
A more recent article on COPD is available.
Am Fam Physician. 2013;88(10):655-663
Related editorial: Choosing the Right Inhaled Medication Device for COPD
Author disclosure: No relevant financial affiliations.
Chronic obstructive pulmonary disease (COPD) is a common problem in primary care. COPD is diagnosed with spirometry only in clinically stable patients with a postbronchodilator forced expiratory volume in one second/forced vital capacity ratio of less than 0.70. All patients with COPD who smoke should be counseled about smoking cessation. Influenza and pneumococcal vaccinations are recommended for all patients with COPD. The Global Initiative for Chronic Obstructive Lung Disease assigns patients with COPD into four groups based on the degree of airflow restriction, symptom score, and number of exacerbations in one year. Pulmonary rehabilitation is recommended for patients in groups B, C, and D. Those in group A should receive a short-acting anticholinergic or short-acting beta2 agonist for mild intermittent symptoms. For patients in group B, long-acting anticholinergics or long-acting beta2 agonists should be added. Patients in group C or D are at high risk of exacerbations and should receive a long-acting anticholinergic or a combination of an inhaled corticosteroid and a long-acting beta2 agonist. For patients whose symptoms are not controlled with one of these regimens, triple therapy with an inhaled corticosteroid, long-acting beta2 agonist, and anticholinergic should be considered. Prophylactic antibiotics and oral corticosteroids are not recommended for prevention of COPD exacerbations. Continuous oxygen therapy improves mortality rates in patients with severe hypoxemia and COPD. Lung volume reduction surgery can improve survival rates in patients with severe, upper lobe–predominant COPD with heterogeneous emphysema distribution.
Chronic obstructive pulmonary disease (COPD) is a common problem in primary care. The estimated prevalence is 6.3% (15 million persons) in the United States,1 with more than 126,000 deaths each year.2 COPD treatments aim to improve quality of life and control symptoms while reducing exacerbation risk, which can lead to increased morbidity and mortality.
This article summarizes expert consensus guidelines from the Global Initiative for Chronic Obstructive Lung Disease (GOLD) for nonpharmacologic and pharmacologic interventions for patients with stable COPD.3 The GOLD guidelines are widely used in the management of COPD. (Disclosure: the GOLD program is funded by pharmaceutical companies that make medications for COPD, and the board of directors, committee members, and reviewers have ties to the pharmaceutical industry. See http://www.goldcopd.org/disclosure-statements.html.)
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Suspected COPD should be confirmed by spirometry in stable patients with a postbronchodilator forced expiratory volume in one second/forced vital capacity ratio of less than 0.70. | C | 3 |
| Smoking cessation is recommended for all patients with COPD who smoke. | C | 14, 15 |
| Patients in GOLD group A should be treated with a short-acting anticholinergic or short-acting beta2 agonist on an as-needed basis. | A | 19–21 |
| Patients in GOLD group B should be treated with a long-acting anticholinergic or long-acting beta2 agonist. | A | 22–29 |
| Patients in GOLD group C or D should be treated with a long-acting anticholinergic or a combination of an inhaled corticosteroid and long-acting beta2 agonist. | B | 3, 24, 28, 34, 37, 38 |
| Long-term oxygen therapy improves mortality rates in patients with severe hypoxemia and COPD. | A | 42, 43 |
Although some of the GOLD recommendations are derived from outcome-oriented evidence, the guidelines have not been shown to provide better clinical outcomes than other guidelines on COPD management, such as those from the National Institute for Health and Care Excellence4 or the joint guidelines from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society.5 A comparison of treatment guidelines is shown in Table 1.3–5 The joint guideline from the American College of Physicians uses the forced expiratory volume in one second (FEV1) to guide treatment decisions, whereas the National Institute for Health and Care Excellence guideline focuses on symptoms of breathlessness and exacerbations. The GOLD guideline combines the subjective and objective components of COPD to classify severity and guide treatment recommendations.

