
Am Fam Physician. 2012;86(12):1118-1124
A more recent article on cutaneous cryosurgery is available.
Author disclosure: No relevant financial affiliations to disclose.
Cutaneous cryosurgery refers to localized application of freezing temperatures to achieve destruction of skin lesions. It can be used to treat a broad range of benign and premalignant skin conditions, and certain malignant skin conditions, with high cure rates. Cellular destruction is accomplished by delivery of the cryogen via dipstick, probe, or spray techniques. It is widely used in primary care because of its safety, effectiveness, low cost, ease of use, good cosmetic results, and lack of need for anesthesia. Cryosurgery is as effective as alternative therapies for most cases of molluscum contagiosum, dermatofibromas, keloids, and plantar or genital warts. It is a more effective cure for common warts than salicylic acid or observation. Cryosurgery is generally the treatment of choice for actinic keratosis. Contraindications to cryosurgery include cryofibrinogenemia, cryoglobulinemia, Raynaud disease, agammaglobulinemia, and multiple myeloma. Complications from cryosurgery include hypopigmentation and alopecia, and can be avoided by limiting freeze times to less than 30 seconds. Referral to a dermatologist should be considered in cases of diagnostic uncertainty or for treatment of skin cancer, which requires larger amounts of tissue destruction, resulting in higher complication rates.
Cryosurgery refers to localized application of freezing temperatures to achieve destruction of body tissue. It is used primarily for cutaneous lesions, but also has wider applications in ophthalmology, gynecology, neurosurgery, cardiology, and oncology.1–3 Cryosurgery has been used for more than 150 years to treat a broad range of benign and premalignant skin conditions, and certain malignant conditions, with high cure rates.1 Liquid nitrogen—the modern cryogen of choice—became commercially available in the 1940s. Since that time, cryosurgery has been commonly performed in the outpatient setting because of its safety, effectiveness, low cost, ease of use, good cosmetic results, and lack of need for anesthesia.1,4
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Malignant skin lesions should not be treated using the dipstick method because of inadequate depth of freezing. | C | 1 |
| Cryosurgery is more effective than salicylic acid or observation for the cure of common warts, but not plantar warts. | B | 16, 17 |
| Cryosurgery is highly effective for actinic keratosis and is the treatment of choice for most patients. | C | 5 |
Mechanism and Goals
Cryosurgery can be accomplished through an open spray technique, or through direct application of a dipstick or cooled probe. Liquid nitrogen has a boiling point of –195.6°C (–320.1°F). When tissue is cooled, it is injured by ice crystal formation within cells, vascular thrombosis and stasis, and the release of electrolytes and toxins. An open spray transfers heat (causes cooling of the skin) faster and is more damaging to tissues, but firm pressure and use of lubricating jelly can speed freezing time when using a probe.1
Destruction of malignant cells requires a final tissue temperature of –60°C (–76°F).1 To treat malignant lesions other than melanoma, clinicians should use intermittent spraying to allow deep penetration of the ice ball, which can result in a maximum depth of 10 mm. An ice ball is the total volume of tissue destroyed by freezing; if viewed in cross-section, it would appear as a sphere. The same amount of tissue destruction should occur as would be removed during an excision, which may require debulking before freezing. The total thaw time between freezes for a malignant lesion should be at least 90 seconds after reaching adequate temperature at the base of the ice ball, because slow thawing increases the concentrations of toxic levels of electrolytes in the surrounding tissues.1 Shorter thaw times are associated with incomplete eradication of malignant cells.
Conversely, when treating a superficial lesion, a continuous spray technique will limit the depth of freezing due to deflection of the gases. Treatment of benign lesions is simpler because the rapid freezing causes sloughing of the epidermis from the dermis.
