
Am Fam Physician. 2020;101(7):399-406
Related letter: Is Cutaneous Cryosurgery the Best Treatment Option for Cutaneous Warts?
Author disclosure: No relevant financial affiliations.
Cryosurgery is the application of freezing temperatures to achieve the destruction of tissue. Cutaneous cryosurgery has become a commonly performed outpatient procedure because of the combination of its safety, effectiveness, low cost, ease of use, lack of need for injectable anesthetic, and good cosmetic results. Cryosurgery may be performed in the outpatient setting using dipstick, spray, or cryoprobe techniques to treat a variety of benign, premalignant, and malignant skin lesions with high cure rates. Benign lesions such as common and plantar warts, anogenital condylomas, molluscum contagiosum, and seborrheic keratoses can be treated with cryotherapy. Basal and squamous cell carcinomas with low-risk features may be treated with cryosurgery. Contraindications to cryosurgery include neoplasms with indefinite margins or when pathology is desired, basal cell or squamous cell carcinomas with high-risk features, and prior adverse local reaction or hypersensitivity to cryosurgery. Potential adverse effects include bleeding, blistering, edema, paresthesia, and pain and less commonly include tendon rupture, scarring, alopecia, atrophy, and hypopigmentation.
Cryosurgery is the application of freezing temperatures to achieve the destruction of tissue.1 Cryosurgery is an effective and efficient method for treating a wide range of cardiac, dermatologic, ophthalmic, gynecologic, oncologic, neurologic, and urologic conditions. Cutaneous cryosurgery has become a commonly performed outpatient procedure because of its safety, effectiveness, low cost, ease of use, lack of need for injectable anesthetic, and good cosmetic results.1
| Clinical recommendation | Evidence rating | Comments |
|---|---|---|
| Cryosurgery is useful in the treatment of actinic keratoses with cure rates reported between 69% for freeze times of more than five seconds and 83% for freeze times of more than 20 seconds.18 | B | Limited evidence from single randomized controlled trial |
| Warts may be treated with cryotherapy administered at two-, three-, or four-week intervals without differences in cure rates.21 | A | Cochrane review with clear recommendation |
| Cryosurgery is as effective as daily treatment with salicylic acid in the treatment of plantar warts, with higher reported patient satisfaction.22 | B | Limited evidence from single randomized controlled trial |
Mechanism of Action
Commonly available cryogens include Freon 12, Freon 22, solid carbon dioxide, liquid nitrous oxide, liquid nitrogen, and liquid helium.2 The freons are typically used for skin anesthesia. Liquid nitrous oxide is effective in treating benign skin lesions; however, it is more commonly used for ophthalmic and gynecologic lesions. Liquid nitrogen has become the cryogen of choice in most clinical situations.3
Temperatures of −13°F to −58°F (−25°C to −50°C) can be achieved with liquid nitrogen within 30 seconds when using a spray or probe. Effective removal of malignant lesions typically requires lower temperatures (−40°F to −58°F [−40°C to −50°C]) achieved with the application of spray or probe. Liquid nitrogen used with the applicator method is useful in treating premalignant and benign lesions, requiring slightly higher temperatures of −4°F to −22°F (−20°C to −30°C).3
Equipment/Techniques
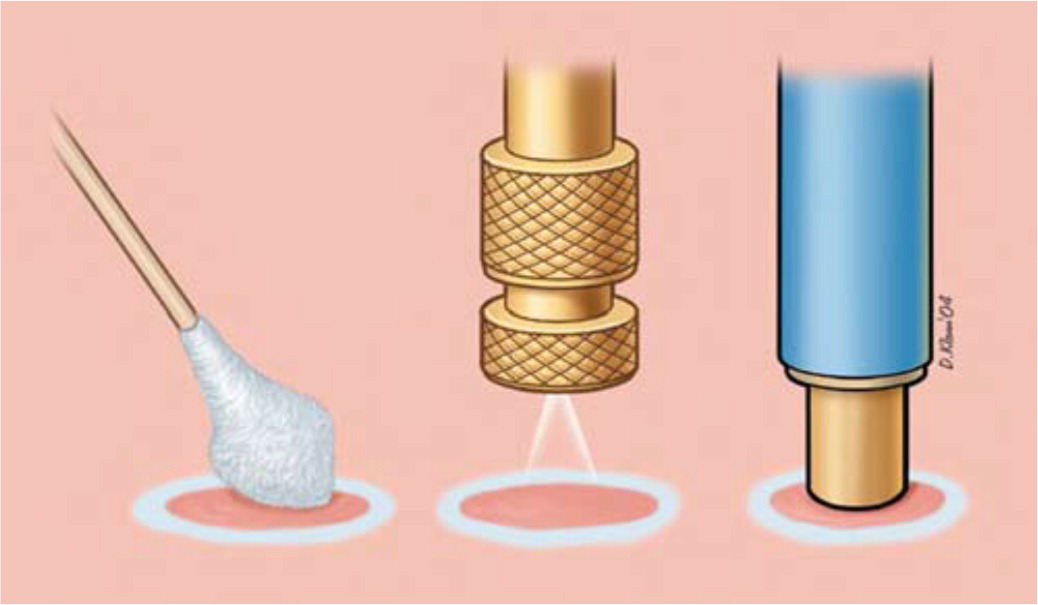
DIPSTICK
The dipstick technique involves dipping a cotton- or synthetic-tipped applicator into liquid nitrogen and applying it directly to the lesion. Applicators may be created using synthetic material balls and rolling on the end of an applicator stick; similarly, nonsterile 8-inch rayon-tipped swabs may be used.6 If greater application accuracy is required, the tip of the applicator may be rolled to a point or a “hard tail.”7 This method creates sufficient depth of freezing to treat nonmalignant skin lesions, such as actinic keratoses, angiomas, molluscum contagiosum, verrucae, and lentigo simplex.2,4 The choice of treatment technique is based on physician preference, available clinical resources, and the size and location of the lesions. Ordinary steel hot/cold liquid containers may be used to transport liquid nitrogen when using the dipstick method8 (Figure 3). Ensure that the cap to the container remains loose, allowing gas to escape, because building pressure from the nitrogen gas expansion can result in explosion and injury.

