
Am Fam Physician. 2013;87(4):267-273
A more recent article on Parkinson disease is available.
Related letter: Risks of Deep Brain Stimulation for Parkinson Disease.
Patient information: See related handout on Parkinson disease, written by the authors of this article.
Author disclosure: No relevant financial affiliations to disclose.
Parkinson disease is a progressive neurologic disorder afflicting approximately 1 percent of Americans older than 60 years. The cardinal features of Parkinson disease are bradykinesia, rigidity, tremor, and postural instability. There are a number of neurologic conditions that mimic the disease, making it difficult to diagnose in its early stages. Physicians who rarely diagnose Parkinson disease should refer patients suspected of having it to physicians with more experience in making the diagnosis, and should periodically reevaluate the accuracy of the diagnosis. Treatment is effective in reducing motor impairment and disability, and should be started when a patient begins to experience functional impairment. The combination of carbidopa and levodopa is the most effective treatment, but dopamine agonists and monoamine oxidase-B inhibitors are also effective, and are less likely to cause dyskinesias. For patients taking carbidopa/levodopa who have motor complications, adjunctive therapy with a dopamine agonist, a monoamine oxidase-B inhibitor, or a catechol O-methyltransferase inhibitor will improve motor symptoms and functional status, but with an increase in dyskinesias. Deep brain stimulation is effective in patients who have poorly controlled symptoms despite optimal medical therapy. Occupational, physical, and speech therapy improve patient function. Fatigue, sleep disturbances, dementia, and depression are common in patients with Parkinson disease. Although these conditions are associated with significantly lower quality of life, they may improve with treatment.
Parkinson disease is a progressive neurodegenerative disorder that is pathologically defined by degeneration of the dopaminergic neurons in the substantia nigra and development of Lewy bodies in the residual dopaminergic neurons.1 Pathologic changes may be detected up to 20 years before the onset of motor symptoms, and are accompanied by a clinical prodrome of nonspecific symptoms such as hyposmia, constipation, and fatigue.2 The disease affects approximately 1 percent of persons older than 60 years, and up to 4 percent of those older than 80 years.3