| American College of Physicians/American College of Chest Physicians/American Thoracic Society/European Respiratory Society guideline5 | |
| FEV1 = 60% to 80% predicted: inhaled bronchodilators may be used | |
| FEV1 < 60% predicted: long-acting anticholinergic or long-acting beta2 agonist recommended; combination therapy may be used (long-acting anticholinergic, long-acting beta2 agonist, or inhaled corticosteroid) | |
| Global Initiative for Chronic Obstructive Lung Disease guideline3 | |
| Patient group A*: short-acting anticholinergic or short-acting beta2 agonist as needed | |
| Patient group B*: long-acting anticholinergic or long-acting beta2 agonist | |
| Patient group C or D*: long-acting anticholinergic or combination of long-acting beta2 agonist plus inhaled corticosteroid | |
| National Institute for Health and Care Excellence guideline4 | |
| Patients with breathlessness and exercise limitation: short-acting beta2 agonist or short-acting anticholinergic as needed | |
| Patients with persistent breathlessness and exacerbations despite therapy above: | |
| FEV1 ≥ 50% predicted: long-acting anticholinergic or long-acting beta2 agonist | |
| FEV1 < 50% predicted: long-acting anticholinergic or combination of long-acting beta2 agonist and inhaled corticosteroid | |
| Patients with persistent breathlessness or exacerbations despite therapy above: | |
| FEV1 ≥ 50% predicted: long-acting beta2 agonist plus inhaled corticosteroid, or combination of long-acting anticholinergic, long-acting beta2 agonist, and inhaled corticosteroid | |
| FEV1 < 50% predicted: long-acting anticholinergic, long-acting beta2 agonist, and inhaled corticosteroid | |
Diagnosis
A diagnosis of COPD should be considered in patients with progressive dyspnea, chronic cough, or increased sputum production with risk factors (e.g., smoking). COPD can be diagnosed with spirometry only in stable patients (i.e., those not experiencing an acute exacerbation of symptoms) with a postbronchodilator FEV1/forced vital capacity ratio of less than 0.70.3 The diagnosis of COPD and interpretation of spirometry results have been reviewed previously.6,7
