceived risks of an intravenous contrast study outweigh the benefits. Efforts to minimize the risk of contrast-induced nephropathy include avoidance of dehydration and nonsteroidal anti-inflammatory agents, and use of the lowest possible doses of low or iso-osmolal nonionic contrast agents.44 Isotonic intravenous hydration with sodium bicarbonate or saline has been shown to prevent contrast-induced nephropathy, although there is conflicting evidence over which fluid and administration protocol is superior.
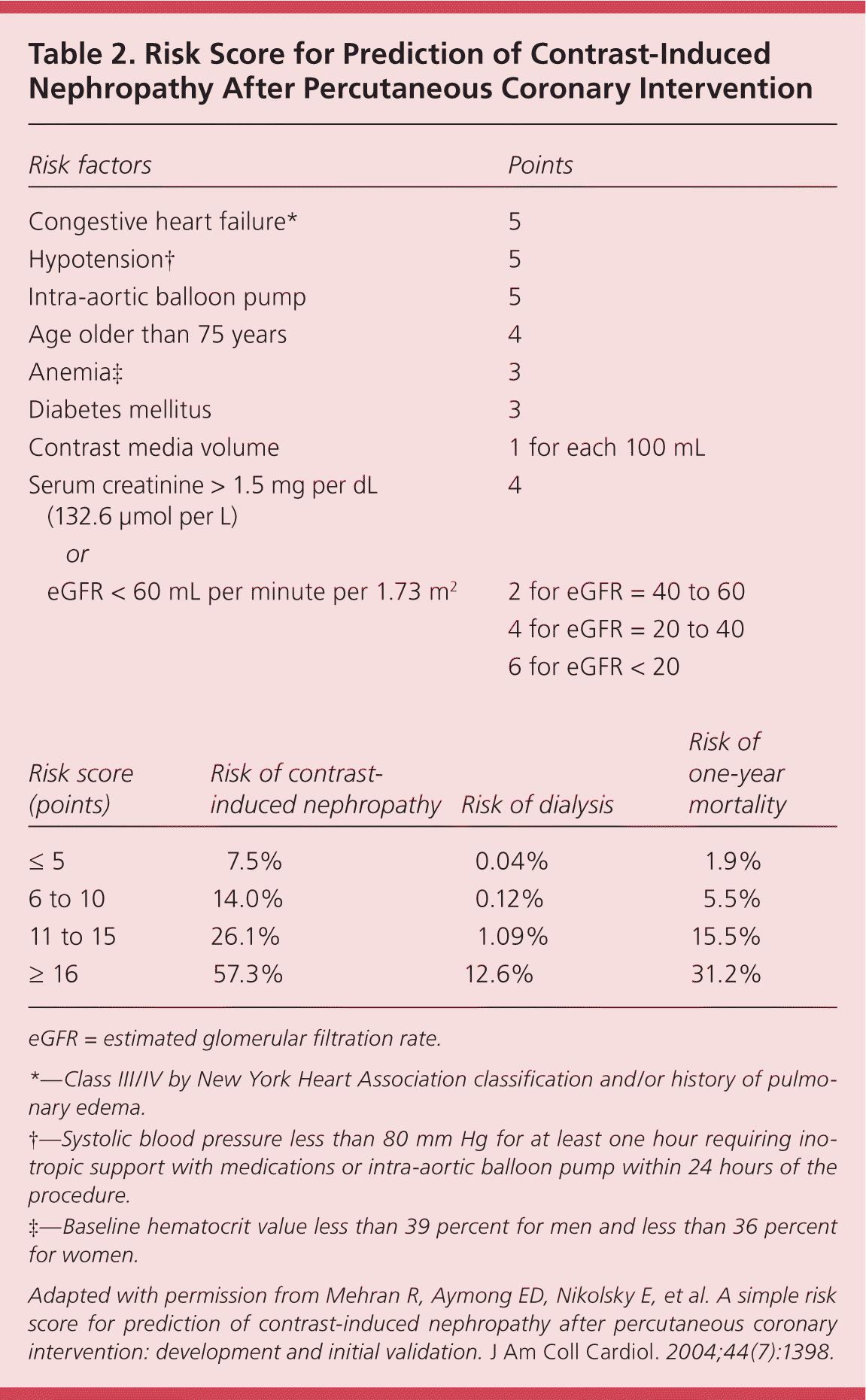
| Risk factors | Points | |
|---|---|---|
| Congestive heart failure* | 5 | |
| Hypotension† | 5 | |
| Intra-aortic balloon pump | 5 | |
| Age older than 75 years | 4 | |
| Anemia‡ | 3 | |
| Diabetes mellitus | 3 | |
| Contrast media volume | 1 for each 100 mL | |
| Serum creatinine > 1.5 mg per dL (132.6 μmol per L) | 4 | |
| or | ||
| eGFR < 60 mL per minute per 1.73 m2 | 2 for eGFR = 40 to 60 | |
| 4 for eGFR = 20 to 40 | ||
| 6 for eGFR < 20 | ||
Data on the effectiveness of N-acetylcysteine for preventing contrast-induced nephropathy are also inconsistent, although a meta-analysis of randomized trials demonstrated that high doses decrease the incidence of contrast-induced nephropathy.45 Given the relatively low cost and favorable safety profile of N-acetylcysteine, some experts recommend giving 1,200 mg orally twice daily on the day before and the day of contrast administration.46 Because the effect of intravenous contrast on kidney function can be observed within 48 to 72 hours, it is important to check the serum creatinine level in high-risk patients within this time frame.
Magnetic resonance imaging using gadolinium-based contrast agents is no longer considered the safer alternative to iodinated contrast in patients with CKD. Gadolinium-based contrast agents have been associated with acute kidney injury and with nephrogenic systemic fibrosis, a progressive multiorgan system fibrosing disease. Most patients who have developed this disease after exposure to gadolinium were receiving long-term dialysis, whereas the remainder were patients with CKD and a GFR less than 30 mL per minute per 1.73 m2.47 The risk in patients with a GFR greater than 30 mL per minute per 1.73 m2 is not known, but the American College of Radiology considers these persons to be at no or extremely low risk of developing nephrogenic systemic fibrosis.48 The pathogenesis is unknown, and there is no treatment for this debilitating disease. The FDA recommends avoidance of gadolinium in patients with a GFR less than 30 mL per minute per 1.73 m2, or with acute kidney injury caused by hepatorenal syndrome or in the perioperative liver transplantation period.49
Data Sources: A PubMed search was completed for each subsection of the manuscript, using the key terms chronic kidney disease, chronic renal failure, prevalence, diabetes, hypertension, proteinuria, dyslipidemia, anemia, hemoglobin goals, contrast nephropathy, and nephrogenic systemic fibrosis. Additional data sources searched included the Agency for Healthcare Research and Quality Evidence Reports, the Cochrane Database of Systematic Reviews, the National Guidelines Clearinghouse, the Institute for Clinical Systems Improvement, the U.S. Preventive Services Task Force, Bandolier, Database of Abstracts of Reviews of Effects, Effective Health Care, EBM Online/Evidence-Based Medicine, Essential Evidence Plus, UpToDate, and the AFP By Topic collection on kidney disease. Search dates: July 2010 to November 2010.