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Physicians who have limited experience caring for patients with Parkinson disease should consider referring a patient with suspected disease to a physician who has expertise in movement disorders to confirm the diagnosis. | C | 4, 11 |
| Carbidopa/levodopa (Sinemet), nonergot dopamine agonists, or monoamine oxidase-B inhibitors should be used for initial treatment of Parkinson disease. | A | 4, 11, 15 |
| Nonergot dopamine agonists, catechol O-methyltransferase inhibitors, or monoamine oxidase-B inhibitors should be added to levodopa to treat motor complications in advanced Parkinson disease. | A | 4, 11, 21, 23 |
| Amantadine should be considered for treatment of dyskinesias in patients with advanced Parkinson disease. | B | 4, 11, 21 |
| Deep brain stimulation should be offered to patients with functional impairment despite optimal medical treatment, but it should be performed in experienced centers, and it carries a risk of serious adverse effects. | B | 25, 26 |
| Physical therapy should be offered to patients with Parkinson disease to improve gait, and speech therapy should be offered to improve speech volume. | B | 11, 20 |
| Occupational therapy may help patients with Parkinson disease to maintain family, social, and work roles; continue activities of daily living; and improve safety and motor function. | C | 11 |
Diagnosis
The diagnosis of Parkinson disease is clinical, and relies on the presence of the cardinal features of bradykinesia, rigidity, tremor, and postural instability, coupled with gradual symptom progression and a sustained response to therapy with levodopa.4 However, some of these features are shared by other neurologic conditions. Conditions commonly misdiagnosed as Parkinson disease include nonparkinsonian tremors such as essential tremor, and diseases with parkinsonian features such as vascular parkinsonism, progressive supranuclear palsy, and drug-induced parkinsonism.5,6 Table 1 describes common disorders to consider in the differential diagnosis.1,4,7–9
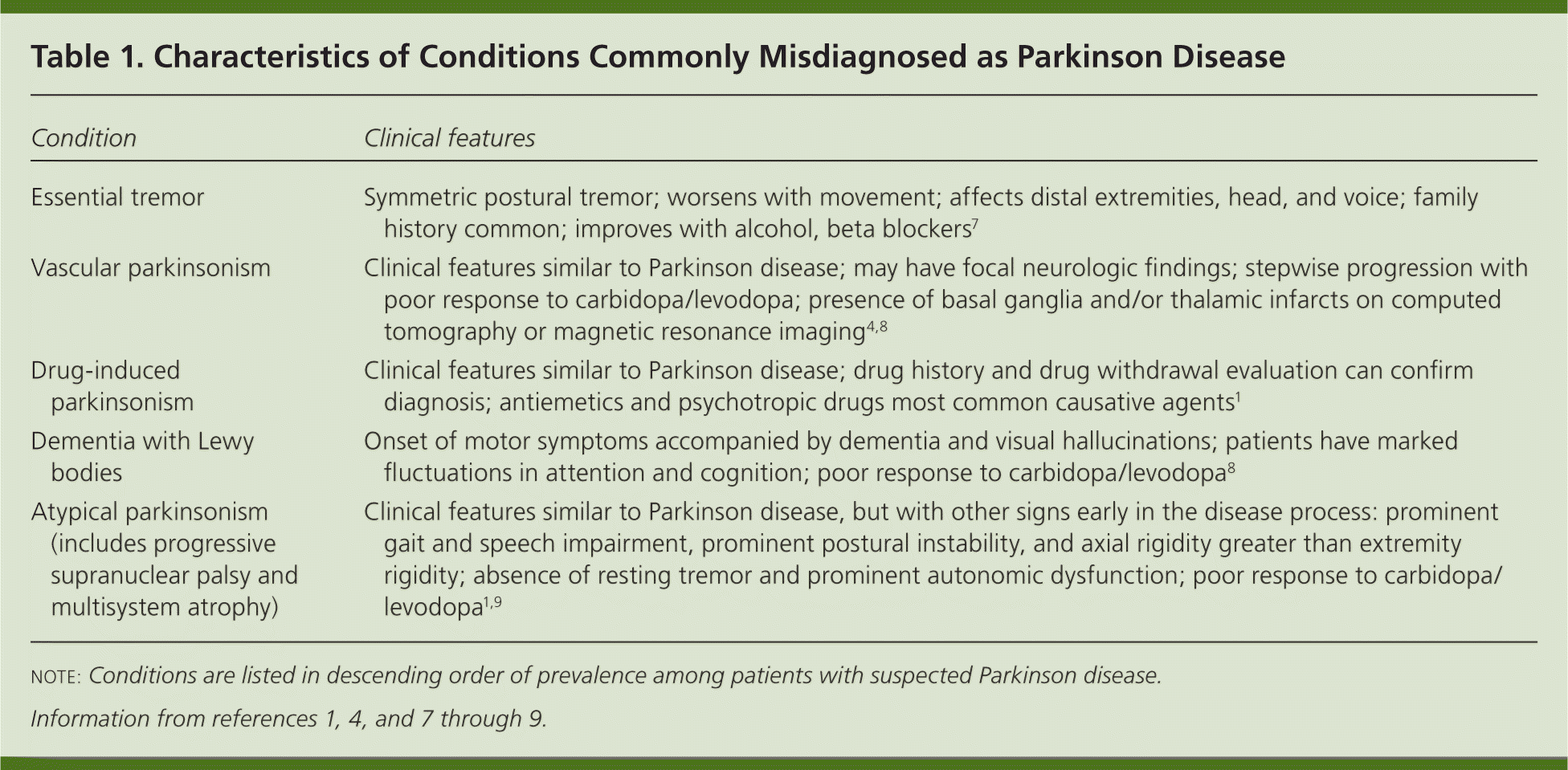
| Condition | Clinical features |
|---|---|
| Essential tremor | Symmetric postural tremor; worsens with movement; affects distal extremities, head, and voice; family history common; improves with alcohol, beta blockers7 |