Assessment
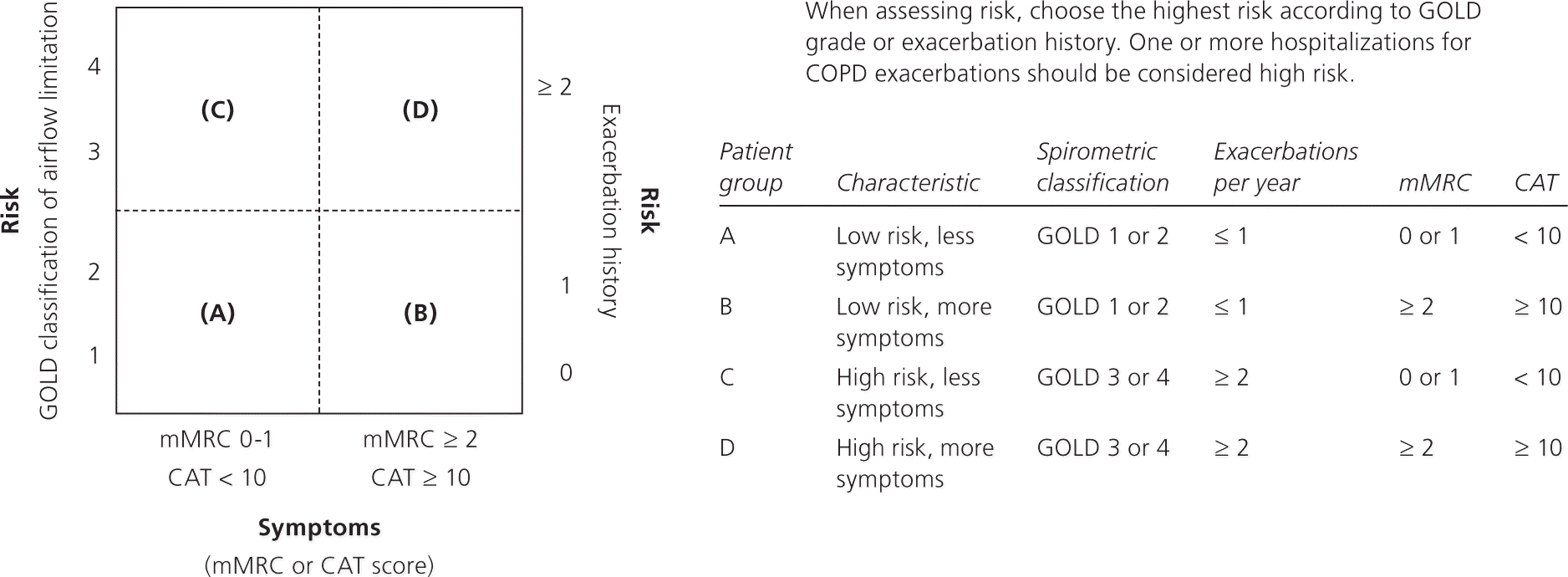
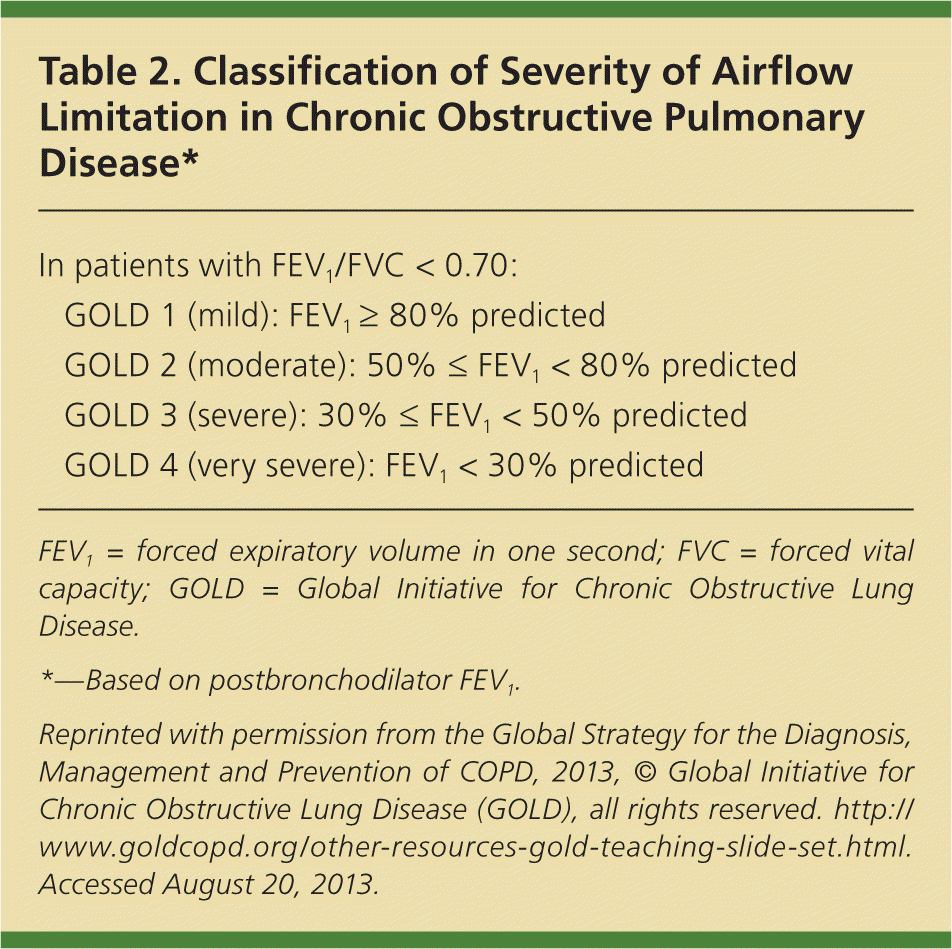
GOLD classifies persons with COPD into four groups based on the severity of disease, as assessed by the following criteria: the degree of airflow restriction, a patient symptom score, and the number of exacerbations in one year (Figure 1).8 This grading system uses objective spirometry data and subjective symptoms because the degree of airflow restriction does not always correlate well with symptoms.9 The degree of airflow restriction is graded as mild, moderate, severe, or very severe (Table 2).8 Persons with mild or moderate airflow restriction are assigned to group A or B, whereas those with severe or very severe airflow restriction are assigned to group C or D.
| In patients with FEV1/FVC < 0.70: | |
| GOLD 1 (mild): FEV1 ≥ 80% predicted | |
| GOLD 2 (moderate): 50% ≤ FEV1 < 80% predicted | |
| GOLD 3 (severe): 30% ≤ FEV1 < 50% predicted | |
| GOLD 4 (very severe): FEV1 < 30% predicted | |
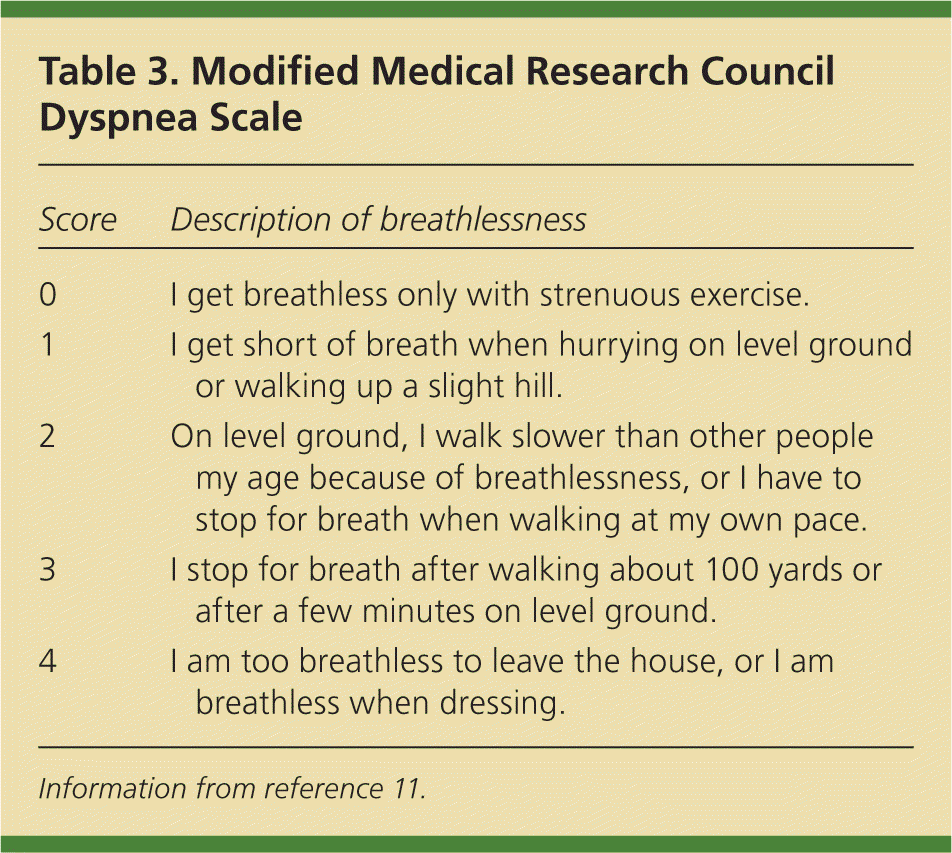
COPD symptoms are assessed subjectively using one of two validated patient symptom questionnaires.10 Because FEV1 does not necessarily correlate with patient symptoms, and because improvement of a patient's health status and reduction in symptoms are the goals of treatment, the inclusion of symptom questionnaires allows for the diagnostic assessment to match treatment goals, similar to the guidelines from the National Institute for Health and Care Excellence.3 GOLD recommends the use of the COPD Assessment Test (CAT) or the modified Medical Research Council Dyspnea Scale (mMRC, Table 3).11 The CAT is available at http://www.catestonline.org/ (eFigure A), and the CAT and the mMRC are available in the smartphone app COPD Pocket Consultant Guide (http://bit.ly/1aTrkIs). Patients with a CAT score less than 10 or an mMRC score of 0 or 1 are assigned to group A or C. Those with a CAT score of 10 or more or an mMRC score of 2 or more are assigned to group B or D.