Equipment and Design
DIPSTICK
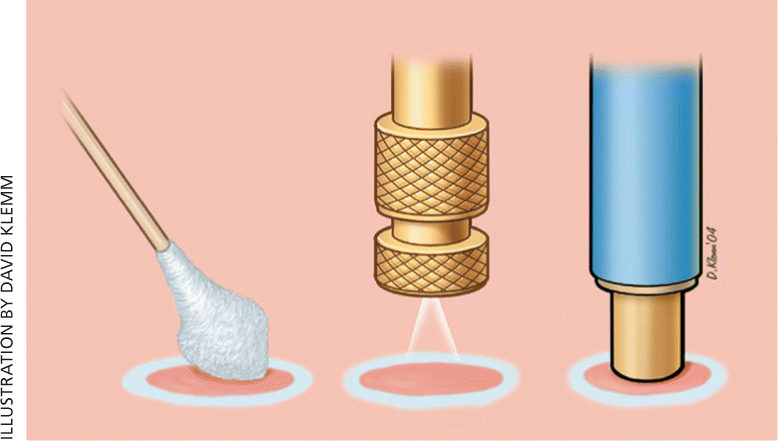
OPEN SPRAY
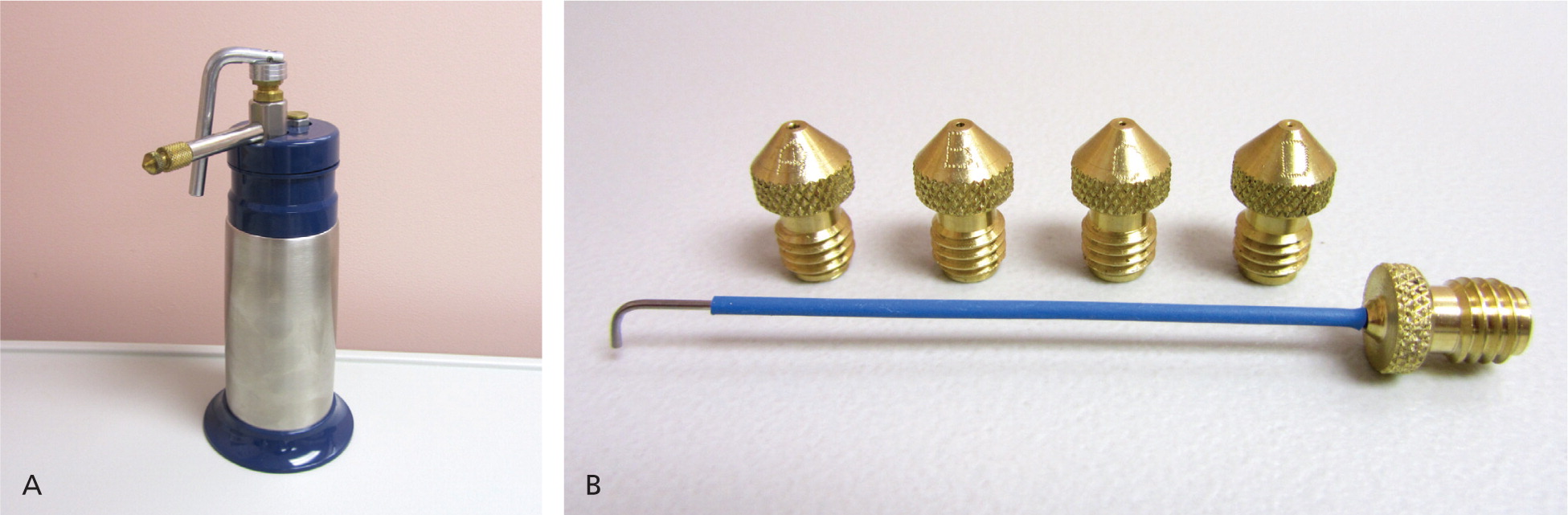
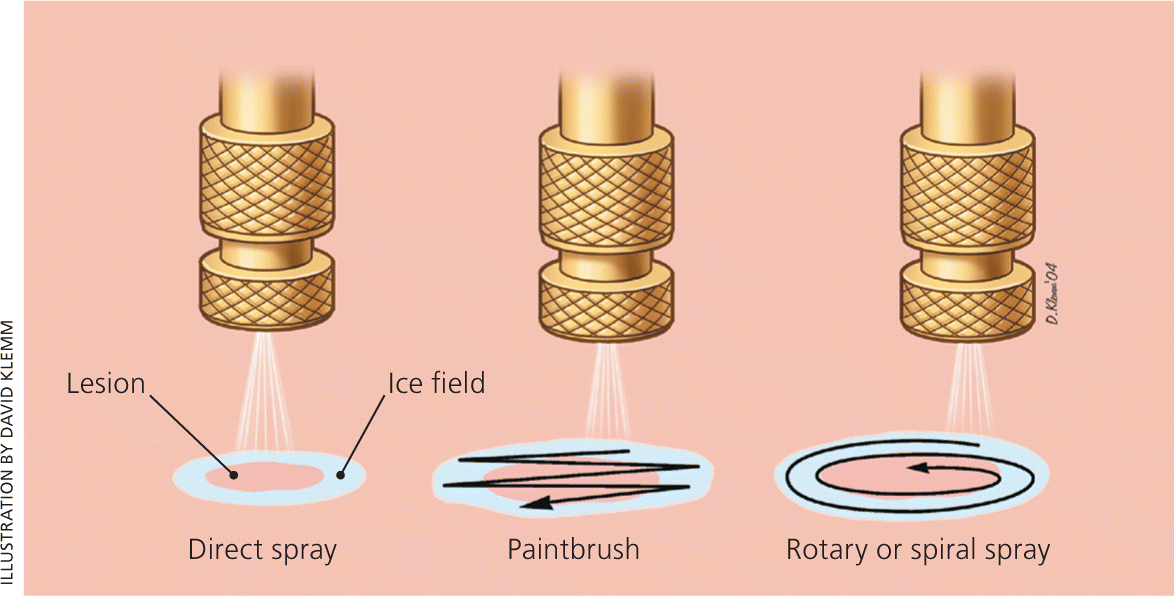
Using one of several commercially available spray guns (Figure 2), a fine spray of liquid nitrogen can be directed at any skin lesion, whether benign or malignant. Superficial and irregular lesions are amenable to this therapy as well. Different techniques (e.g., pulse, continuous, spiral, paint brush) can be used to adequately freeze1 (Figure 3). The spray is directed from a 90-degree angle at a distance of 1 to 2 cm. A video of the open spray technique is available at http://www.youtube.com/watch?v=CQxQ3ucfis4.