| Vascular parkinsonism | Clinical features similar to Parkinson disease; may have focal neurologic findings; stepwise progression with poor response to carbidopa/levodopa; presence of basal ganglia and/or thalamic infarcts on computed tomography or magnetic resonance imaging4,8 |
| Drug-induced parkinsonism | Clinical features similar to Parkinson disease; drug history and drug withdrawal evaluation can confirm diagnosis; antiemetics and psychotropic drugs most common causative agents1 |
| Dementia with Lewy bodies | Onset of motor symptoms accompanied by dementia and visual hallucinations; patients have marked fluctuations in attention and cognition; poor response to carbidopa/levodopa8 |
| Atypical parkinsonism (includes progressive supranuclear palsy and multisystem atrophy) | Clinical features similar to Parkinson disease, but with other signs early in the disease process: prominent gait and speech impairment, prominent postural instability, and axial rigidity greater than extremity rigidity; absence of resting tremor and prominent autonomic dysfunction; poor response to carbidopa/levodopa1,9 |
Features that increase the likelihood of Parkinson disease include those associated with bradykinesia, such as micrographia, a shuffling walk, and difficulties performing motor tasks such as turning in bed, rising from a chair, or opening jars.10 Features that make Parkinson disease less likely include falls in the early stages of the disease, poor response to levodopa, symmetry at onset, rapid progression, lack of tremor, and dysautonomia.9
The diagnosis of Parkinson disease is difficult and diagnostic error is common, particularly in the early stages.5,6 A physician who rarely diagnoses Parkinson disease should consider referring a patient suspected of having it to a physician who has more experience with the disease to confirm the diagnosis.4,11 No clinical decision rules are of proven usefulness in diagnosing early disease,4 although the Parkinson's UK Brain Bank criteria improve diagnostic accuracy in patients with advanced disease.11 Given the inherent uncertainty of diagnosis in early disease and the increasing diagnostic accuracy with disease progression, physicians caring for patients with Parkinson disease should periodically reevaluate the diagnosis.4
ROLE OF IMAGING IN DIAGNOSIS