| Score | Description of breathlessness |
|---|---|
| 0 | I get breathless only with strenuous exercise. |
| 1 | I get short of breath when hurrying on level ground or walking up a slight hill. |
| 2 | On level ground, I walk slower than other people my age because of breathlessness, or I have to stop for breath when walking at my own pace. |
| 3 | I stop for breath after walking about 100 yards or after a few minutes on level ground. |
| 4 | I am too breathless to leave the house, or I am breathless when dressing. |
The third component used to determine the GOLD group is the number of COPD exacerbations in one year. GOLD defines an exacerbation as an acute event characterized by worsening of respiratory symptoms beyond normal day-to-day variations that leads to a change in medication.3 Exacerbations are associated with higher mortality.12,13 Patients with no or one exacerbation per year are assigned to group A or B, and those with two or more are assigned to group C or D. If there is a discrepancy when all three components are considered, the patient should be assigned to the higher-risk group.
Patients with COPD should be reassessed every two to three months. Symptom questionnaires (e.g., CAT, mMRC), smoking cessation (if applicable), and exacerbation history should be reviewed. Repeat spirometry is recommended on a yearly basis.3
Treatment
COPD treatment is guided by the patient group assignment. As disease severity increases, long-acting inhalers and combination therapies are added to provide additional symptom control and reduce the risk of exacerbations.
SMOKING CESSATION
Patients who smoke should be assisted with smoking cessation through counseling and effective medications.14,15 The American Academy of Family Physicians' Ask and Act Tobacco Cessation Program provides online resources for physicians and patients (http://bit.ly/1fV71eZ).
IMMUNIZATIONS