CONFINED SPRAY
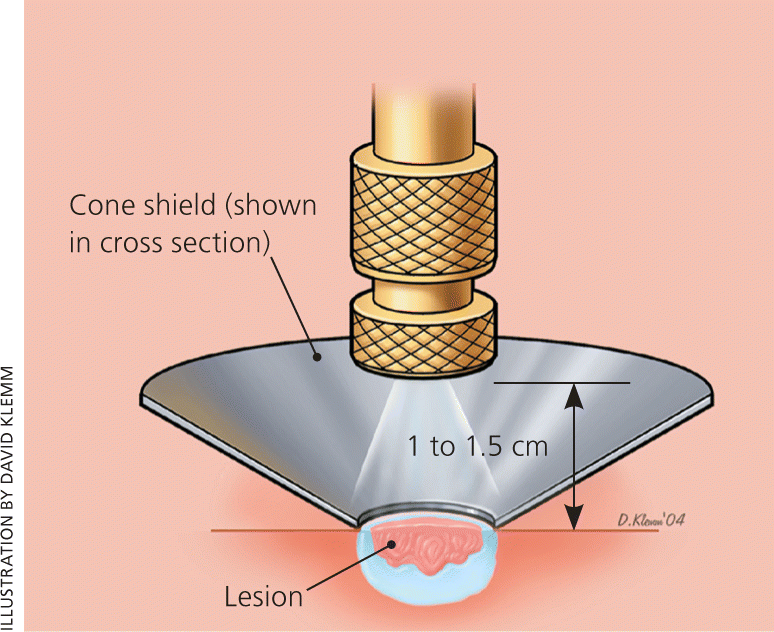
Confined spray is used particularly when sensitive structures are nearby. A cone directs the cryogen to the skin (Figure 4).1 Clinicians can use the standard, common spray gun with an ear speculum, or a commercial product (CryoPen) using small canisters of nitrous oxide, which has a temperature of –127°C (–196.6°F). A video demonstrating the combined spray with a CryoPen is available at http://www.youtube.com/watch?v=7aV_qcgAwKE.
CRYOPROBE
Application of a precooled metal instrument, or cryoprobe, against the lesion is useful for round lesions on flat surfaces.1
Clinical Applications
Because there are often multiple possible treatment modalities for cutaneous lesions, clinicians should consider factors unique to the patient when selecting treatment. For example, a patient who is averse to pain or who has large areas of affected skin might be better suited to a topical remedy than to cryosurgery.5 However, several factors may make cryotherapy a better option than surgical intervention: a history of poor wound healing, intolerance to local anesthesia injection, anticoagulant use, indwelling pacemakers, or the presence of several scattered lesions.2 Some general advantages of cryosurgery are listed in Table 1.1,6

| Anesthesia optional |
| Excellent cosmetic results |
| Low cost |
| Low risk of infection |
| Minimal wound care |
| No need for suture removal |
| No work or sport restrictions |
| Portable to multiple treatment settings |
| Safe procedure |
| Short preparation time |
| Useful in pregnancy |