Imaging plays a limited role in diagnosis and should not be used routinely.4,11 Imaging may help when the clinical presentation makes it difficult to differentiate Parkinson disease from another disorder with similar characteristics. For example, limited-quality studies indicate that magnetic resonance imaging may help differentiate the disease from progressive supranuclear palsy.4 Better data support using single-photon emission computed tomography to distinguish the disease from essential tremor.4,11 Table 2 summarizes current recommendations for imaging in the diagnosis of Parkinson disease.4,9,11
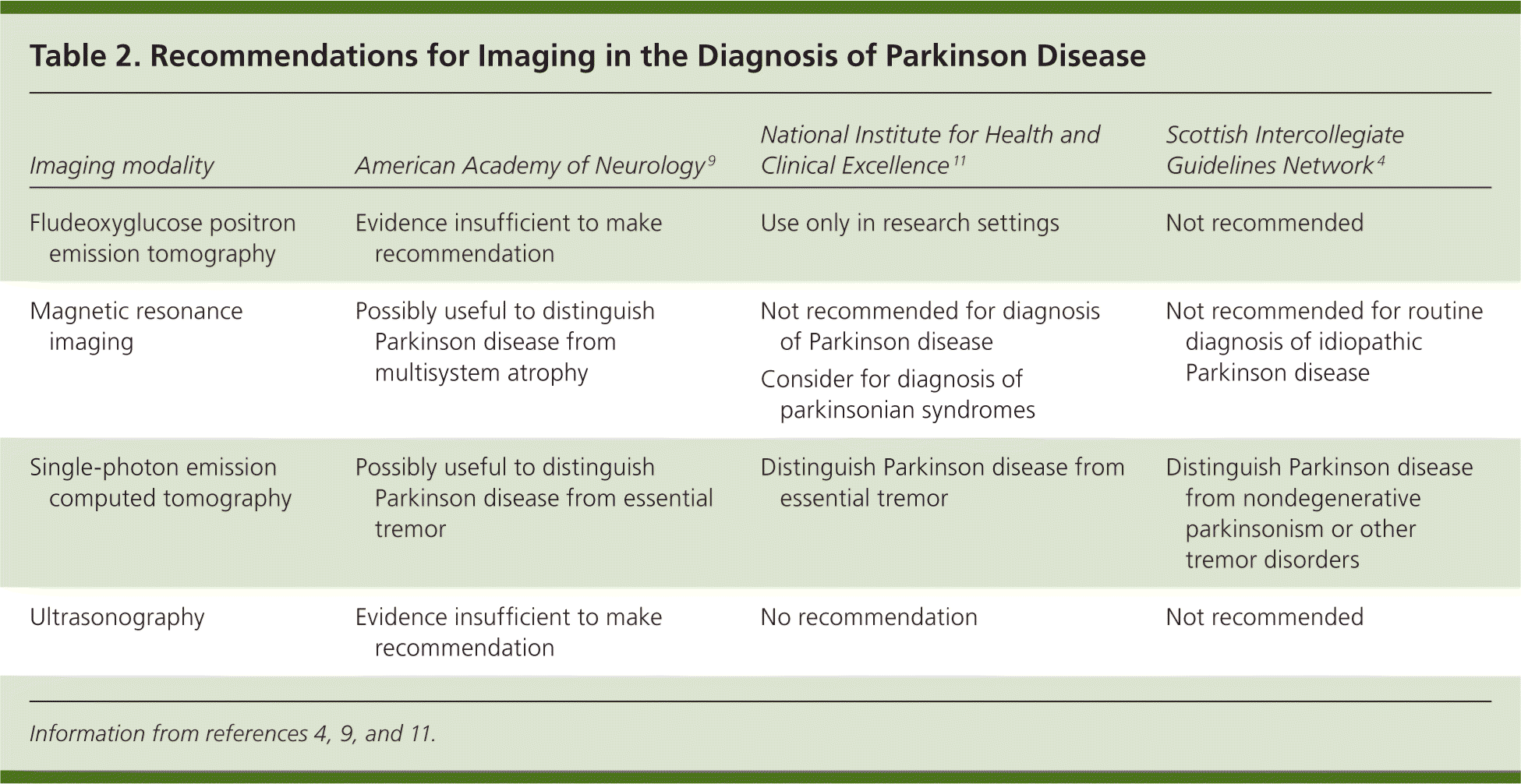
| Imaging modality | American Academy of Neurology9 | National Institute for Health and Clinical Excellence11 | Scottish Intercollegiate Guidelines Network4 |
|---|---|---|---|
| Fludeoxyglucose positron emission tomography | Evidence insufficient to make recommendation | Use only in research settings | Not recommended |
| Magnetic resonance imaging | Possibly useful to distinguish Parkinson disease from multisystem atrophy | Not recommended for diagnosis of Parkinson disease | Not recommended for routine diagnosis of idiopathic Parkinson disease |
| Consider for diagnosis of parkinsonian syndromes | |||
| Single-photon emission computed tomography | Possibly useful to distinguish Parkinson disease from essential tremor | Distinguish Parkinson disease from essential tremor | Distinguish Parkinson disease from nondegenerative parkinsonism or other tremor disorders |
| Ultrasonography | Evidence insufficient to make recommendation | No recommendation | Not recommended |
Prognosis
Patients with Parkinson disease experience progressive decline in motor and cognitive function and increased mortality. Risk factors for more rapid decline in motor function include older age at diagnosis, and prominent bradykinesia and rigidity at diagnosis. Prominent tremor at diagnosis may predict a slower rate of disease progression.9 The incidence of dementia increases with patient age and duration of Parkinson disease, with 60 percent of patients who have the disease developing dementia within 12 years of diagnosis.12 In the Physicians' Health Study, which enrolled 22,071 male physicians between 40 and 83 years of age, the adjusted relative risk of mortality for the 560 men who developed the disease during 23 years of follow-up was 2.3.13 The relative risk of mortality was 1.8 in a longitudinal Dutch cohort of 6,969 men and women.14 In a community-based cohort in Norway, men with Parkinson disease at age 70 had a median life expectancy of eight years, and women with Parkinson disease at age 70 had a median life expectancy of 11 years.12