Influenza vaccination reduces COPD exacerbations and is recommended yearly.16 The Centers for Disease Control and Prevention recommends pneumococcal vaccination for all adults 19 years and older who have chronic lung disease, including COPD. However, a meta-analysis of seven studies did not show a decrease in pneumonia rates, hospital admissions, or emergency department visits in patients with COPD who received the pneumococcal vaccine.17
PULMONARY REHABILITATION
Pulmonary rehabilitation has been shown to improve exercise tolerance, reduce dyspnea, and improve health-related quality of life in patients similar to those in GOLD groups B through D.18
INHALED MEDICATIONS
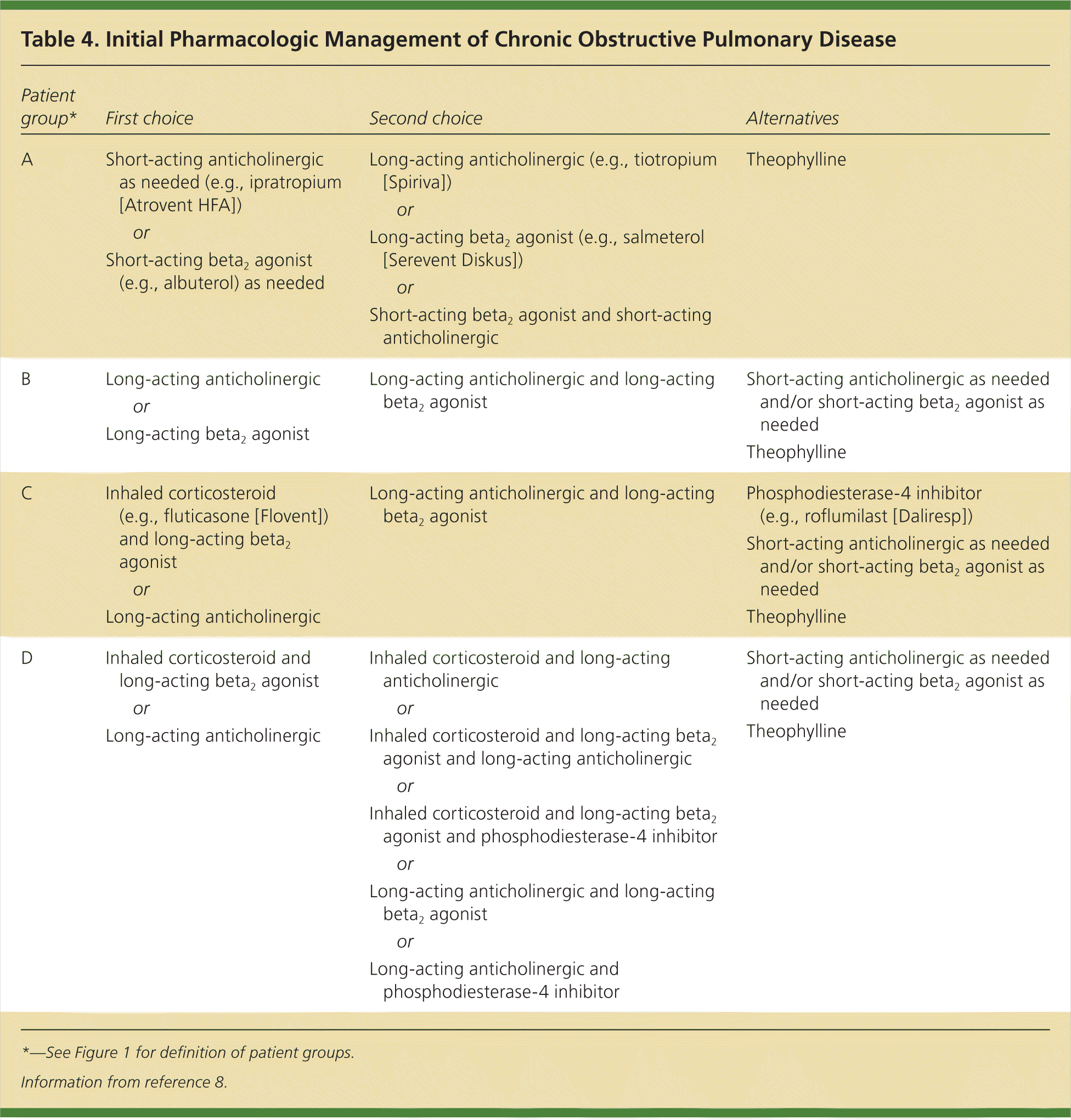
For patients in group A, a short-acting anticholinergic (e.g., ipratropium [Atrovent HFA]) or short-acting beta2 agonist (e.g., albuterol, levalbuterol [Xopenex HFA], pirbuterol [Maxair Autohaler]) is recommended on an as-needed basis for mild intermittent symptoms. A meta-analysis of 13 studies found that short-acting beta2 agonists improved lung function, dyspnea, and fatigue, and decreased breathlessness compared with placebo.19 A 2006 Cochrane review that included 3,912 patients showed a small benefit in quality of life and lung function in those receiving ipratropium compared with albuterol.20 Combination therapy with scheduled albuterol and ipratropium has been shown to increase FEV1 but does not affect patient symptom scores.21 It is not known if as-needed dosing is more or less effective than scheduled administration.
For patients in group B, long-acting inhaled medications should be used. Options include long-acting anticholinergics (e.g., tiotropium [Spiriva], aclidinium [Tudorza Pressair]) or long-acting beta2 agonists (e.g., arformoterol [Brovana], formoterol [Foradil], indacaterol [Arcapta], salmeterol [Serevent Diskus]). Tiotropium has been shown to improve quality-of-life scores, with a number needed to treat of 14 to prevent one exacerbation and 30 to prevent one hospitalization over one year.22 If tiotropium is prescribed, patients should be switched from ipratropium or ipratropium/albuterol (Combivent) to albuterol alone as short-acting rescue medication.