Contraindications to cryosurgery are fairly rare, but there are situations in which it should be used with caution (Table 2).2,7,8 It should not be offered to patients with a proven sensitivity to the cryogen. Neither should it be used on a skin lesion for which the diagnosis is uncertain.7 Cryosurgery should not be attempted on children younger than seven years because of the pain associated with the procedure.9 Some older children also may be reluctant to undergo cryosurgery for the same reason. However, topical application of lidocaine tape or lidocaine/prilocaine cream (Emla) before or immediately after freezing may help children tolerate the procedure better.10,11
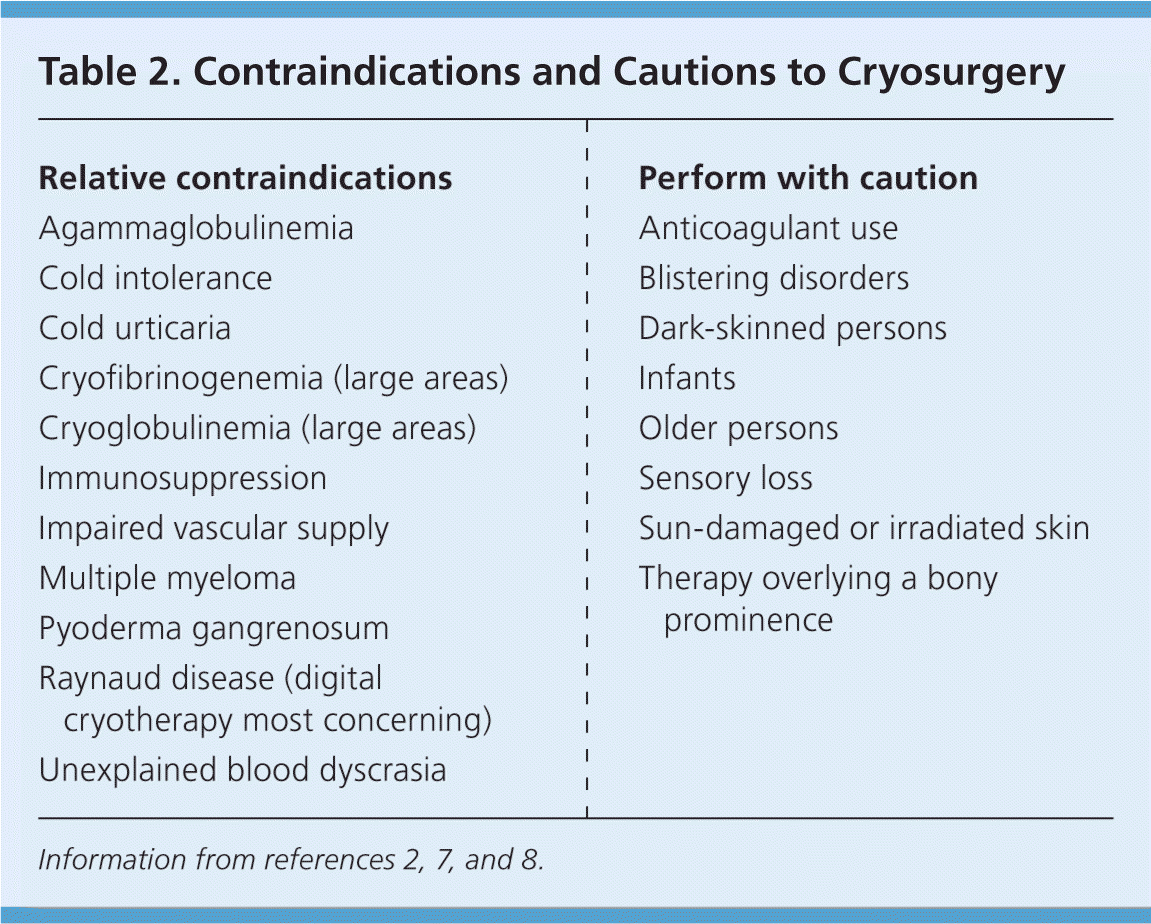
| Relative contraindications |
| Agammaglobulinemia |
| Cold intolerance |
| Cold urticaria |
| Cryofibrinogenemia (large areas) |
| Cryoglobulinemia (large areas) |
| Immunosuppression |
| Impaired vascular supply |
| Multiple myeloma |
| Pyoderma gangrenosum |
| Raynaud disease (digital cryotherapy most concerning) |
| Unexplained blood dyscrasia |
| Perform with caution |
| Anticoagulant use |
| Blistering disorders |
| Dark-skinned persons |
| Infants |
| Older persons |
| Sensory loss |
| Sun-damaged or irradiated skin |
| Therapy overlying a bony prominence |
BENIGN LESIONS
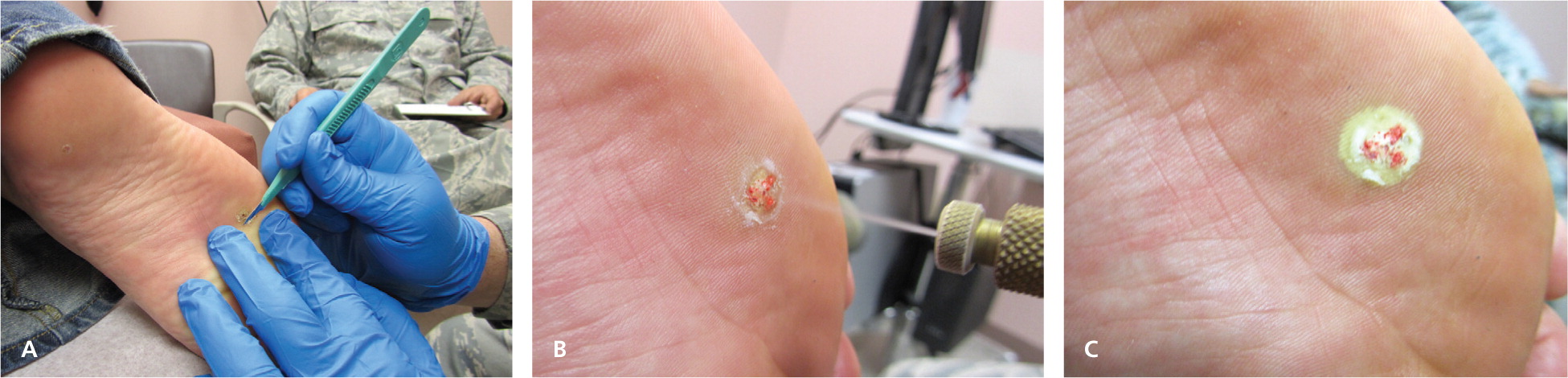
Cryosurgery for genital warts and common warts has a cure rate of 60 to 86 percent.11–13 Several treatment sessions with long freeze times may be necessary depending on the size and location of the warts, with plantar warts requiring the longest course of treatment (Table 31,2,7,9,14 ). Topical keratolytics and simple occlusive therapy also have reasonable effectiveness for warts; hence, cryosurgery is sometimes combined with these other modalities. Shaving a hyperkeratotic wart down to its base (Figure 5) and pretreating with salicylic acid are commonly employed strategies before application of the cryogen.2,11–13,15 There is recent evidence that cryosurgery is more effective than other forms of treatment (i.e., salicylic acid or observation) for common warts, but not plantar warts.16,17