Treatment of Motor Symptoms
EARLY MEDICAL THERAPY
The American Academy of Neurology recommends initiating treatment once patients develop functional disability.15 Levodopa, nonergot dopamine agonists, and monoamine oxidase-B inhibitors can be used for initial therapy 4,11,15 (Table 3). Levodopa is administered with carbidopa, which inhibits the peripheral metabolism of levodopa, thereby allowing therapeutic concentrations of levodopa to enter the brain without disabling adverse effects. The combination of carbidopa and levodopa (Sinemet) is the most effective agent available for the treatment of motor symptoms. However, its early use is associated with earlier development of dyskinesias (abnormal involuntary movements). Dopamine agonists such as pramipexole (Mirapex) and ropinirole (Requip) directly stimulate dopamine receptors. They are less effective than levodopa in treating motor symptoms of Parkinson disease, but have a lower incidence of dyskinesias. Compared with carbidopa/levodopa, dopamine agonists cause more sleepiness, edema, nausea, and hallucinations, and have higher dropout rates in clinical trials.16
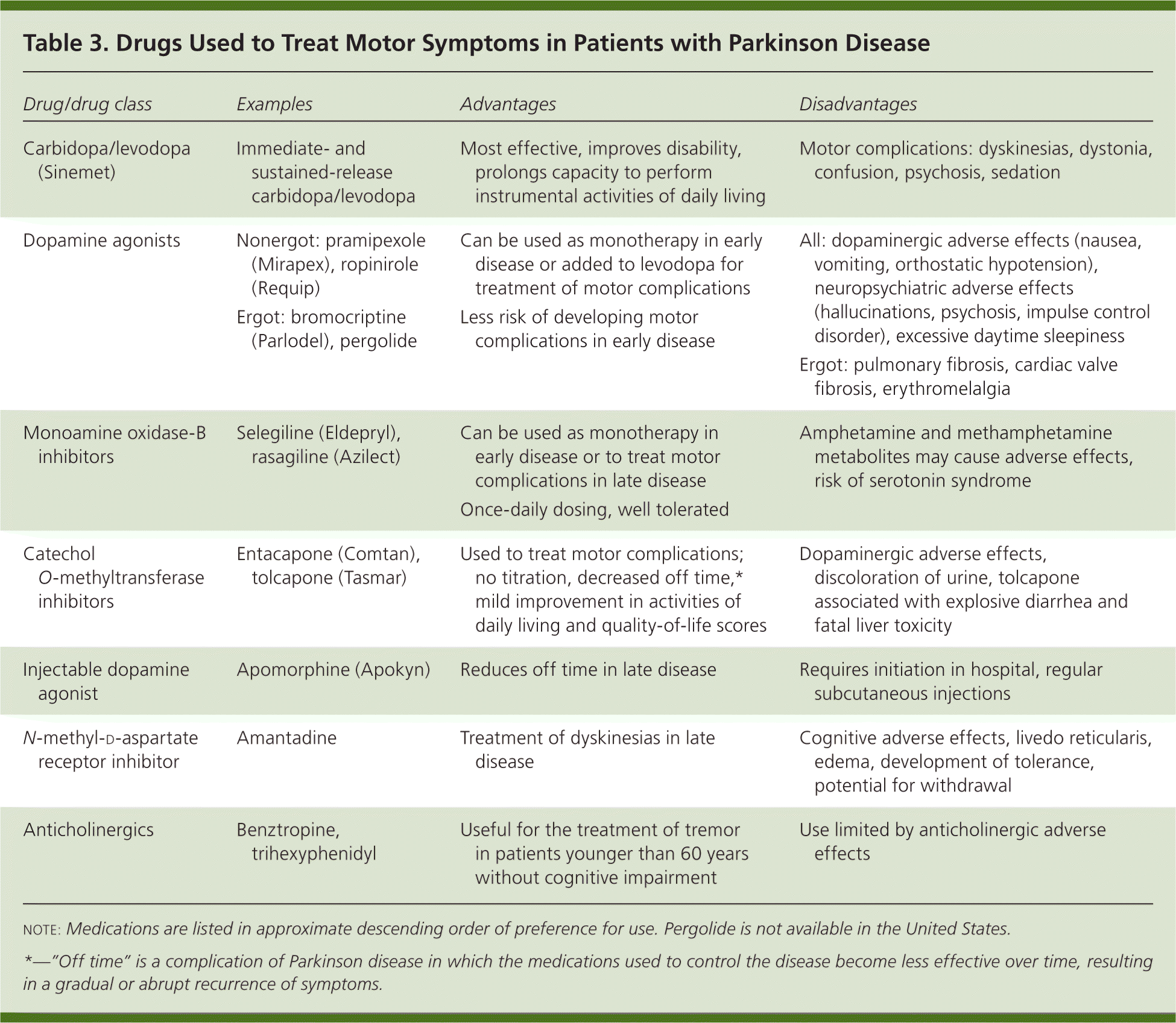
| Drug/drug class | Examples | Advantages | Disadvantages |
|---|---|---|---|
| Carbidopa/levodopa (Sinemet) | Immediate- and sustained-release carbidopa/levodopa |
|
|
| Dopamine agonists | Nonergot: pramipexole (Mirapex), ropinirole (Requip) |
|
|
| Ergot: bromocriptine (Parlodel), pergolide | |||
| Monoamine oxidase-B inhibitors | Selegiline (Eldepryl), rasagiline (Azilect) |
|
|
| Catechol O-methyltransferase inhibitors | Entacapone (Comtan), tolcapone (Tasmar) |
|
|
| Injectable dopamine agonist | Apomorphine (Apokyn) |
|
|
| N-methyl-D-aspartate receptor inhibitor | Amantadine |
|
|
| Anticholinergics | Benztropine, trihexyphenidyl |
|
|
Ergot-derived dopamine agonists such as cabergoline, bromocriptine (Parlodel), lisuride, and pergolide should not be used as first-line treatments because of the risk of serosal fibrosis and cardiac valvulopathies. (note: Lisuride and pergolide are not available in the United States.) If ergot-derived dopamine agonists are used, baseline and annual echocardiography, chest radiography, and testing of erythrocyte sedimentation rate and renal function should be performed.4,11 Monoamine oxidase-B inhibitors are less effective than either carbidopa/levodopa or dopamine agonists in treating motor symptoms of Parkinson disease, cause less dyskinesia than carbidopa/levodopa, and generate fewer adverse effects than dopamine agonists.4,11,15,17 Administering carbidopa/levodopa in combination with a dopamine agonist in early disease does not delay the development of dyskinesias.18