Long-acting beta2 agonists reduce exacerbation risk and improve FEV1 and daily symptom scores.23 A randomized, double-blind trial of 6,112 patients with moderate to severe COPD showed that salmeterol improved FEV1 and decreased exacerbation risk, but did not reduce mortality.24 Indacaterol is a once-daily long-acting beta2 agonist that improves FEV1 and reduces rescue use of albuterol.25 In patients with comorbid asthma or an unclear diagnosis, monotherapy with a long-acting beta2 agonist is contraindicated because it may increase cardiovascular mortality.26
Tiotropium reduces exacerbations and COPD-related hospitalizations compared with long-acting beta2 agonists, but does not affect mortality.27 For patients whose symptoms are not controlled with tiotropium or a long-acting beta2 agonist alone, a combination of tiotropium and a long-acting beta2 agonist is recommended based on short-term outcomes of improved symptom scores and higher FEV1.28,29
A 2008 meta-analysis found an association between the use of inhaled anticholinergics (ipratropium and tiotropium) and cardiovascular mortality in patients with COPD.30 However, a subsequent randomized, double-blind trial with 5,993 patients demonstrated decreased cardiovascular and overall mortality with tiotropium after four years of follow-up.31 A large cohort study of U.S. veterans showed an increased risk of cardiovascular events with the use of ipratropium in the previous six months.32 Given this association, ipratropium should be avoided in patients with cardiovascular disease.
Patients in GOLD groups C and D should be prescribed a long-acting anticholinergic or a combination of an inhaled corticosteroid and long-acting beta2 agonist.3 Compared with tiotropium alone, fluticasone/salmeterol (Advair) improved daily symptom scores and decreased mortality (number needed to treat = 40), but increased the incidence of pneumonia (number needed to harm = 25) and did not change the rate of exacerbations.33 Patients with poorly controlled symptoms should start triple therapy with an inhaled corticosteroid, long-acting anticholinergic, and long-acting beta2 agonist. The data for triple therapy are inconsistent, with studies showing improvement in lung function and symptom scores but conflicting results regarding reduction in exacerbation rates compared with tiotropium alone.28,34 A summary of initial treatment options and common medications is presented in Table 48 andTable 5,35 and patient instructions for inhaler use are reviewed in eFigure B.
| Patient group* | First choice | Second choice | Alternatives | ||
|---|---|---|---|---|---|
| A |
|
|
| ||
| B |
|
|
| ||
| C |
|
|
| ||
| D |
|
|
| ||
| Medication | Dosage | Cost* | Potential adverse effects | |
|---|---|---|---|---|
| Short-acting anticholinergic | ||||
| Ipratropium (Atrovent HFA) | Two puffs every six hours as needed | $236 per inhaler | Anaphylaxis, angioedema, bronchospasm (paradoxical), glaucoma (narrow-angle), hypersensitivity reaction, laryngospasm | |
| Short-acting beta2 agonists | ||||
| Albuterol | Two puffs every four to six hours as needed | $33 per inhaler (generic) | Angina, angioedema, arrhythmias, bronchospasm (paradoxical), hypertension, hypokalemia, QT-interval prolongation, seizures | |
| Levalbuterol (Xopenex HFA) | Two puffs every four to six hours as needed | $55 per inhaler | Anaphylaxis, arrhythmias, bronchospasm (paradoxical), hypersensitivity reaction, hypertension, hypokalemia, metabolic acidosis, paresthesia, syncope | |
| Pirbuterol (Maxair Autohaler) | One or two puffs every four to six hours as needed | $450 per inhaler | Arrhythmias, bronchospasm (paradoxical), hypersensitivity reaction, hypertension, hypokalemia, seizures | |
| Long-acting anticholinergics | ||||
| Aclidinium (Tudorza Pressair) | One dose twice per day | $237 for 60 doses | Atrioventricular block, bronchospasm (paradoxical), cardiopulmonary arrest, heart failure, hypersensitivity reaction | |
| Tiotropium (Spiriva) | One dose per day | $282 for 30 doses | Angioedema, bronchospasm (paradoxical), glaucoma, hypersensitivity reaction | |
| Long-acting beta2agonists | ||||
| Arformoterol (Brovana) | 15 mcg twice per day (nebulizer only) | $249 for 30 vials (15 mcg per vial) | Arrhythmias, bronchospasm (paradoxical), hypersensitivity reaction, hypokalemia, lung cancer | |
| Formoterol (Foradil) | One dose every 12 hours | $200 for 60 doses | Anaphylaxis, arrhythmias, asthma exacerbation, atrial fibrillation, bronchospasm (paradoxical), hypertension, hypokalemia, metabolic acidosis | |