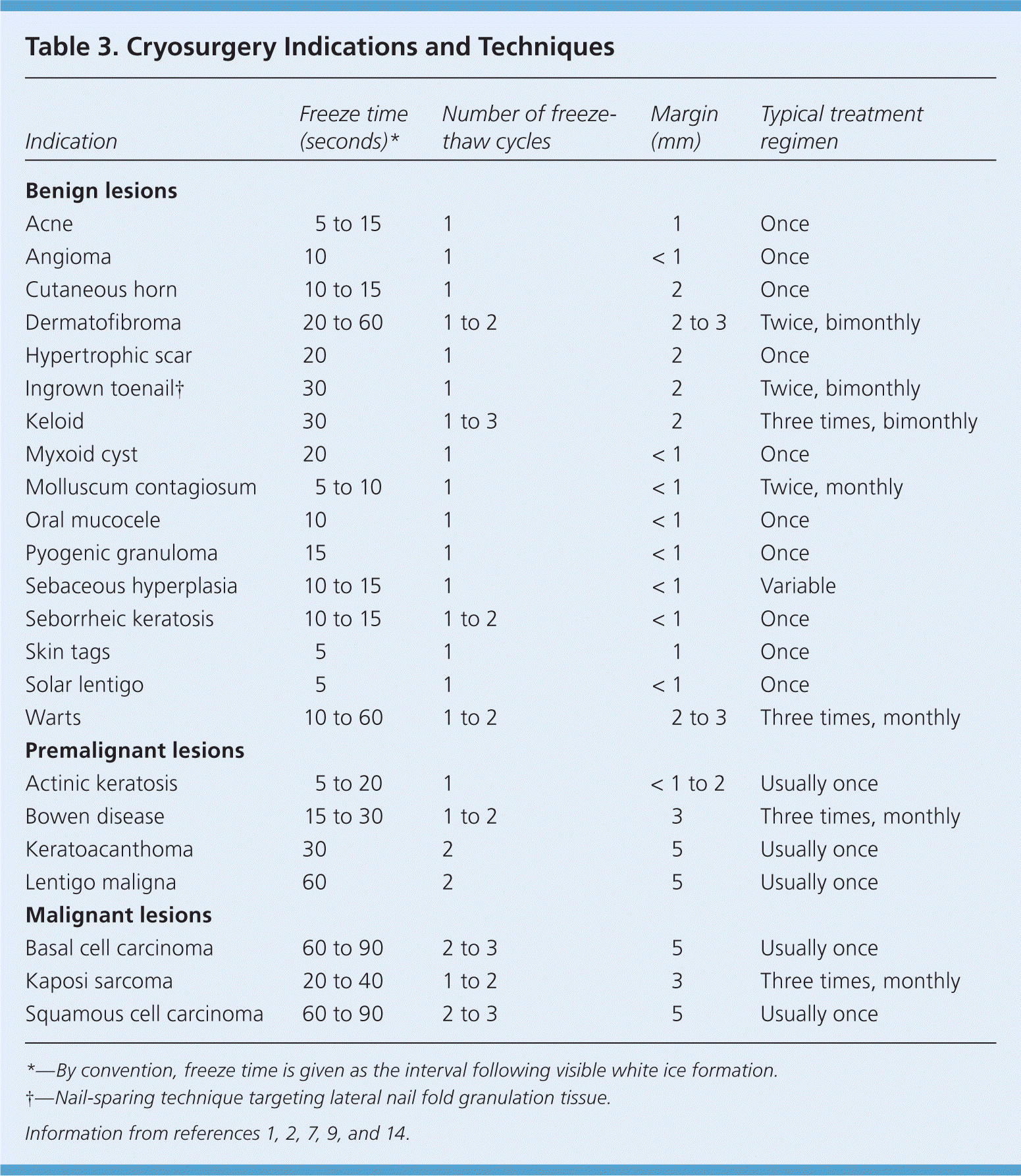
| Indication | Freeze time (seconds)* | Number of freeze-thaw cycles | Margin (mm) | Typical treatment regimen |
|---|---|---|---|---|
| Benign lesions | ||||
| Acne | 5 to 15 | 1 | 1 | Once |
| Angioma | 10 | 1 | < 1 | Once |
| Cutaneous horn | 10 to 15 | 1 | 2 | Once |
| Dermatofibroma | 20 to 60 | 1 to 2 | 2 to 3 | Twice, bimonthly |
| Hypertrophic scar | 20 | 1 | 2 | Once |
| Ingrown toenail† | 30 | 1 | 2 | Twice, bimonthly |
| Keloid | 30 | 1 to 3 | 2 | Three times, bimonthly |
| Myxoid cyst | 20 | 1 | < 1 | Once |
| Molluscum contagiosum | 5 to 10 | 1 | < 1 | Twice, monthly |
| Oral mucocele | 10 | 1 | < 1 | Once |
| Pyogenic granuloma | 15 | 1 | < 1 | Once |
| Sebaceous hyperplasia | 10 to 15 | 1 | < 1 | Variable |
| Seborrheic keratosis | 10 to 15 | 1 to 2 | < 1 | Once |
| Skin tags | 5 | 1 | 1 | Once |
| Solar lentigo | 5 | 1 | < 1 | Once |
| Warts | 10 to 60 | 1 to 2 | 2 to 3 | Three times, monthly |
| Premalignant lesions | ||||
| Actinic keratosis | 5 to 20 | 1 | < 1 to 2 | Usually once |
| Bowen disease | 15 to 30 | 1 to 2 | 3 | Three times, monthly |
| Keratoacanthoma | 30 | 2 | 5 | Usually once |
| Lentigo maligna | 60 | 2 | 5 | Usually once |
| Malignant lesions | ||||
| Basal cell carcinoma | 60 to 90 | 2 to 3 | 5 | Usually once |
| Kaposi sarcoma | 20 to 40 | 1 to 2 | 3 | Three times, monthly |
| Squamous cell carcinoma | 60 to 90 | 2 to 3 | 5 | Usually once |
Cryosurgery is also a standard treatment for molluscum contagiosum, especially for a small number of larger lesions that could easily be treated in one session. Before consenting to cryosurgery for molluscum and warts, patients should be informed that many of these lesions resolve on their own within six to 24 months.12
