A more recent article on pancreatic cancer is available.
Am Fam Physician. 2014;89(8):626-632
Related Curbside Consultation: The Hospice Referral
Patient information: See related handout on pancreatic cancer, written by the authors of this article
Author disclosure: No relevant financial affiliations.
Pancreatic cancer remains the fourth leading cause of cancer-related deaths in the United States. Risk factors include family history, smoking, chronic pancreatitis, obesity, diabetes mellitus, heavy alcohol use, and possible dietary factors. Because more than two-thirds of adenocarcinomas occur in the head of the pancreas, abdominal pain, jaundice, pruritus, dark urine, and acholic stools may be presenting symptoms. In symptomatic patients, the serum tumor marker cancer antigen 19-9 can be used to confirm the diagnosis and to predict prognosis and recurrence after resection. Pancreas protocol computed tomography is considered standard for the diagnosis and staging of pancreatic cancer. Although surgical resection is the only potentially curative treatment for pancreatic ductal adenocarcinomas, less than 20% of surgical candidates survive five years. The decision on resectability requires multidisciplinary consultation. Pancreatic resections should be performed at institutions that complete at least 15 of the surgeries annually. Postoperatively, use of gemcitabine or fluorouracil/leucovorin as adjuvant chemotherapy improves overall survival by several months. However, more than 80% of patients present with disease that is not surgically resectable. For patients with locally advanced or metastatic disease, chemoradiotherapy with gemcitabine or irinotecan provides clinical benefit and modest survival improvement. Palliation should address pain control, biliary and gastric outlet obstruction, malnutrition, thromboembolic disease, and depression.
The American Cancer Society estimated 43,920 cases of pancreatic cancer, with approximately 37,390 deaths, in the United States in 2012. It remains the fourth leading cause of cancer-related deaths.1 The age-adjusted annual incidence rates of pancreatic cancer in men and women have been slowly increasing, but it remains an uncommon cancer. Although there is an equal prevalence in both sexes, there is a slightly higher occurrence in black persons compared with white persons.2 More than 90% of these cancers are pancreatic ductal adenocarcinomas, which are the focus of this review.3
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Pancreas protocol computed tomography is considered the standard for diagnosis and staging of pancreatic cancer. | C | 14, 15 |
| The decision on resectability requires multidisciplinary consultation, and distinction should be made between tumors that are resectable, borderline resectable, or unresectable. | C | 20 |
| Pancreatic resections should be performed at institutions that complete at least 15 of these surgeries annually. | B | 22–26 |
| Adjuvant chemotherapy with gemcitabine (Gemzar) or fluorouracil/leucovorin improves overall survival in patients with resected pancreatic ductal adenocarcinomas (two to three months). | B | 28–30 |
| Irinotecan (Camptosar), a new chemotherapeutic agent, is an option for patients with metastatic pancreatic adenocarcinoma to improve progression-free and overall survival. | B | 31 |