The choice of initial therapy should be guided by the patient's preferences after a discussion of the risks and benefits of each class of medications, taking into account the degree of the patient's functional disability. Although up to 40 percent of patients who have Parkinson disease use an alternative therapy,19 no good evidence shows that any herbal medication or supplement is effective for treatment of the disease, and there is no convincing evidence that any such treatment is neuroprotective.20 In particular, vitamin E should not be used for neuroprotection because there is good evidence indicating that it does not slow disease progression.4,20
LATE MEDICAL THERAPY
As Parkinson disease progresses, initial therapy becomes less effective and additional motor complications develop, including dyskinesias and motor fluctuations. The patient's “on time,” when medication is effectively controlling the disease's symptoms, becomes shorter, and “off time” occurs when disease symptoms recur gradually or abruptly. These complications impair function and quality of life.21
Several medications are used as adjunctive therapy with levodopa to help reduce motor fluctuations. Dopamine agonists decrease off time in patients and improve function. The nonergot dopamine agonists pramipexole and ropinirole are preferred to the ergot agonists, for reasons previously noted.4,11,21 Apomorphine (Apokyn) is a nonergot dopamine agonist injected subcutaneously that decreases off time. It has significant adverse effects, and treatment should be started in an experienced center.4,11 Monoamine oxidase-B inhibitors also decrease off time in patients. Catechol O-methyltransferase inhibitors decrease levodopa metabolism, allowing for more levodopa to enter the brain. They also modestly decrease off time.4,11,21,22 The catechol O-methyltransferase inhibitor tolcapone (Tasmar) is associated with fatal hepatotoxicity and should be avoided.4,11 These treatments all increase dyskinesias and other adverse effects, including hallucinations, nausea, vomiting, constipation, hypotension, insomnia, and somnolence.4,11,21,23 A Cochrane review that indirectly compared these drugs concluded that dopamine agonists were most effective at reducing off time.23 Only amantadine has been shown to reduce dyskinesias. This effect is modest and may last less than eight months.4,11,21