Dermatofibromas have a propensity for regrowth, but when treated with cryotherapy can be eradicated in most cases.18 Cryotherapy has been used alone, after debulking, or in conjunction with intralesional steroids to treat keloids and hypertrophic scars. It is more successful in the treatment of scars that are recent and smaller in size.1,19–21 Dermatofibromas and keloid scarring often require multiple treatment sessions with a probe or spray technique.14
PREMALIGNANT LESIONS
Cryosurgery is the preferred and most effective method for treating all forms of actinic keratosis. The lesions can be thick or thin, and well or poorly demarcated. Freeze times are highly variable and depend on the size of the lesion, but overall eradication rates are excellent (close to 99 percent).2,5
Two other conditions are precursors to, or low-grade forms of, squamous cell carcinoma. Bowen disease and keratoacanthoma should be treated with a destructive treatment such as cryosurgery. Any raised portion of the lesion is usually excised first to allow better delivery of the cryogen to the base.1,22
The preferred treatment for lentigo maligna is complete excision because of the risk of transformation to melanoma. In instances in which surgery is not practical, extensive cryosurgery provides a 90 percent cure rate.1,23 Freezing pigmented nevi should be avoided because regrowth with atypical features may require surgical excision to rule out malignancy. Also, it can be difficult for a pathologist to distinguish between a melanoma and altered histology from freezing.9 Tissue diagnosis from a biopsy should always be obtained before a suspected malignant lesion is treated with cryosurgery. In cases in which there is uncertainty about the diagnosis, consultation with a dermatologist is indicated.