| For patients with locally advanced or metastatic pancreatic cancer, chemoradiotherapy with gemcitabine provides clinical benefit and modest survival improvement (two to three months). | B | 30–33 |
| Placement of endoscopic biliary or enteral stents for biliary and gastric outlet obstruction provides palliative relief for persons with unresectable pancreatic cancer. | B | 37–40 |
| For prevention of recurrent thromboembolic disease in persons with pancreatic cancer, low-molecular-weight heparin is preferred over warfarin (Coumadin). | B | 42 |
Risk Factors
There are several risk factors associated with the development of pancreatic ductal adenocarcinoma3–5 (Table 16 ). Although the risk from smoking is relatively low compared with other risks, based on the current smoking prevalence rates, 25% to 30% of all pancreatic cancer is attributable to tobacco exposure. In familial pancreatic cancer, two or more first-degree relatives have pancreatic cancer in the absence of other cancer syndromes or other known genetic defects. In contrast, the very high-risk genetic and familial syndromes are rare, ranging from one in 280,000 persons for Peutz-Jeghers syndrome to 1% of the Ashkenazi Jewish population for BRCA1 and BRCA2. Therefore, these syndromes account for 10% or less of pancreatic cancers.4
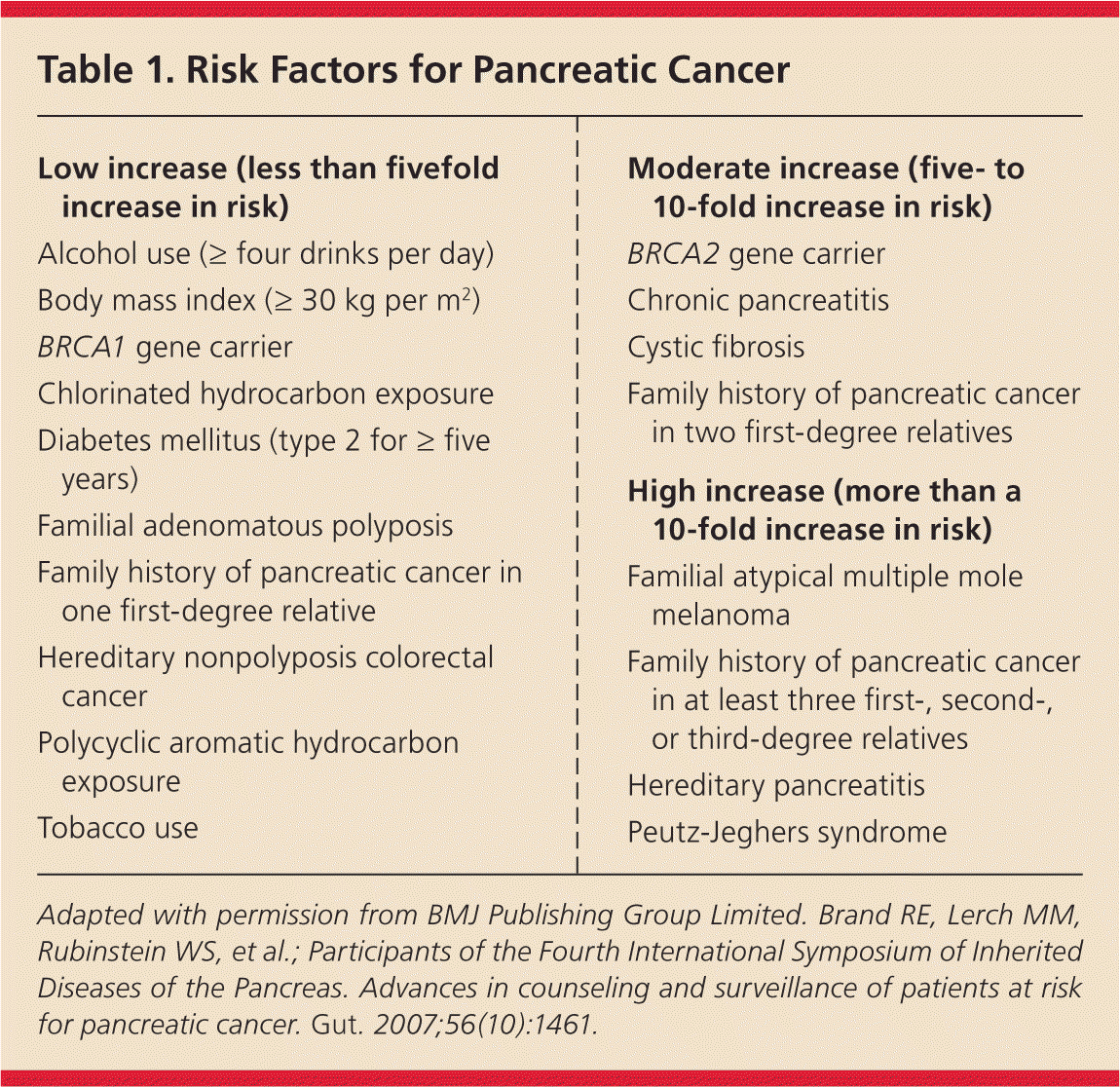
| Low increase (less than fivefold increase in risk) |
| Alcohol use (≥ four drinks per day) |
| Body mass index (≥ 30 kg per m2) |
| BRCA1 gene carrier |
| Chlorinated hydrocarbon exposure |
| Diabetes mellitus (type 2 for ≥ five years) |
| Familial adenomatous polyposis |
| Family history of pancreatic cancer in one first-degree relative |
| Hereditary nonpolyposis colorectal cancer |
| Polycyclic aromatic hydrocarbon exposure |
| Tobacco use |
| Moderate increase (five- to 10-fold increase in risk) |
| BRCA2 gene carrier |
| Chronic pancreatitis |
| Cystic fibrosis |
| Family history of pancreatic cancer in two first-degree relatives |
| High increase (more than a 10-fold increase in risk) |
| Familial atypical multiple mole melanoma |
| Family history of pancreatic cancer in at least three first-, second-, or third-degree relatives |
| Hereditary pancreatitis |
| Peutz-Jeghers syndrome |
Screening and Primary Prevention
The U.S. Preventive Services Task Force recommends against routine screening for pancreatic cancer in asymptomatic adults who are at average risk because of lack of mortality benefit.7 However, it may be reasonable to consider screening persons at high risk of developing pancreatic ductal adenocarcinomas, such as persons from families with known genetic defects predisposing them to pancreatic cancer or with familial pancreatic cancer. Expert opinion recommends screening and surveillance with computed tomography (CT) or endoscopic ultrasonography.8
Diagnosis
DIFFERENTIAL DIAGNOSIS
The differential diagnosis of suspected pancreatic cancer is broad given the wide range of nonspecific symptoms. Possibilities include, but are not limited to, cholangitis, cholecystitis, cholelithiasis, choledocholithiasis, choledochal cysts, duodenal or gastric ulcers, gastritis, pancreatitis, abdominal aortic aneurysm, lymphomas, and primary or secondary cancers of the biliary tree, liver, pancreas, stomach, or intestine.