SPRAY
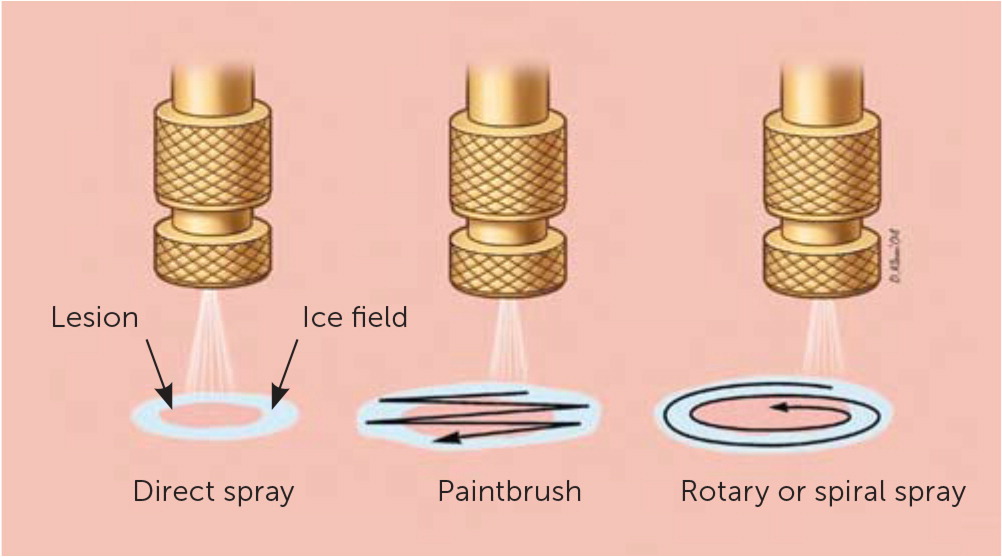
Commercially produced spray canisters apply a fine spray to the skin lesion with a variety of nozzles. The open-spray technique is useful in treating multiple, superficial, irregular skin lesions and lesions located on curved body surfaces. Lesions treated with the spray technique include actinic keratoses, seborrheic keratoses, cystic acne, verrucae, keratoacanthoma, and neoplasms.4
The cryoblast technique is useful for thick, hyperkeratotic lesions. To perform the cryoblast technique, the standard spray tip is removed from the handheld cryosurgery canister. The liquid nitrogen is applied in short one- to two-second pulses until the desired ice ball has been created.9 One small randomized controlled trial (n = 40) found higher cure rates at nine weeks with the cryoblast technique compared with the traditional cryospray technique in treating plantar warts10 (Figure 5).

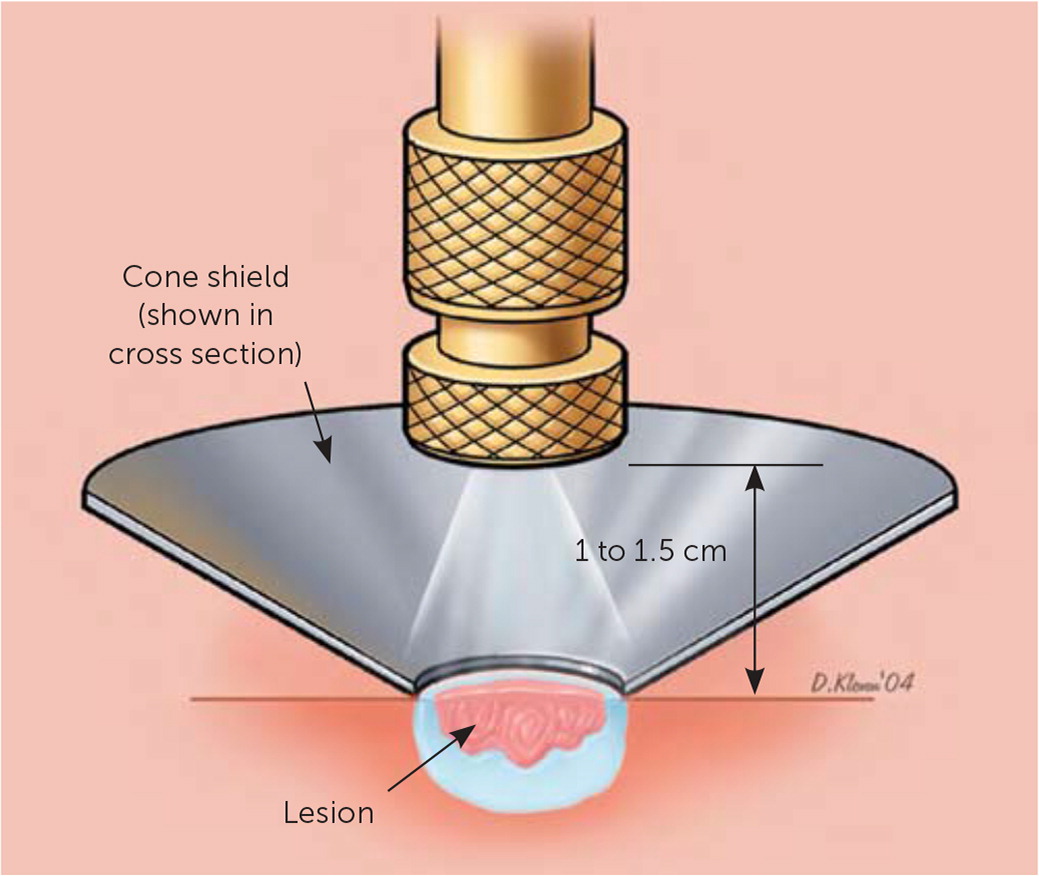
The timed spot-freeze technique provides for standardization of cryosurgery treatment. The freezing time is based on skin thickness, vascularity, tissue type, and lesion characteristics3 (Table 11–4,12,13). The liquid nitrogen is applied via a spray canister fitted with a variety of available nozzles. The nozzle is positioned 1 to 2 cm from the skin surface and aimed at the center of the lesion. The spray trigger is depressed, and liquid nitrogen is applied until the ice ball encompasses the lesion and the desired margin is established. Margins for benign lesions are commonly 1 to 2 mm, premalignant lesions require margins of 2 to 3 mm, and malignant lesions require margins of 4 to 5 mm to ensure adequate depth of freeze.1–4,12,13 Margins of this size allow for depth of freeze to ensure that a temperature of −58°F is reached to a depth of 4 to 5 mm.