SURGERY
Most patients will develop disabling symptoms despite optimal medical therapy, and are candidates for deep brain stimulation, which targets either the subthalamic nucleus or the globus pallidus interna.24 Factors that predict a good response to surgery for advanced Parkinson disease include good response to levodopa, few comorbidities, absence of cognitive impairment, and absence of (or well-controlled) depression.24 Risks of surgery include intracranial hemorrhage; stroke; infection; lead migration, misplacement, or fracture; and death.24
A recent randomized multicenter trial compared best medical therapy to deep brain stimulation over six months. Patients receiving deep brain stimulation had significant gains in on time, and improvements in motor function and quality of life. However, adverse effects were more frequent in the surgical group; these included surgical site infection, falls, and depression.25 Deep brain stimulation does not slow disease progression, and patients eventually develop treatment-resistant symptoms such as gait freezing.24–26
PHYSICAL, OCCUPATIONAL, AND SPEECH THERAPY
Physical therapy improves balance, muscle strength, and walking speed in patients with Parkinson disease.11,20 No evidence shows that one type of physical therapy is better than another.11 Although there is less evidence that occupational therapy is beneficial,11,20 it may help patients maintain family, social, and work roles and improve safety and motor function, and should be offered to those having difficulty performing tasks of daily living.11 Many patients who have the disease develop dysarthria, with low speech volume, decreased pitch, and pronunciation difficulties. Speech therapy, particularly therapy aimed at improving the volume of speech, is effective.11,20
Management of Nonmotor Symptoms
Even early in the course of Parkinson disease, nonmotor symptoms such as fatigue are common. Later in the course, nonmotor symptoms significantly lower patients' quality of life. Recognizing and treating these symptoms improve the quality of life for patients who have Parkinson disease, as well as for their caregivers.