CLINICAL EXAMINATION
Initial presenting symptoms may vary according to tumor location9 (Table 210 ). More than 90% of these cancers are ductal adenocarcinomas, with more than two-thirds occurring in the head of the pancreas.3 Abdominal pain, jaundice, pruritus, dark urine, and acholic stools may be presenting symptoms as a result of obstruction within the biliary tree.11 Nonspecific findings from cancers of the pancreatic body or tail include unexplained weight loss, anorexia, early satiety, dyspepsia, nausea, and depression.9 Also, a sudden onset of atypical type 2 diabetes mellitus that is difficult to control in a thin patient 50 years or older suggests pancreatic cancer.12
Physical examination findings for pancreatic cancer are also variable. Patients may present in early stages with normal examination findings or in advanced stages with manifestations of liver involvement such as abdominal tenderness, jaundice, and cachexia. A nontender, distended, palpable gallbladder in a patient with jaundice (Courvoisier sign) is 83% to 90% specific, but is only 26% to 55% sensitive for a biliary obstruction from malignancy.11 Advanced pancreatic cancer, like other abdominal malignancies, can be associated with recurring superficial thrombophlebitis (Trousseau sign) or left supraclavicular lymphadenopathy (Virchow node). Subcutaneous areas of nodular fat necrosis (pancreatic panniculitis) may be evident in rare cases.13
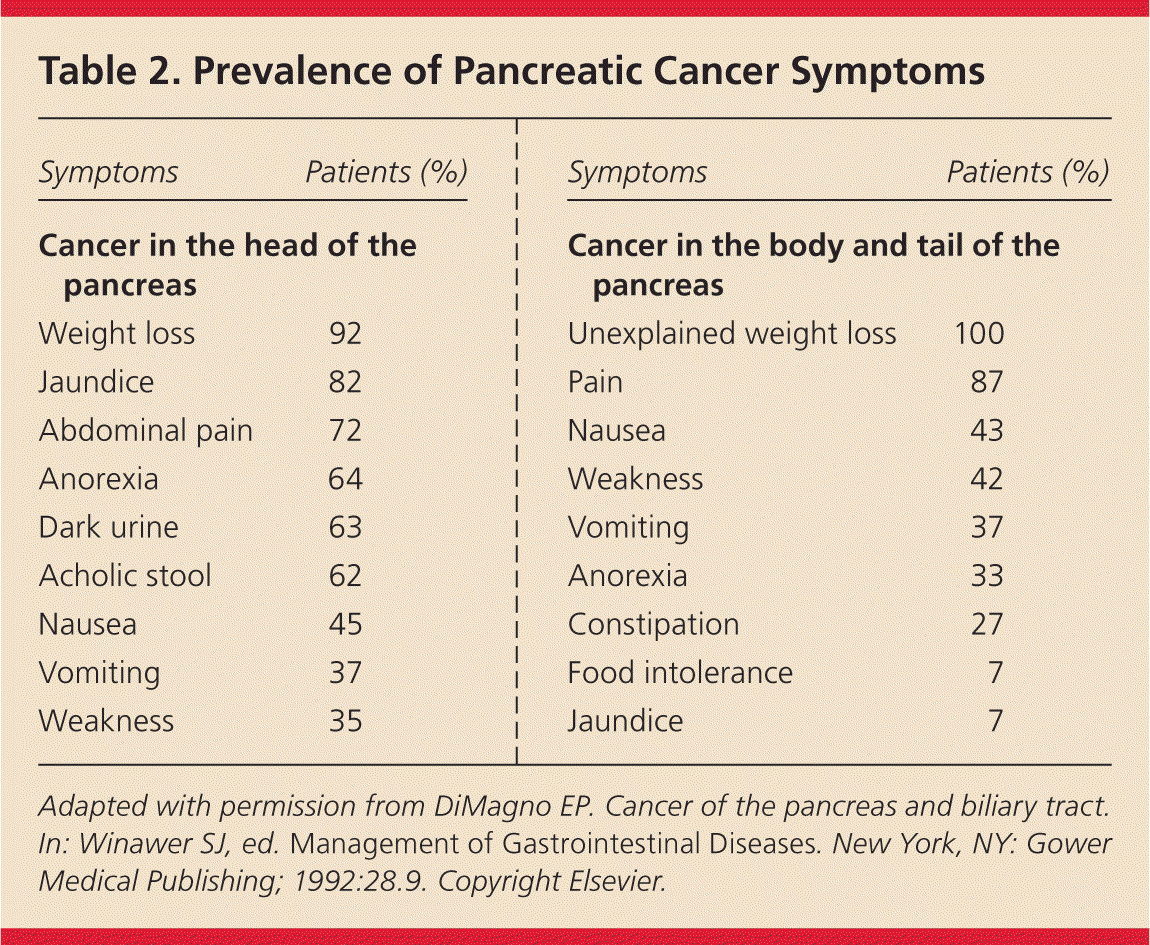
| Symptoms | Patients (%) |
|---|---|
| Cancer in the head of the pancreas | |
| Weight loss | 92 |
| Jaundice | 82 |
| Abdominal pain | 72 |
| Anorexia | 64 |
| Dark urine | 63 |
| Acholic stool | 62 |
| Nausea | 45 |
| Vomiting | 37 |
| Weakness | 35 |
| Cancer in the body and tail of the pancreas | |
| Unexplained weight loss | 100 |
| Pain | 87 |
| Nausea | 43 |
| Weakness | 42 |
| Vomiting | 37 |
| Anorexia | 33 |
| Constipation | 27 |
| Food intolerance | 7 |
| Jaundice | 7 |
DIAGNOSTIC TESTS