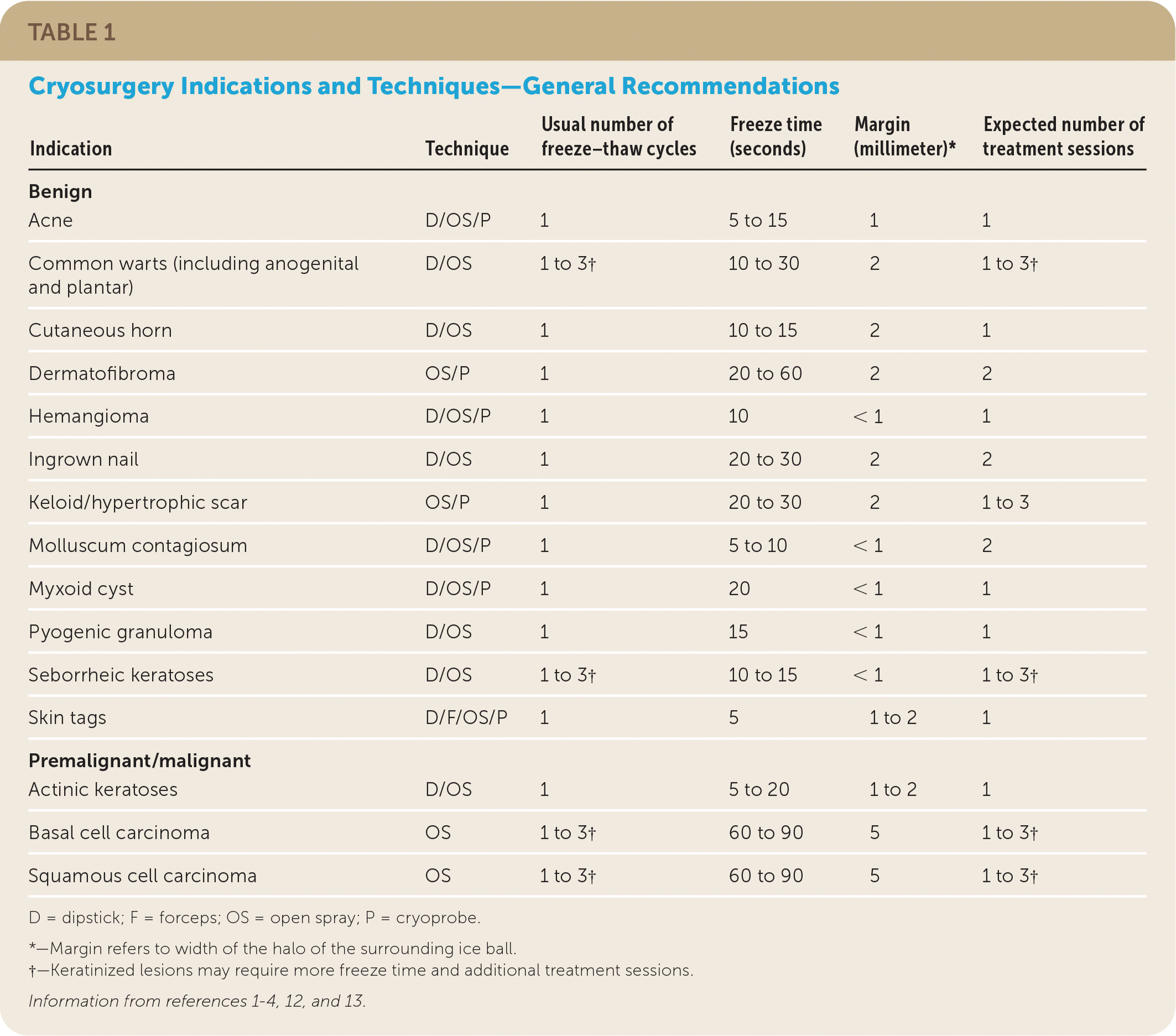
| Indication | Technique | Usual number of freeze–thaw cycles | Freeze time (seconds) | Margin (millimeter)* | Expected number of treatment sessions |
|---|---|---|---|---|---|
| Benign | |||||
| Acne | D/OS/P | 1 | 5 to 15 | 1 | 1 |
| Common warts (including anogenital and plantar) | D/OS | 1 to 3† | 10 to 30 | 2 | 1 to 3† |
| Cutaneous horn | D/OS | 1 | 10 to 15 | 2 | 1 |
| Dermatofibroma | OS/P | 1 | 20 to 60 | 2 | 2 |
| Hemangioma | D/OS/P | 1 | 10 | < 1 | 1 |
| Ingrown nail | D/OS | 1 | 20 to 30 | 2 | 2 |
| Keloid/hypertrophic scar | OS/P | 1 | 20 to 30 | 2 | 1 to 3 |
| Molluscum contagiosum | D/OS/P | 1 | 5 to 10 | < 1 | 2 |
| Myxoid cyst | D/OS/P | 1 | 20 | < 1 | 1 |
| Pyogenic granuloma | D/OS | 1 | 15 | < 1 | 1 |
| Seborrheic keratoses | D/OS | 1 to 3† | 10 to 15 | < 1 | 1 to 3† |
| Skin tags | D/F/OS/P | 1 | 5 | 1 to 2 | 1 |
| Premalignant/malignant | |||||
| Actinic keratoses | D/OS | 1 | 5 to 20 | 1 to 2 | 1 |
| Basal cell carcinoma | OS | 1 to 3† | 60 to 90 | 5 | 1 to 3† |
| Squamous cell carcinoma | OS | 1 to 3† | 60 to 90 | 5 | 1 to 3† |
After the desired margin of the ice ball has been achieved, the liquid nitrogen spray should be maintained by varying trigger pressure and flow of the liquid nitrogen spray to maintain the target margins for adequate freeze time. The time that is required to sustain the ice ball varies based on lesion type and may range from five to 30 seconds beyond the time the initial margins have been reached. If more than one freeze–thaw cycle is required, complete thawing should be allowed before the initiation of the next treatment (typically one to two minutes). This technique may be used to treat lesions up to 2 cm.