| Indacaterol (Arcapta) | One capsule per day | $187 for 30 capsules | Arrhythmias, bronchospasm (paradoxical), hypersensitivity reaction, hypokalemia, seizure disorder | |
| Salmeterol (Serevent Diskus) | One puff every 12 hours | $205 per inhaler | Anaphylaxis, angioedema, arrhythmias, bronchospasm (paradoxical), fever, glaucoma, hypersensitivity reaction, hypertension, hypokalemia, paresthesia, pelvic inflammatory disease, vasculitis | |
| Inhaled corticosteroids | ||||
| Beclomethasone (Qvar, 40 to 80 mcg per puff) | 40 to 320 mcg twice per day | $176 per inhaler | Anaphylaxis, angioedema, bronchospasm, hypersensitivity reaction, glaucoma, suicidal ideation | |
| Budesonide (Pulmicort, 90 to 180 mcg per puff) | 180 to 360 mcg twice per day | $120 to $135 per inhaler, depending on dosage | Adrenal insufficiency, angioedema, benign intracranial hypertension, bronchospasm, glaucoma, hypersensitivity reaction, hypertension, hypokalemia, leukocytosis | |
| Ciclesonide (Alvesco, 80 to 160 mcg per puff) | 80 to 160 mcg twice per day | $188 per inhaler | Angioedema, bronchospasm (paradoxical), elevated liver enzymes, increased intraocular pressure | |
| Fluticasone (Flovent HFA, 44 to 220 mcg per puff; Flovent Diskus, 100 to 250 mcg per puff) | 44 to 500 mcg twice per day | $130 to $275 per inhaler, depending on dosage and delivery system | Anaphylaxis, angioedema, asthma exacerbation, bronchospasm (paradoxical), Churg-Strauss syndrome, fever, hypersensitivity reaction, muscle injury, vasculitis, wheezing | |
| Mometasone (Asmanex, 220 mcg per puff) | One or two puffs per day | $181 per inhaler | Anaphylaxis, angioedema, bronchospasm, fever, hypersensitivity reaction, increased intraocular pressure, wheezing | |
| Combination medications | ||||
| Budesonide/formoterol (Symbicort) | Two puffs twice per day | $222 to $253, depending on dosage | Anaphylaxis, angioedema, arrhythmias, bronchospasm, glaucoma, hypersensitivity reaction, hypertension, hypokalemia, hypotension, increased intraocular pressure, tachycardia | |
| Fluticasone/salmeterol (Advair) | One puff twice per day (Diskus); two puffs twice per day (HFA) | $235 to $380 per inhaler, depending on dosage and delivery system | Anaphylaxis, angioedema, arrhythmias, asthma exacerbation, bronchospasm, hypertension, hypokalemia, myocardial ischemia, stridor, tachycardia, wheezing | |
| Ipratropium/albuterol (Combivent Respimat) | One or two puffs every six hours as needed | $280 per inhaler | Anaphylaxis, angioedema, arrhythmias, exacerbation of chronic obstructive pulmonary disease, glaucoma, hypersensitivity reaction, hypertension, hypokalemia, increased intraocular pressure, metabolic acidosis, myocardial ischemia, tachycardia | |
| Mometasone/formoterol (Dulera) | Two puffs twice per day | $241 per inhaler | Adrenal suppression, angioedema, arrhythmias, asthma exacerbation, bronchospasm, glaucoma, hypersensitivity reaction, hypokalemia, seizures, vasculitis | |
| Other | ||||
| Roflumilast (Daliresp) | 500 mcg per day | $215 for 30 500-mcg tablets | Arrhythmias, elevated liver enzymes, hypersensitivity reaction, lung cancer, paresthesia, prostate cancer, renal failure, suicidal ideation | |
| Theophylline (extended-release) | 300 mg per day initially, then titrate by serum levels | $10 (generic) for 30 300-mg tablets | Arrhythmias, hyperthyroidism, intractable vomiting, peptic ulcer disease, seizures, status epilepticus | |
ORAL MEDICATIONS
Theophylline can be added or used as an alternative in patients whose symptoms are not controlled with triple therapy or who cannot afford inhaler therapy. Theophylline requires drug level monitoring and improves lung function parameters, but has uncertain effects on symptoms and exacerbations.36
Roflumilast (Daliresp), an oral phosphodiesterase-4 inhibitor approved for use in patients with COPD and chronic bronchitis symptoms, can also be added to long-acting bronchodilators in patients in group C or D. Studies have demonstrated improvement in FEV1 but inconsistent results regarding reduction of exacerbation rates.37,38
Prophylactic antibiotic therapy is not recommended to prevent COPD exacerbations. Although erythromycin and azithromycin (Zithromax) have shown a reduced risk of exacerbations,39,40 there are insufficient data about the effects on macrolide resistance and long-term adverse effects to recommend their use.