For many patients presenting with the common symptoms of pancreatic cancer, abdominal ultrasonography is a reasonable first imaging test. However, if ultrasonography is not diagnostic or pancreatic cancer is highly suggested by findings on the clinical examination, then pancreas protocol CT is the standard for diagnosis and staging.14,15 Pancreas protocol CT involves triphasic (i.e., arterial, late, and venous phases) cross-sectional imaging that allows for enhancement between the parenchyma and adenocarcinoma.
If CT is not possible because of lack of availability or allergy to contrast media, magnetic resonance imaging with contrast media can be used to diagnose and stage pancreatic cancer. Magnetic resonance imaging, as well as magnetic resonance cholangiopancreatography, can be performed as an adjunct to CT in detecting extrapancreatic disease.3
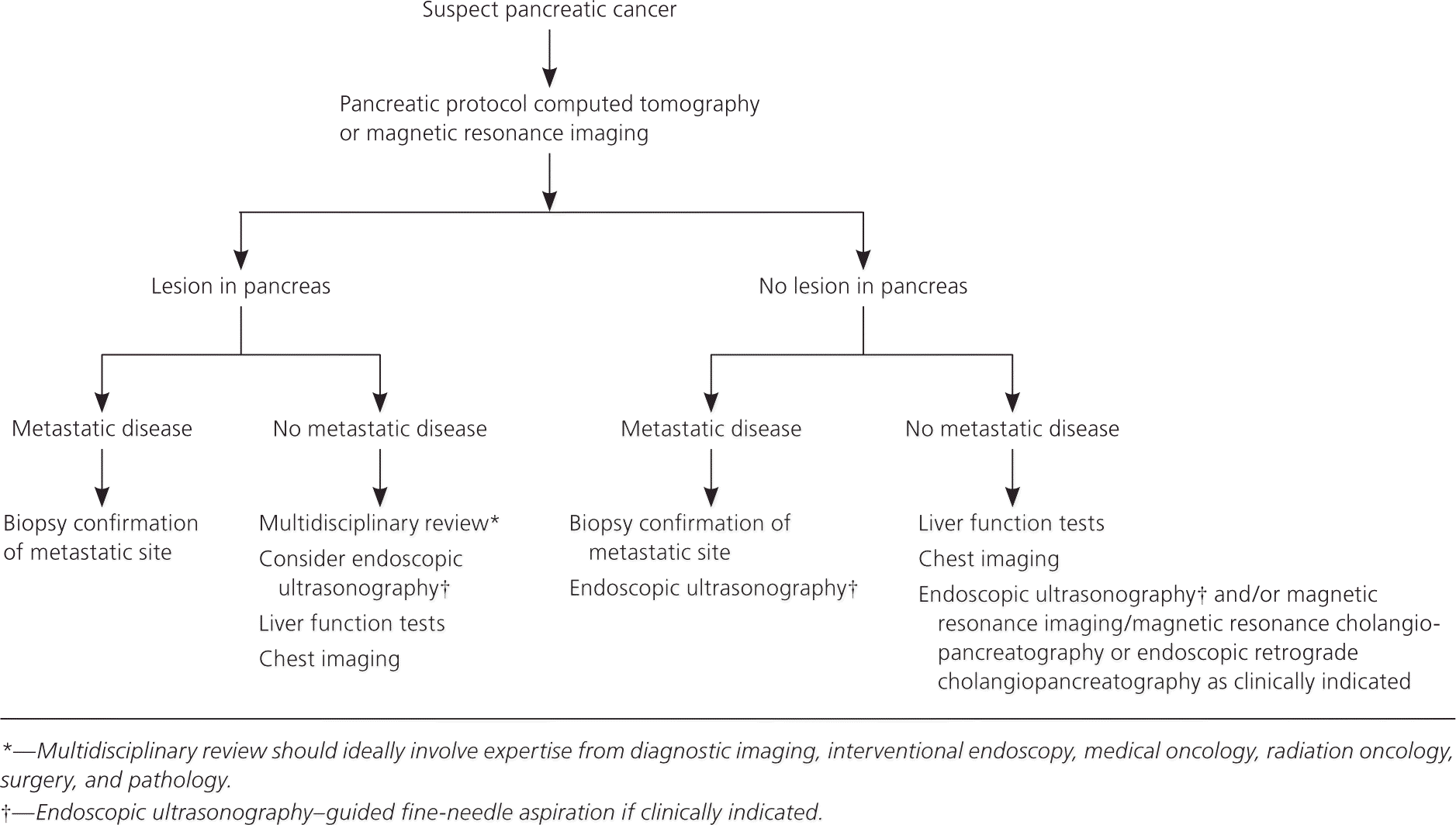
If a pancreatic mass is identified, subsequent endoscopic ultrasonography and fine-needle aspiration are indicated. Pancreatic cysts identified on imaging also require endoscopic ultrasonography and fine-needle aspiration. Concerning cystic lesions of the pancreas include serous cystadenoma, mucinous cystic neoplasm, cyst-like dilatations of the pancreatic duct such as intraductal papillary mucinous neoplasm, cystic degenerations or necrosis, cystic endocrine tumor, and ductal adenocarcinoma.16 If no mass is identified on cross-sectional imaging and no evidence of metastatic disease is present, further endoscopic ultrasonography, endoscopic retrograde cholangiopancreatography, magnetic resonance imaging, or magnetic resonance cholangiopancreatography is indicated.3
The most common serum tumor marker used for pancreatic ductal adenocarcinomas is cancer antigen 19-9, which is expressed in pancreatic and hepatobiliary disease. In symptomatic patients, it can help confirm the diagnosis and predict prognosis and recurrence after resection.3 However, cancer antigen 19-9 is not tumor-specific; therefore, it is not a sufficient individual screening tool for asymptomatic patients. Cancer antigen 19-9 has a limited sensitivity of 50% to 75% and specificity of 80% to 85%; it cannot distinguish between cancer and chronic pancreatitis and possibly other disease states with chronic inflammation.17