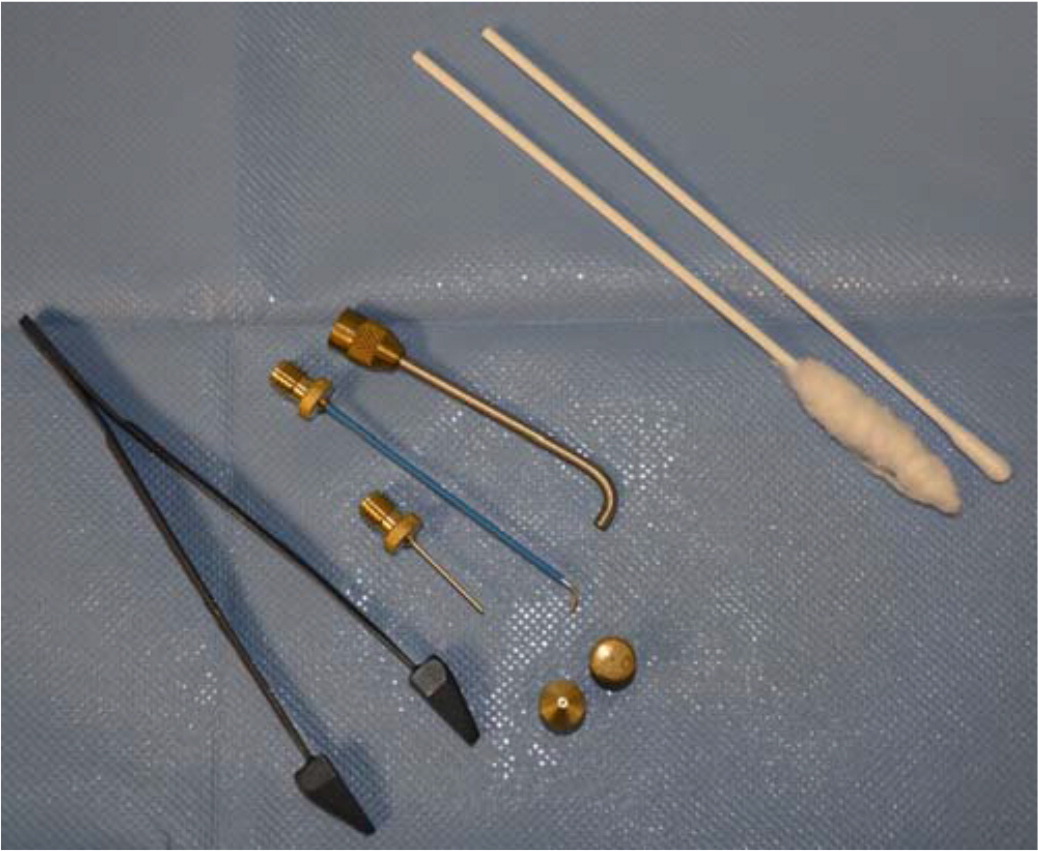
CRYOPROBE
The cryoprobe technique (i.e., contact therapy) involves the direct application of a cooled metal accessory directly against the skin lesion.4 Cryoprobes are useful for lesions that appear on flat surfaces, including the face or eyelids, and wherever there is concern regarding possible over-spray to sensitive nearby structures.1,3,4 A gel medium is routinely applied between the probe and the skin surface to improve surface contact.
Treatment/Clinical Application
Cryotherapy remains a useful approach for the treatment of many benign, premalignant, and malignant skin lesions and is a viable alternative to excision, electrodesiccation, and curettage. A useful video demonstrating the various cryosurgery techniques can be found at https://www.youtube.com/watch?v=K7DkK8myhj4. Physicians should engage their patients in shared decision-making to determine the most appropriate treatment modality. Biopsy should be performed before cryosurgery when neoplasm is suspected and to confirm the diagnosis when it is uncertain.
MALIGNANT LESIONS: BASAL CELL AND SQUAMOUS CELL CARCINOMAS
When a malignant skin lesion is suspected, biopsy should be performed to confirm the diagnosis and to establish the depth of tumor invasion. Cryosurgery is not a first-line treatment for malignant lesions; however, it remains a treatment option for low-risk lesions. Cryotherapy is not indicated for basal cell carcinoma lesions that are larger than 3 mm in depth, for recurrent lesions, or in areas of the body with a high risk of occurrence14,15 (low-risk features of basal and squamous cell carcinoma amenable to cryotherapy are summarized in Table 21,15–18). The goal of treatment with cryosurgery is to destroy the same amount of tissue that would otherwise be removed via local excision.4 A surrounding ice ball of 3 to 5 mm greater than the lesion is recommended with use of any of the cryosurgical techniques. Although expert opinion varies on the number of required freeze–thaw cycles, all of the opinions support multiple cycles in the treatment of malignant lesions. The need for repeat treatment sessions is dependent on clinical response. Significantly raised lesions can benefit from curettage and debulking before cryotherapy.1 Clinical monitoring after treatment for recurrence is recommended because recurrence typically occurs within the first two years following cryotherapy.4 A systematic review of 118 observational studies found that the recurrence of squamous cell carcinoma was lowest after cryotherapy; however, the lesions were small and low risk.17
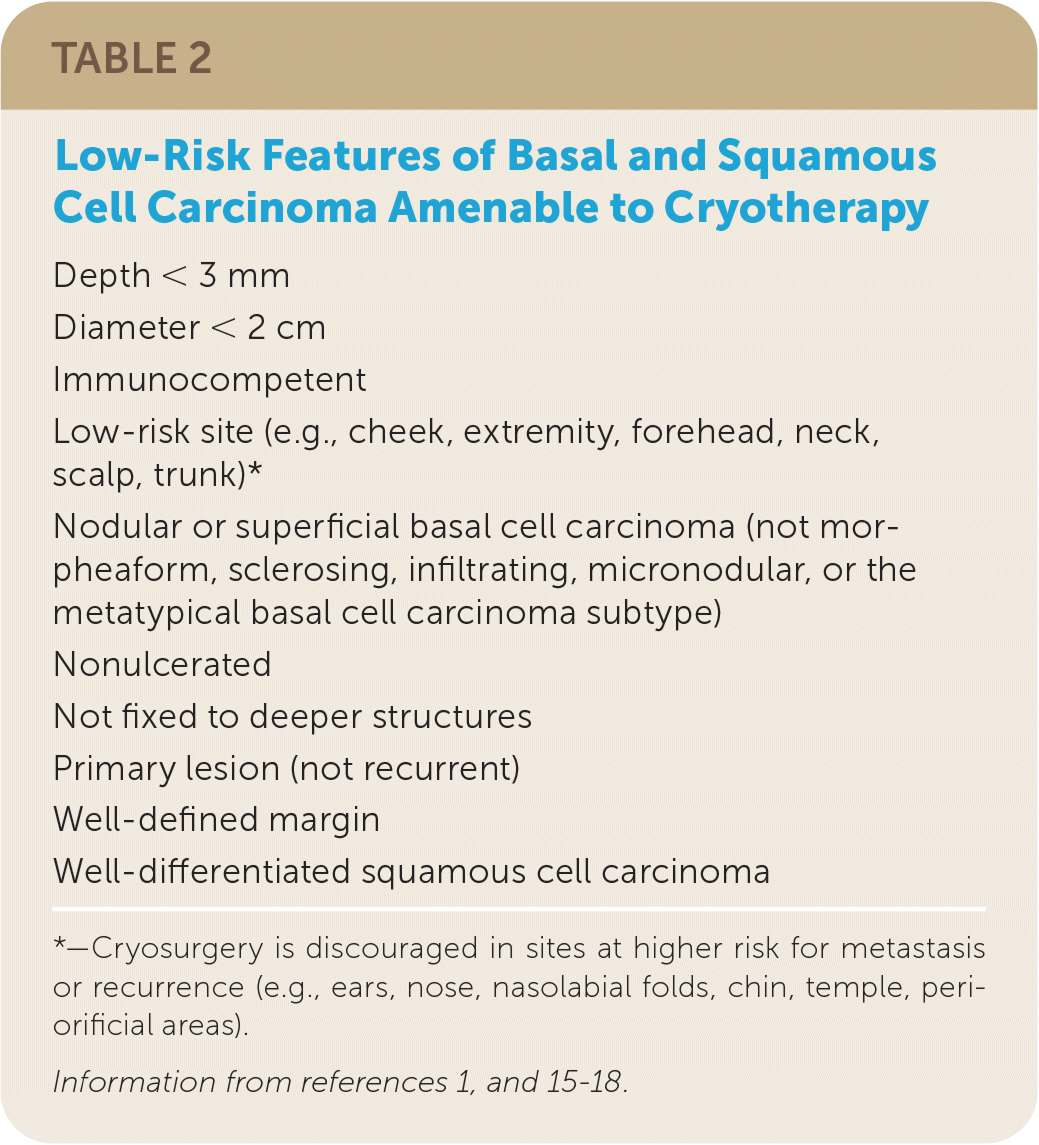
| Depth < 3 mm |
| Diameter < 2 cm |
| Immunocompetent |