MALIGNANT LESIONS
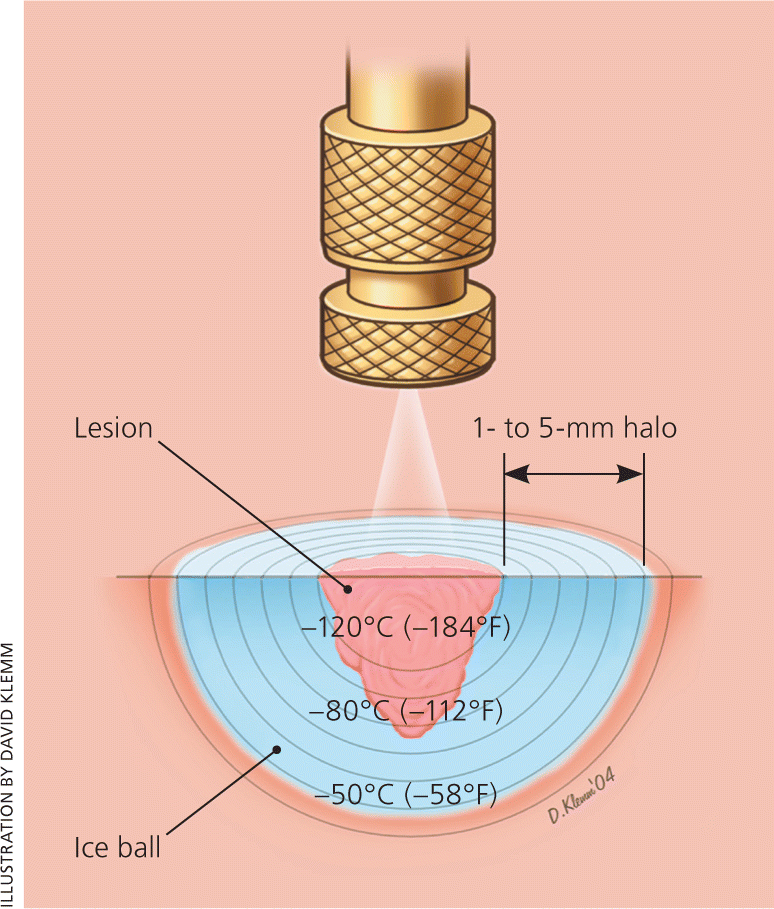
Treatment of skin cancer requires complete destruction of the tumor while minimizing damage to normal tissue. Risk of complications is higher with cryosurgery techniques, and referral to a dermatologist is typically recommended. It is an acceptable treatment for many basal cell carcinomas and, to a lesser extent, squamous cell carcinomas with low-risk features24,25 (Table 424 ). Some experts recommend shave excision or curettage before freezing to debulk the tumor and make it more amenable to cryosurgery.1 Freezing to a target temperature of –50°C to –60°C (–58°F to –76°F) and a 5-mm margin or depth is advisable (Figure 6). Inserting a needle with a thermocouple beneath the lesion can help to assure the clinician that adequate depth has been achieved.4 Cure rates of approximately 99 percent for nonmelanoma skin cancers have been documented with proper technique and are comparable with alternative treatments.1,2,26,27

| Depth < 3 mm |
| Diameter < 2 cm |
| Low-risk site (e.g., trunk, extremity, cheek, forehead, neck, scalp)* |
| Nodular or superficial basal cell carcinoma subtype (not sclerosing) |
| Not fixed to deeper structures |
| Primary lesion (not recurrent) |
| Well-defined margin |
| Well-differentiated squamous cell carcinoma |
Although not necessarily curable, Kaposi sarcoma, associated with human immunodeficiency virus infection, can be readily treated with cryosurgery via the removal of smaller lesions.28
Postoperative Care and Complications
Before cryosurgery, patients should be informed of the potential complications and expected adverse effects (Table 5).7,8,14 The freeze-thaw cycle immediately produces a moderate-intensity localized burning pain, which changes to a throbbing sensation within a few minutes and is accompanied by erythema and edema, especially following facial treatments.8,29 Hours later, a serous or hemorrhagic blister typically forms, which sloughs and dries over one to two weeks, producing an eschar.8 Complete healing typically occurs within two to four weeks for benign lesions, but may take up to six weeks for extensively treated malignant lesions.4
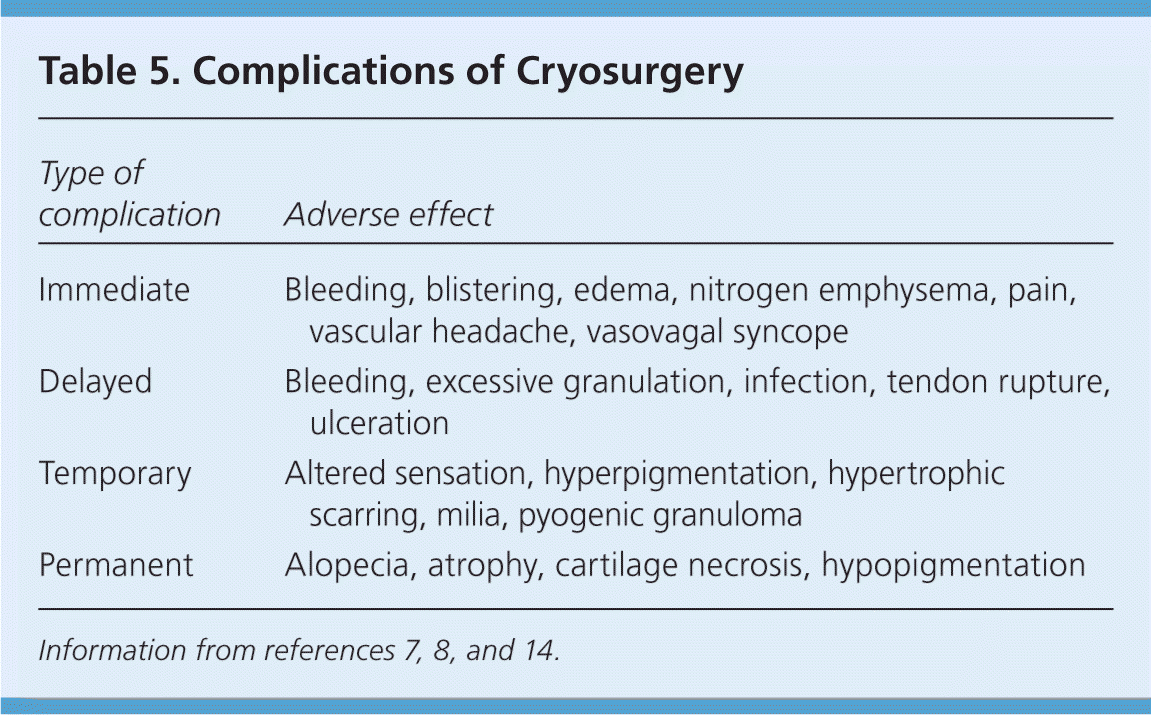
| Type of complication | Adverse effect |
|---|---|
| Immediate | Bleeding, blistering, edema, nitrogen emphysema, pain, vascular headache, vasovagal syncope |