Staging
Detection of pancreatic cancer at an early stage is critical to curative treatment. Once a mass is identified and fine-needle aspiration confirms the diagnosis, endoscopic ultrasonography can determine tumor size and extent of lymph node metastases, and assess for portal venous system involvement to complete staging.3 In addition to endoscopic ultrasonography, chest CT and serum liver enzyme tests are useful to determine if a patient is a candidate for surgery.3 Diagnostic staging laparoscopy can be considered for detection of occult peritoneal metastases. Table 3 lists the American Joint Committee on Cancer staging classification for pancreatic tumors, with data on pancreatic cancer distribution by stage and survival.18,19 A multidisciplinary team with expertise in surgery, diagnostic imaging, pathology, interventional endoscopy, and medical and radiation oncology is highly recommended to determine which patients are candidates for surgery.3
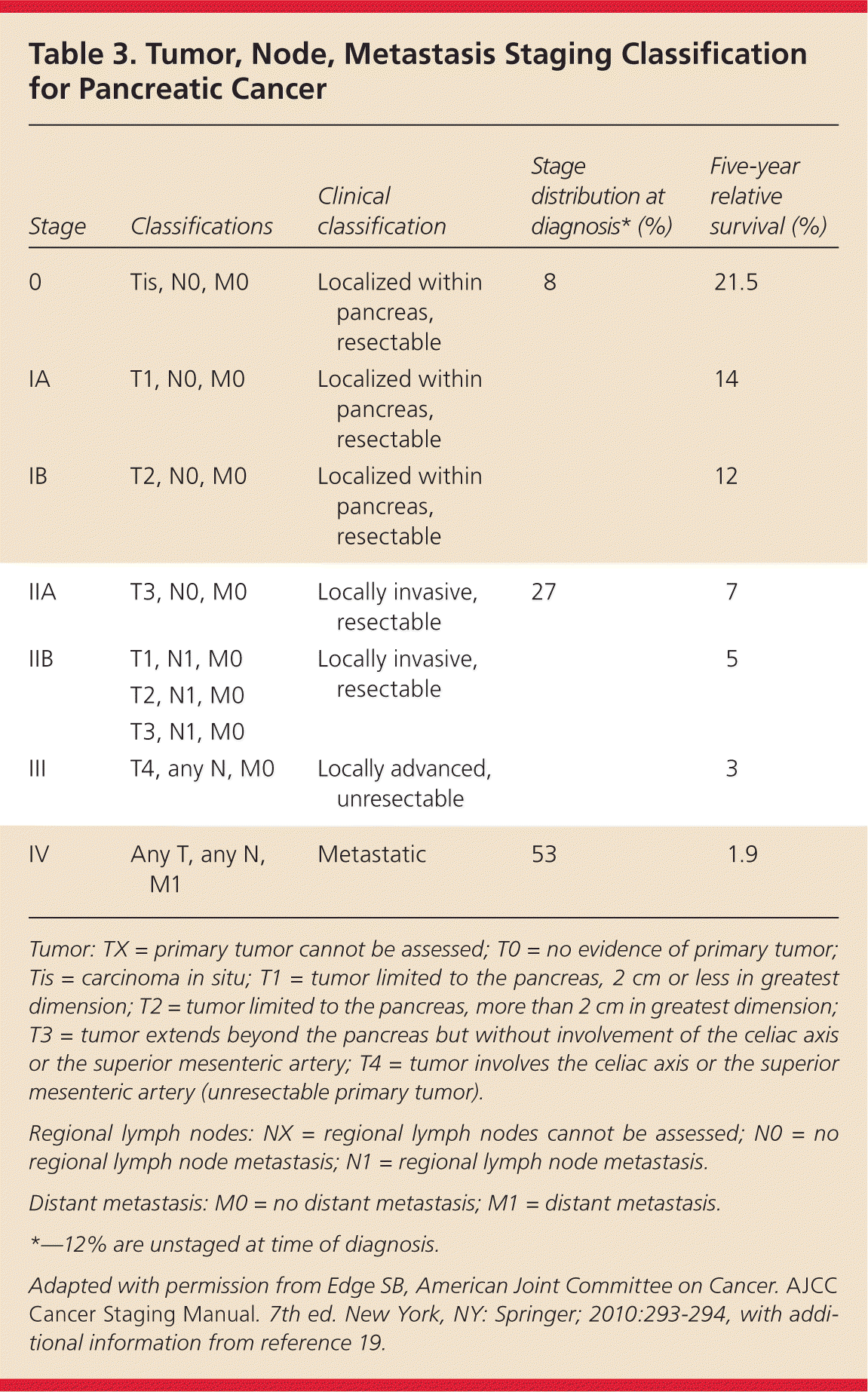
| Stage | Classifications | Clinical classification | Stage distribution at diagnosis* (%) | Five-year relative survival (%) |
|---|---|---|---|---|
| 0 | Tis, N0, M0 | Localized within pancreas, resectable | 8 | 21.5 |
| IA | T1, N0, M0 | Localized within pancreas, resectable | 14 | |
| IB | T2, N0, M0 | Localized within pancreas, resectable | 12 | |
| IIA | T3, N0, M0 | Locally invasive, resectable | 27 | 7 |
| IIB | T1, N1, M0 | 5 | ||
| T2, N1, M0 | ||||
| T3, N1, M0 | ||||
| III | T4, any N, M0 | Locally advanced, unresectable | 3 | |
| IV | Any T, any N, M1 | Metastatic | 53 | 1.9 |
Treatment
RESECTABLE LESIONS
Surgical resection is the only potentially curative treatment for pancreatic ductal adenocarcinomas. Approximately 15% to 20% of patients have resectable disease, but less than 20% of patients who undergo surgery survive five years.3 The decision on resectability requires a multidisciplinary consultation, and distinction should be made between tumors that are resectable, borderline resectable, or unresectable (locally advanced and metastatic).20 An expert consensus group has developed criteria to define tumor resectability, thereby identifying patients who will benefit from surgery (Table 4).3,21