| Low-risk site (e.g., cheek, extremity, forehead, neck, scalp, trunk)* |
| Nodular or superficial basal cell carcinoma (not morpheaform, sclerosing, infiltrating, micronodular, or the metatypical basal cell carcinoma subtype) |
| Nonulcerated |
| Not fixed to deeper structures |
| Primary lesion (not recurrent) |
| Well-defined margin |
| Well-differentiated squamous cell carcinoma |
LESIONS DUE TO SUN DAMAGE: ACTINIC AND SEBORRHEIC KERATOSES
Cryotherapy is indicated in the treatment of benign lesions associated with sun damage, such as premalignant actinic keratoses. Treatment of such lesions typically requires one freeze–thaw cycle with an adequate amount of freeze time.4 In a prospective, multicenter study (N = 90; 421 actinic keratoses), complete cure rates for actinic keratoses treated with cryotherapy were found to be 39% for freeze times of less than five seconds, 69% for freeze times of more than five seconds, and 83% for freeze times of more than 20 seconds.18 However, a Cochrane review found that in the treatment of an individual actinic keratosis, photodynamic therapy appears to be more effective with better cosmetic outcomes than cryotherapy.19
Seborrheic keratoses often require cryotherapy with longer treatment times for adequate ice ball development and multiple freeze–thaw cycles. Repeat treatments may be needed. In seborrheic keratoses that are flat and small, a single cycle of shorter duration is likely adequate, dependent on the characteristics of the tissue and response.4
VIRAL LESIONS: VERRUCA AND MOLLUSCUM
Clearance of verrucous lesions is variable, depending on the size of the lesion and the degree of hyperkeratosis. Clearance rates following cryosurgery range from 39% to 84% at three months. Several treatment sessions may be required; therefore, expectations should be discussed with the patient.20 For deep plantar warts, improved cure rates can be achieved by pretreatment with keratolytics (i.e., salicylic acid) followed by shaving the hyperkeratotic tissue and subsequent cryotherapy treatment. A Cochrane review found no significant difference in cure rates between cryotherapy administered at two-, three-, and four-week intervals for the treatment of warts.21 Also, the Cochrane review did not demonstrate significant difference between salicylic acid and cryotherapy or difference between cryotherapy and placebo.21 A randomized controlled trial (n = 240) found no difference in clearance rates in the treatment of warts comparing repeat cryosurgery with daily salicylic acid (34% vs. 31% at six months, P = .64), although patient satisfaction was higher with cryosurgery.22