FATIGUE AND SLEEP DISTURBANCE
Fatigue is present in one-third of patients with Parkinson disease at diagnosis, and is associated with severity of illness. It is less common in patients treated with carbidopa/levodopa.27 Methylphenidate (Ritalin) may improve fatigue in patients with the disease.28 Excessive daytime sleepiness occurs in more than one-half of patients who have Parkinson disease, and is caused by both the disease itself and the adverse effects of medications, such as dopamine agonists.29 Physicians should educate patients about good sleep hygiene.11 Melatonin is not effective for improving sleep.4 Three small randomized trials show that modafinil (Provigil) improves subjective measures of sleepiness without changing objective measures of sleep.28 It should not be used to prevent sleep attacks that may interfere with potentially hazardous activities.4 Physicians should advise patients with sleep attacks to refrain from hazardous activities, such as driving or operating machinery.11
In one study, rapid eye movement sleep behavior disorder was found in 46 percent of patients with Parkinson disease.30 This disorder is characterized by dramatic and potentially violent behaviors that occur during sleep, such as yelling, kicking, or jumping, and diagnosis is confirmed by video polysomnography in a sleep laboratory.31 Limited data suggest that rapid eye movement sleep behavior disorder may respond to low-dose clonazepam (Klonopin).31 Other movement disorders affecting sleep, such as restless legs syndrome and periodic limb movement disorder, occur in almost 20 percent of patients with Parkinson disease. One small study showed that taking carbidopa/levodopa at bedtime decreased the number of movements in patients with restless legs syndrome.28
DISORDERS OF AUTONOMIC FUNCTION
Autonomic dysfunction, evidenced by orthostatic hypotension, erectile dysfunction, urinary incontinence, and constipation, is present in most patients late in the disease. No treatments have demonstrated effectiveness in treating either orthostatic hypotension or urinary incontinence in Parkinson disease.4,28 Sildenafil (Viagra) may improve erectile dysfunction in patients with the disease,28 and one randomized trial showed that polyethylene glycol (Miralax) improved stool frequency and consistency.28 Drooling can be treated with either onabotulinumtoxinA (Botox)28 or glycopyrrolate.32
PSYCHIATRIC DISORDERS
Depression and psychosis occur in up to 50 percent of patients who have Parkinson disease.33 Mild depression can be difficult to diagnose, because some of the motor symptoms of Parkinson disease and depression overlap.4 Physicians should have a high index of suspicion for depression, and consider screening with the Beck Depression Inventory.34 Amitriptyline, desipramine (Norpramin), and nortriptyline (Pamelor) improve depression in patients with Parkinson disease.4,34,35 However, tricyclic antidepressants can cause anticholinergic adverse effects and should not be used in patients with cognitive impairment. When choosing an antidepressant, physicians should take into consideration comorbid conditions and the potential for drug interactions.4,11,34
Psychosis, manifested by visual or auditory hallucinations and delusions, is treated most effectively with clozapine (Clozaril). It requires weekly monitoring because of the risk of agranulocytosis.4,11,34 If regular monitoring is not possible, quetiapine (Seroquel) is modestly effective.4,11,34 Olanzapine (Zyprexa) worsens motor symptoms and is not effective for psychosis in patients with Parkinson disease.4,11,34 Typical antipsychotics (e.g., haloperidol) should be avoided because they will worsen motor symptoms.4
DEMENTIA
Dementia becomes more prevalent as Parkinson disease progresses. In two studies of patients with Parkinson disease, 44 percent of whom met the Diagnostic and Statistical Manual of Mental Disorders, 4th ed., criteria for dementia, the Mini-Mental State Examination had a sensitivity of 98 percent and a specificity of 77 percent, and the longer Cambridge Cognitive Examination had a sensitivity of 95 percent and a specificity of 98 percent.34 Clinicians should evaluate patients for other causes of dementia, and consider discontinuing anticholinergic or dopaminergic medications that may contribute to cognitive impairment.4,11 In controlled trials, rivastigmine (Exelon) therapy has led to small but clinically significant improvements in cognitive, clinical, and activities of daily living scales, but has caused increased tremor and vomiting.4,11,34 Donepezil (Aricept) also improves cognitive function. Although dropout rates in placebo-controlled trials with donepezil are lower than those with rivastigmine, no studies have directly compared the two agents. Either drug may be used for the treatment of dementia in patients with Parkinson disease.4,11,34
Data Sources: Ovid searches of Medline were completed using the terms Parkinson's disease, diagnosis, prognosis, and controlled clinical trial. Ovid searches of the Cochrane Database of Systematic Reviews were completed using the term Parkinson's disease. Searches of Dynamed, Essential Evidence Plus, and the National Guideline Clearinghouse were completed using the search term Parkinson's disease. Search date: January 2011.