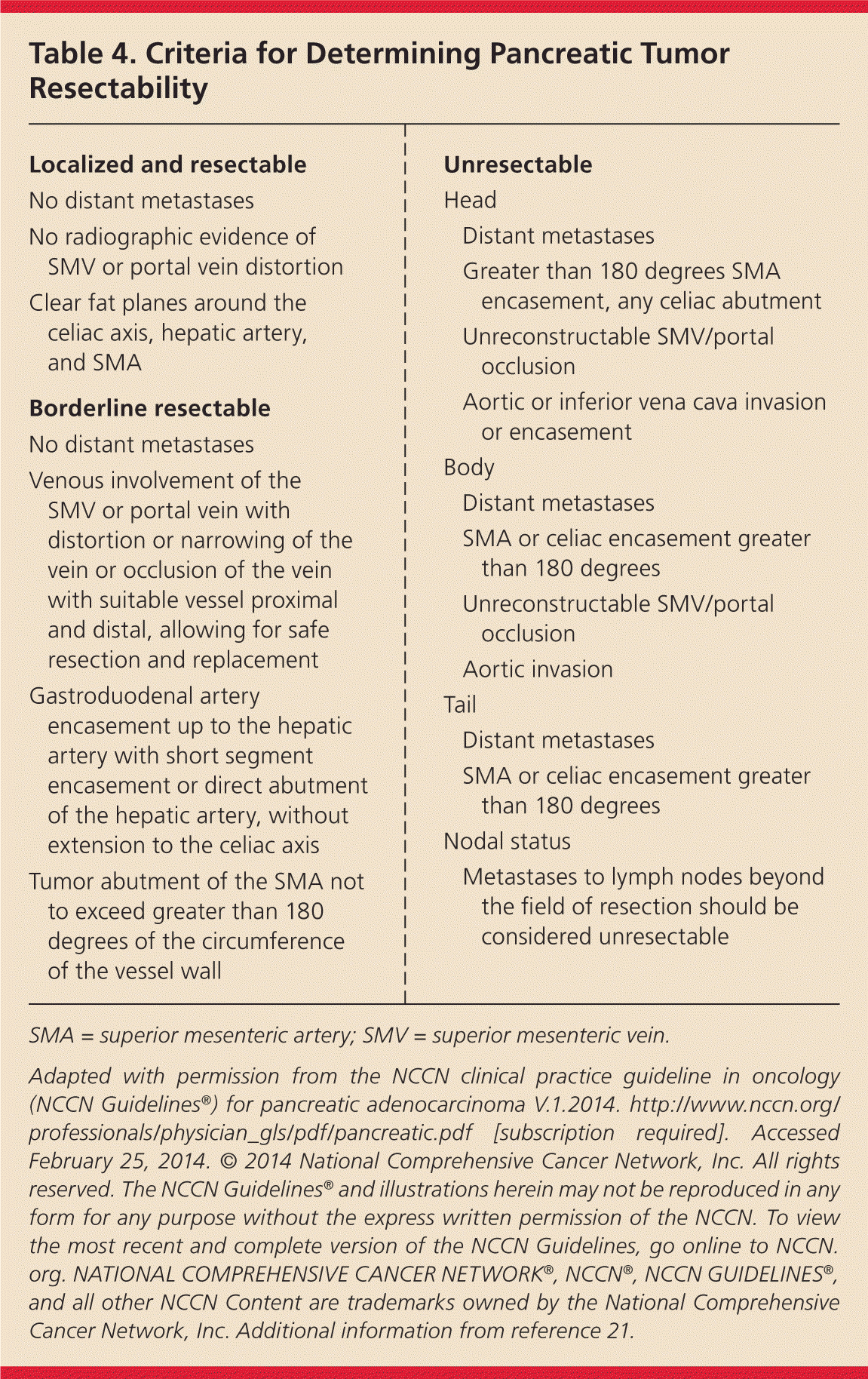
| Localized and resectable | |
| No distant metastases | |
| No radiographic evidence of SMV or portal vein distortion | |
| Clear fat planes around the celiac axis, hepatic artery, and SMA | |
| Borderline resectable | |
| No distant metastases | |
| Venous involvement of the SMV or portal vein with distortion or narrowing of the vein or occlusion of the vein with suitable vessel proximal and distal, allowing for safe resection and replacement | |
| Gastroduodenal artery encasement up to the hepatic artery with short segment encasement or direct abutment of the hepatic artery, without extension to the celiac axis | |
| Tumor abutment of the SMA not to exceed greater than 180 degrees of the circumference of the vessel wall | |
| Unresectable | |
| Head | |
| Distant metastases | |
| Greater than 180 degrees SMA encasement, any celiac abutment | |
| Unreconstructable SMV/portal occlusion | |
| Aortic or inferior vena cava invasion or encasement | |
| Body | |
| Distant metastases | |
| SMA or celiac encasement greater than 180 degrees | |
| Unreconstructable SMV/portal occlusion | |
| Aortic invasion | |
| Tail | |
| Distant metastases | |
| SMA or celiac encasement greater than 180 degrees | |
| Nodal status | |
| Metastases to lymph nodes beyond the field of resection should be considered unresectable | |
Although immediate postoperative mortality is less than 5%, the median survival is about 12 to 19 months.3 Studies support the recommendation that pancreatic resections should be performed at high-volume institutions, generally those that complete at least 15 pancreatic resections annually.22–26 Higher-volume centers have reported decreased mortality rates, shorter hospital stay, and lower overall cost compared with low-volume institutions.3