Cryosurgery may be used to treat anogenital condylomas, when podophyllin is ineffective or difficult to apply based on location of the lesions. Molluscum contagiosum can be treated with cryotherapy if the patient is able to tolerate treatment.
Contraindications
Absolute contraindications to cryosurgery are rare. Relative contraindications to cryosurgery are largely associated with concomitant illnesses (Table 3).1–3,23 Cryosurgery should not be performed on skin lesions with irregular borders or on lesions in which subsequent pathology is required because histological examination of the lesion is not possible after cryotherapy.1,2,4 Care should be taken to avoid overtreatment (shorter durations, fewer freeze–thaw cycles, confined spray technique) when treating over bony prominences and in areas where nerves are superficial (such as sides of digits or in areas where the facial nerve may be affected) to avoid full thickness skin loss and damage to underlying structures.23
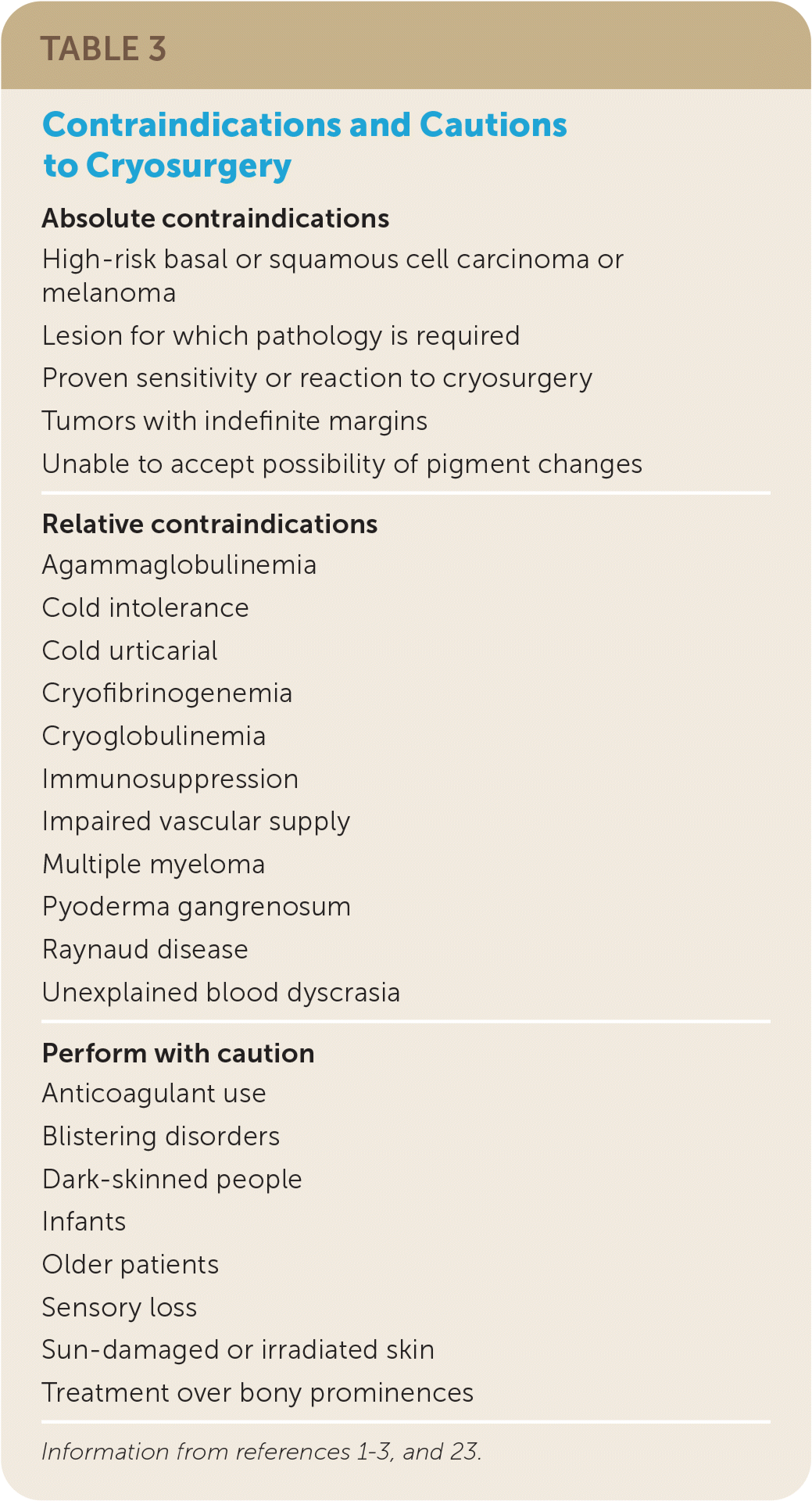
| Absolute contraindications |
| High-risk basal or squamous cell carcinoma or melanoma |
| Lesion for which pathology is required |
| Proven sensitivity or reaction to cryosurgery |
| Tumors with indefinite margins |
| Unable to accept possibility of pigment changes |
| Relative contraindications |
| Agammaglobulinemia |
| Cold intolerance |
| Cold urticarial |
| Cryofibrinogenemia |
| Cryoglobulinemia |
| Immunosuppression |
| Impaired vascular supply |
| Multiple myeloma |
| Pyoderma gangrenosum |
| Raynaud disease |
| Unexplained blood dyscrasia |
| Perform with caution |
| Anticoagulant use |
| Blistering disorders |
| Dark-skinned people |
| Infants |
| Older patients |
| Sensory loss |
| Sun-damaged or irradiated skin |
| Treatment over bony prominences |
Complications
Before performing cryosurgery, all patients should be counseled on possible complications, and informed consent should be obtained1,3,12,23,24 (Table 41,2,12,23,24). The immediate reaction from cryosurgery may be described as a burning pain during freezing that changes to a throbbing pain during the thaw phase.1,12,23 Treatment of the plantar aspect of the foot, pulp, and periungual area of the fingers; helix and concha of the ear; eyelids; lips; and mucous membranes can be the most painful. A local anesthetic can be considered when treating in these areas.2,23 Headaches may develop when treating the forehead, temples, and scalp.12,23,24
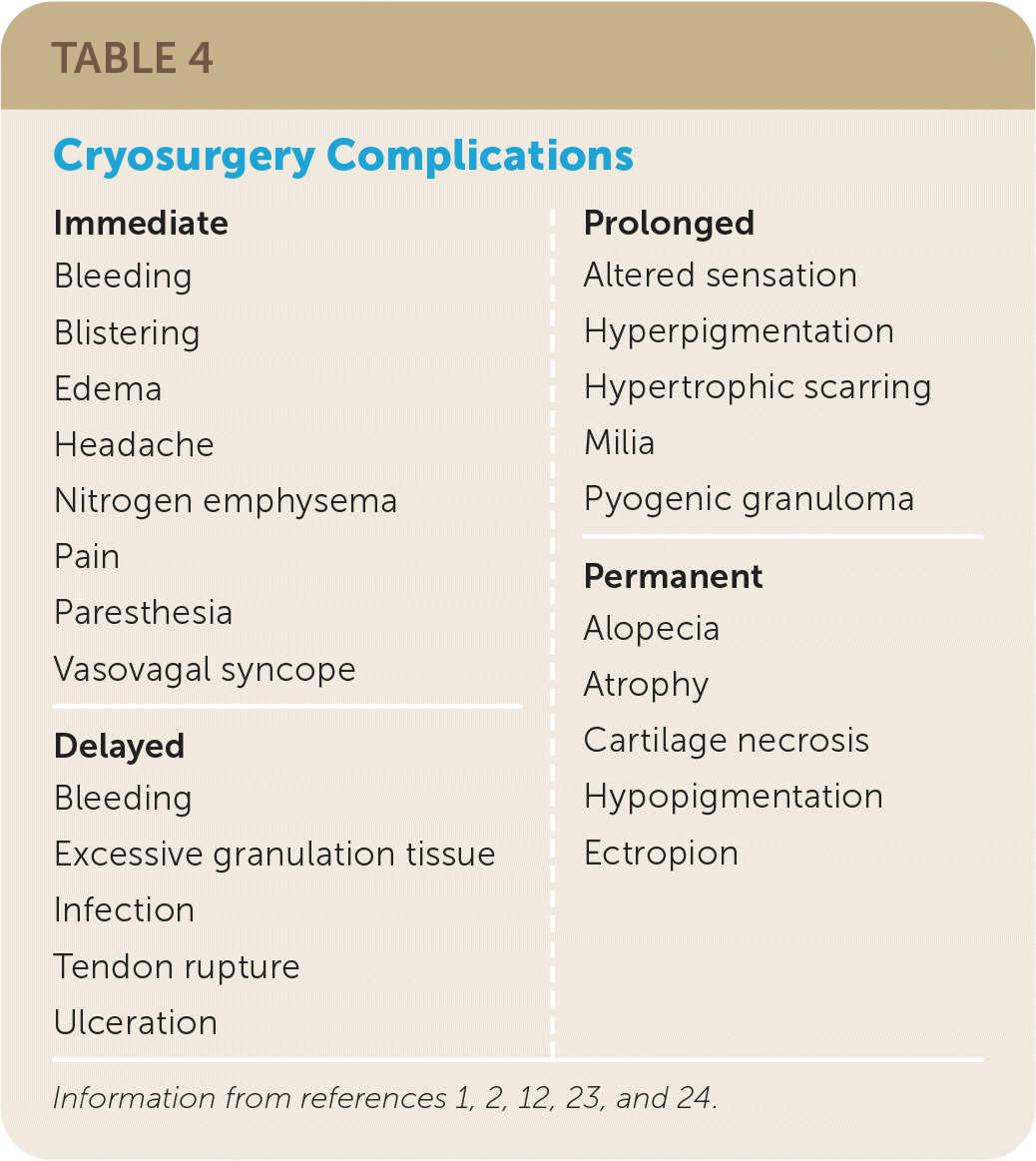
| Immediate | Prolonged |
| Bleeding | Altered sensation |
| Blistering | Hyperpigmentation |
| Edema | Hypertrophic scarring |
| Headache | Milia |
| Nitrogen emphysema | Pyogenic granuloma |
| Pain | Permanent |
| Paresthesia | Alopecia |
| Vasovagal syncope | Atrophy |
| Delayed | Cartilage necrosis |