Oral corticosteroids do not improve quality of life or reduce exacerbation rates, and are not recommended for patients with stable COPD.41
OXYGEN
Long-term oxygen therapy is recommended for patients with COPD and severe hypoxemia (oxygen saturation less than 88% or partial arterial oxygen pressure less than 55 mg Hg). Supplemental oxygen improves endurance and exercise capacity in patients with moderate to severe COPD.42 A multicenter randomized trial with 203 patients who had hypoxemia and COPD demonstrated that continuous oxygen therapy had benefits on survival rates compared with nocturnal oxygen therapy.43 The goal oxygen saturation should be approximately 90% to avoid respiratory acidosis.44
SURGERY
Lung volume reduction surgery improves five-year survival rates in patients with severe COPD and heterogeneous distribution of emphysema with upper lobe predominance.45 Conversely, patients with severe COPD and FEV1 less than 20%, homogenous emphysema, or low carbon monoxide diffusion capacity have increased 30-day mortality after lung volume reduction surgery.46
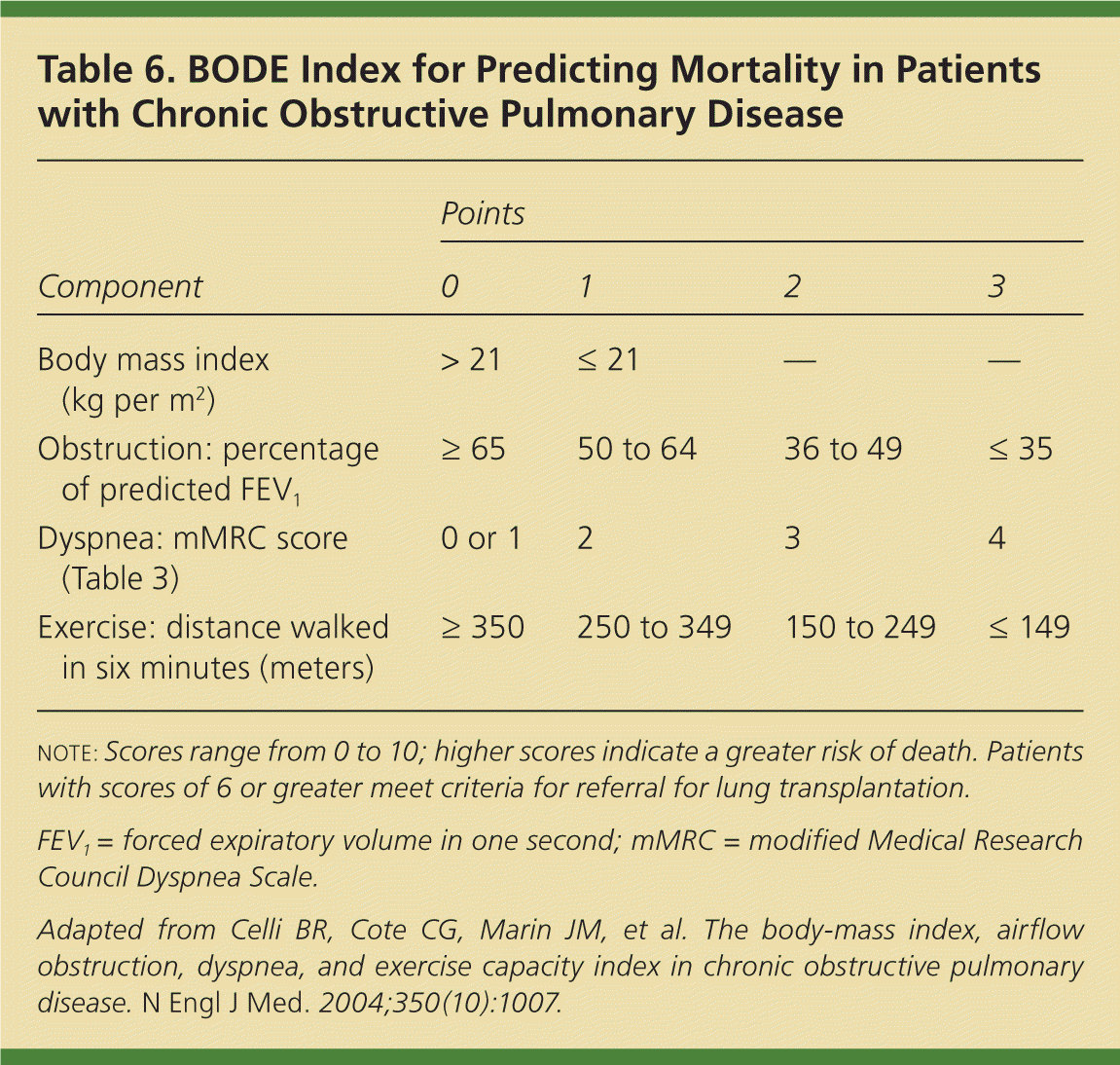
| Component | Points | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Body mass index (kg per m2) | > 21 | ≤ 21 | — | — |
| Obstruction: percentage of predicted FEV1 | ≥ 65 | 50 to 64 | 36 to 49 | ≤ 35 |
| Dyspnea: mMRC score (Table 3) | 0 or 1 | 2 | 3 | 4 |
| Exercise: distance walked in six minutes (meters) | ≥ 350 | 250 to 349 | 150 to 249 | ≤ 149 |
Data Sources: A PubMed search was completed in Clinical Queries using the key terms COPD treatment and COPD therapy. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. Also searched were EBSCO Host Academic Search Premier, DynaMed, Essential Evidence Plus, and UpToDate. Search Date: October 2012.