The classic surgery for resection of a carcinoma of the head of the pancreas is a pancreaticoduodenectomy, also known as a Whipple procedure. In this surgery, the gallbladder, common bile duct, second portion of the duodenum, and the head of the pancreas are resected with the postpyloric duodenum (pylorus-preserving pancreaticoduodenectomy), or the resection is continued proximally to include the distal stomach (classic pancreaticoduodenectomy). No differences in morbidity, mortality, or survival between the two procedures have been found.27
Tumors involving the body or tail of the pancreas are rarely resectable. They are usually advanced at diagnosis and cause symptoms late in their development. For resectable lesions, the surgery performed is typically a distal pancreatectomy with or without splenectomy. Negative margin status, tumor DNA content, tumor size, and absence of lymph node metastasis are the strongest prognostic indicators for long-term survival.3
There is no standard for providing adjuvant treatment of pancreatic ductal adenocarcinomas postoperatively. Adjuvant chemotherapy with gemcitabine (Gemzar) or fluorouracil/leucovorin improves survival by two to three months compared with observation alone, which can also be combined with gemcitabine- or fluoropyrimidine-based chemoradiation.3,28–30
UNRESECTABLE LESIONS
More than 80% of patients present with disease that is not surgically resectable. Although a histologic diagnosis is not necessary before surgery, it is required for treatment of locally advanced, unresectable, or metastatic disease. Some studies have addressed the use of chemoradiation with or without chemotherapy to convert unresectable disease status to resectable. Postresection, these patients have survival rates similar to those with disease initially determined to be resectable.3
LOCALLY ADVANCED LESIONS AND METASTASIS
The primary goals of treatment for advanced pancreatic cancers are palliation and improved survival. In some patients who have good performance status (i.e., adequate nutrition and pain control and patent biliary stent), some effect on survival may be achieved.