| Delayed | Bleeding, excessive granulation, infection, tendon rupture, ulceration |
| Temporary | Altered sensation, hyperpigmentation, hypertrophic scarring, milia, pyogenic granuloma |
| Permanent | Alopecia, atrophy, cartilage necrosis, hypopigmentation |
Most treated lesions require no specific aftercare; dressings are best avoided. However, larger, extensively treated lesions may require soap and water cleansing once or twice daily.2 Large blisters may occasionally need to be lanced.8 Excessive postprocedure pain and swelling can be reduced with a short course of high-potency topical steroid cream.1,29
Serious complications from cryosurgery are rare. Hypopigmentation often occurs, resulting from the higher sensitivity of melanocytes to cold injury. It is much more prominent in patients with darker skin.14,29 Deeper freezes, such as for treatment of carcinoma, can destroy hair follicles, which may produce patches of alopecia. Although uncommon, a prolonged freeze can create scarring caused by disruption of the basement membrane and necrosis of the epidermis.8 Some degree of paresthesia occurs in one-fourth of patients and may persist for one to three months.2,8 Permanent sensory loss is rare, and is best prevented by avoiding cryosurgery over nerve trunks (e.g., preauricular areas).29 The risk and extent of scarring, alopecia, hypopigmentation, and paresthesia can all be reduced by keeping freeze times to less than 30 seconds.8,9
Nitrogen emphysema, produced by subcutaneous tracking and boiling of liquid nitrogen, is a painless yet alarming complication of liquid nitrogen therapy but is seldom encountered. Ulceration and infection are also rare, but are more likely to occur in patients with compromised circulation.8
Data Sources: A PubMed search was completed using the key term skin cryotherapy. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. Also searched were the Agency for Healthcare Research and Quality evidence reports, Bandolier, Clinical Evidence, FPIN's Clinical Inquiries database, the Cochrane database, the Institute for Clinical Systems Improvement, the National Guideline Clearinghouse database, Essential Evidence Plus, EBM Online, and UpToDate. Reference lists of recent review articles were also searched for additional articles. Search date: April 15, 2012.