| Bleeding | Hypopigmentation |
| Excessive granulation tissue | Ectropion |
| Infection | |
| Tendon rupture | |
| Ulceration |
Edema usually occurs within minutes of treatment and can be severe in the periorbital, forehead, lips, or labia minora regions.12,23 Blisters commonly form within a few hours and usually do not require drainage unless they become large and painful.1,23 Hemorrhage is rare and can be controlled by direct pressure immediately after treatment. Delayed hemorrhage may occur up to 14 days posttreatment and is typically a large bleed resulting from arterial or arteriolar necrosis.12,23
Melanocytes have a greater sensitivity to cold injury causing hypopigmentation, especially in darker skin. Alopecia can occur following deeper freezes because of destruction of the hair follicle. Scarring is uncommon but can occur after a prolonged freeze because of destruction of the basement membrane.1,12,23 Paresthesia may occur in the patient or the physician (by inadvertent application or overspray of the cryotherapy) after treatment and can last for one to three months; permanent nerve damage is rare.1,2 Keeping freeze times under 30 seconds can decrease the risk of scarring, alopecia, hypopigmentation, and paresthesia.1,2,12
Nitrogen emphysema is a rare, painless complication that occurs when treating lesions with an open wound. It is produced by the expansion of liquid nitrogen through the subcutaneous tissue as it boils. Nitrogen emphysema can be treated by applying gentle pressure to allow the trapped gas to dissipate.1,2,12,23
Postoperative Care
Postoperative wound care instructions, expectations for routine healing, and signs of complication should be reviewed with the patient. Larger lesions and malignancies should be washed with soap and water one to two times daily.2,24 The treatment site should be left open, but dressings may be used if necessary.1,23 Significant edema can be reduced with a short course of a high-potency topical steroid.1,4,12 Typically, benign and premalignant lesions heal within two to four weeks, but for large areas requiring treatment, healing can take up to 14 weeks.1,4
Billing
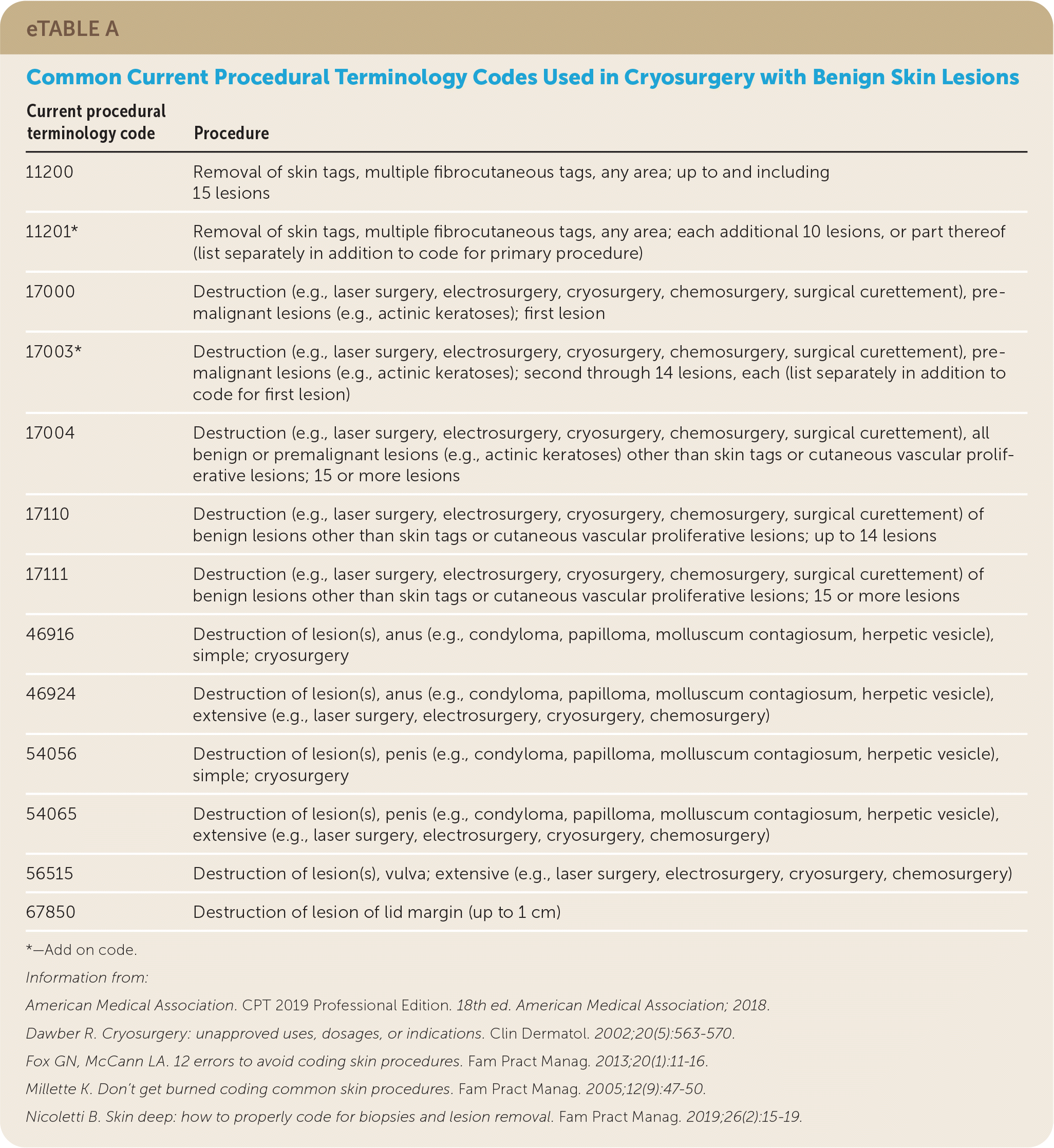
| Current procedural terminology code | Procedure |
|---|---|
| 11200 | Removal of skin tags, multiple fibrocutaneous tags, any area; up to and including 15 lesions |
| 11201* | Removal of skin tags, multiple fibrocutaneous tags, any area; each additional 10 lesions, or part thereof (list separately in addition to code for primary procedure) |
| 17000 | Destruction (e.g., laser surgery, electrosurgery, cryosurgery, chemosurgery, surgical curettement), premalignant lesions (e.g., actinic keratoses); first lesion |