The National Comprehensive Cancer Network recommends systemic chemotherapy followed by consolidation chemoradiation therapy as a treatment option. Concurrent administration of gemcitabine or continuous fluorouracil with radiation is an acceptable regimen.3 A new chemotherapeutic agent, irinotecan (Camptosar), can be used for patients with metastatic pancreatic adenocarcinoma to improve progression-free and overall survival.31 Recent studies have also evaluated combination chemoradiation therapy with FOLFIRINOX (fluorouracil/ leucovorin/oxaliplatin/irinoecan), showing improvements in medial progression-free and medial overall survival rates compared with gemcitabine alone.30,32 However, the toxicity profile of irinotecan may limit its use.
For patients with locally advanced lesions or metastasis, the National Comprehensive Cancer Network panel recommends gemcitabine monotherapy because it may relieve symptoms.3 It also provides clinical benefit and modest survival benefit (two to three months).30–33 Second-line therapy involves combinations of gemcitabine with other agents such as fluorouracil, cisplatin, and oxaliplatin (Eloxatin).3
Intensity-modulated radiotherapy and stereotactic body radiotherapy are two techniques of radiation therapy aimed at increasing the dose to the tumor while sparing radiation to nearby healthy tissue. However, there is no established standard dosage of radiation regimen for either of these techniques.34 The decision on whether to choose up-front chemoradiation or induction chemotherapy followed by consolidation chemoradiation should be based on disease response and patient tolerance. Three phase II trials have assessed up-front chemoradiation in locally advanced cancer with median survival rates ranging from 8.2 to 9 months.3
Because the most recent phase III trials improve survival by only several months (PRODIGE 4/ACCORD 11 median overall survival of 10.5 months vs. 6.9 months),35 the treatment of advanced disease remains controversial. Management of pancreatic ductal adenocarcinomas should be approached from a multidisciplinary stance, with consideration of enrollment in clinical studies, and with goals of care and palliation routinely reassessed.
PALLIATIVE CARE
Palliative care for patients with locally advanced and metastatic disease should address symptoms from biliary obstruction, gastric outlet obstruction, cancer-related pain, malnutrition, thromboembolic disease, and depression. Pain intensity should be quantified and reassessed at specific intervals to determine effectiveness of therapies.36 Early referral to hospice for appropriate patients is important, while they can best benefit from these services.
Approximately 10% to 25% of patients with pancreatic cancer have symptomatic gastric outlet obstruction.37 For patients with a short life expectancy or poor performance status, an enteral stent can be placed. Patients with a life expectancy longer than three to six months can receive an open or laparoscopic gastrojejunostomy with or without a jejunostomy tube, but an enteral stent is also an option.39,40
To prevent recurrent thromboembolic disease, low-molecular-weight heparin is preferred over warfarin (Coumadin).3,42 Finally, depression should be addressed for patients, families, and caregivers. Chaplaincy services should be offered to patients whose spirituality and religion play an important role.43
Surveillance
Data on surveillance for patients with resected pancreatic cancer are limited. Expert consensus recommends history and physical examination every three to six months for two years, then annually.44 Monitoring for recurrence with cancer antigen 19-9 levels, CT, and endoscopic ultrasonography every three to six months can also be considered, although there is limited evidence showing that earlier treatment leads to improved patient outcomes.45
Data Sources: Published literature on this topic was identified by using PubMed (1995 through January 2012), the Cochrane Database of Systematic Reviews, U.S. Preventive Services Task Force, Clinical Evidence, Essential Evidence Plus, and the National Guideline Clearinghouse database. Keywords for search included pancreatic cancer, pancreatic cancer diagnosis, and pancreatic cancer treatment. In addition, we searched for guidelines and systematic reviews related to the diagnosis and management of pancreatic cancer. All guidelines were reviewed for evidence of potential conflict, which might influence the recommendation as well as the use of an evidence-based approach. Search dates: January 2013, and January 31, 2014.