| 17003* | Destruction (e.g., laser surgery, electrosurgery, cryosurgery, chemosurgery, surgical curettement), premalignant lesions (e.g., actinic keratoses); second through 14 lesions, each (list separately in addition to code for first lesion) |
| 17004 | Destruction (e.g., laser surgery, electrosurgery, cryosurgery, chemosurgery, surgical curettement), all benign or premalignant lesions (e.g., actinic keratoses) other than skin tags or cutaneous vascular proliferative lesions; 15 or more lesions |
| 17110 | Destruction (e.g., laser surgery, electrosurgery, cryosurgery, chemosurgery, surgical curettement) of benign lesions other than skin tags or cutaneous vascular proliferative lesions; up to 14 lesions |
| 17111 | Destruction (e.g., laser surgery, electrosurgery, cryosurgery, chemosurgery, surgical curettement) of benign lesions other than skin tags or cutaneous vascular proliferative lesions; 15 or more lesions |
| 46916 | Destruction of lesion(s), anus (e.g., condyloma, papilloma, molluscum contagiosum, herpetic vesicle), simple; cryosurgery |
| 46924 | Destruction of lesion(s), anus (e.g., condyloma, papilloma, molluscum contagiosum, herpetic vesicle), extensive (e.g., laser surgery, electrosurgery, cryosurgery, chemosurgery) |
| 54056 | Destruction of lesion(s), penis (e.g., condyloma, papilloma, molluscum contagiosum, herpetic vesicle), simple; cryosurgery |
| 54065 | Destruction of lesion(s), penis (e.g., condyloma, papilloma, molluscum contagiosum, herpetic vesicle), extensive (e.g., laser surgery, electrosurgery, cryosurgery, chemosurgery) |
| 56515 | Destruction of lesion(s), vulva; extensive (e.g., laser surgery, electrosurgery, cryosurgery, chemosurgery) |
| 67850 | Destruction of lesion of lid margin (up to 1 cm) |
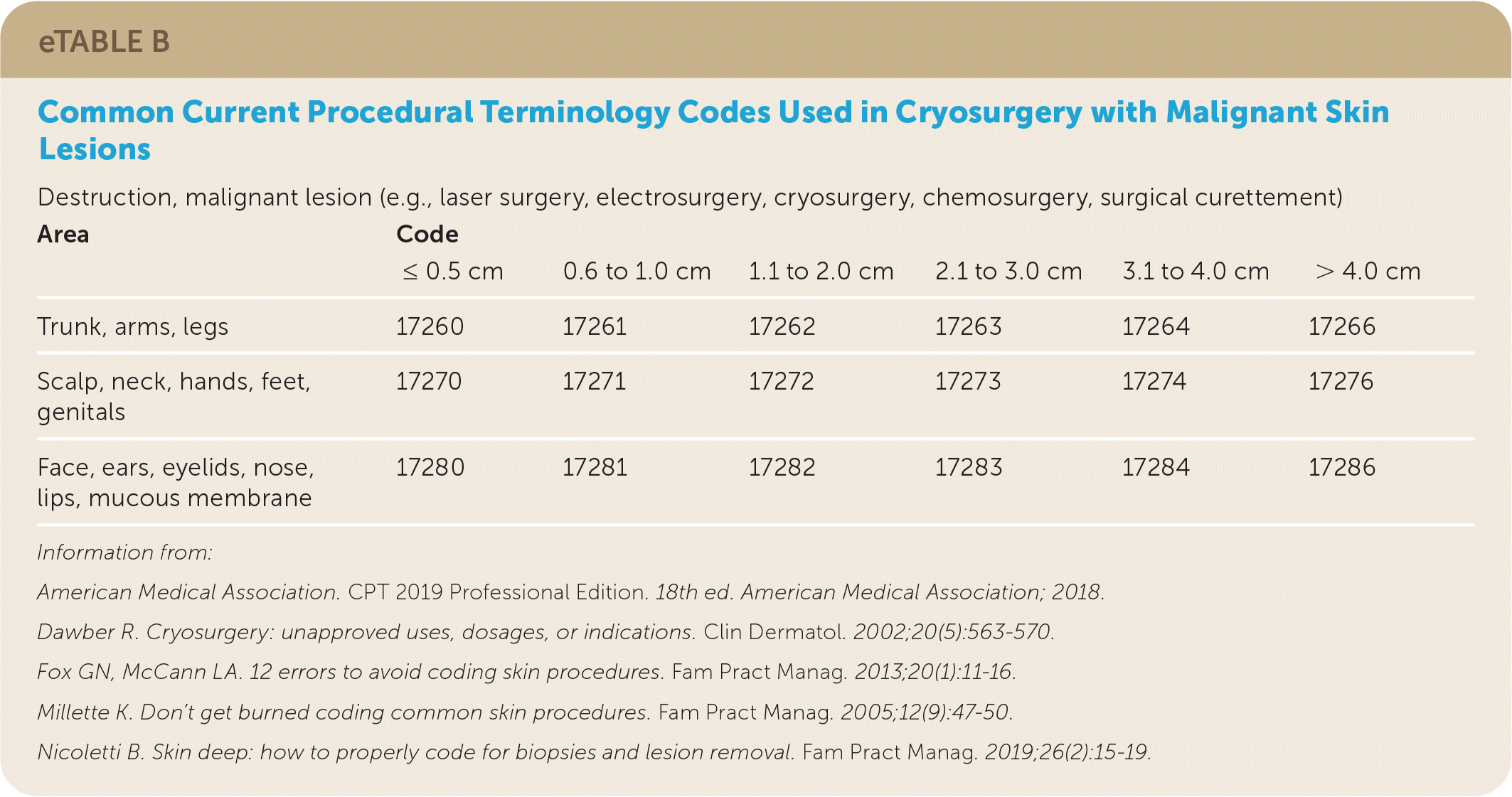
| Area | Code | |||||
|---|---|---|---|---|---|---|
| ≤ 0.5 cm | 0.6 to 1.0 cm | 1.1 to 2.0 cm | 2.1 to 3.0 cm | 3.1 to 4.0 cm | > 4.0 cm | |
| Trunk, arms, legs | 17260 | 17261 | 17262 | 17263 | 17264 | 17266 |
| Scalp, neck, hands, feet, genitals | 17270 | 17271 | 17272 | 17273 | 17274 | 17276 |
| Face, ears, eyelids, nose, lips, mucous membrane | 17280 | 17281 | 17282 | 17283 | 17284 | 17286 |
This article updates previous articles by Zimmerman and Crawford1 and Andrews.3
Data Sources: A PubMed search was completed using the mesh term cryosurgery and the key terms cryotherapy, skin, or dermatology. This search included meta-analyses, randomized controlled trials, and reviews. The authors also searched the Cochrane database, Dynamed, FPIN's Clinical Inquiries database, and Essential Evidence Plus. Reference lists were also searched for additional articles. Search dates: May 12, 2019, and December 11, 2019.
