
A more recent article on pleural effusion is available.
Am Fam Physician. 2014;90(2):99-104
Patient information: See related handout on pleural effusion, written by the authors of this article.
Author disclosure: No relevant financial affiliations.
Pleural effusion affects more than 1.5 million people in the United States each year and often complicates the management of heart failure, pneumonia, and malignancy. Pleural effusion occurs when fluid collects between the parietal and visceral pleura. Processes causing a distortion in body fluid mechanics, such as in heart failure or nephrotic syndrome, tend to cause transudative effusions, whereas localized inflammatory or malignant processes are often associated with exudative effusions. Patients can be asymptomatic or can present with cough, dyspnea, and pleuritic chest pain. Dullness to percussion on physical examination suggests an effusion; chest radiography can confirm the diagnosis. Thoracentesis may be indicated to diagnose effusion and relieve symptoms. Ultrasound guidance is preferred when aspirating fluid. Routine assays for aspirated fluid include protein and lactate dehydrogenase levels, Gram staining, cytology, and pH measurement. Light's criteria should be used to differentiate exudative from transudative effusions. Additional laboratory assays, bronchoscopy, percutaneous pleural biopsy, or thoracoscopy may be required for diagnosis if the initial test results are inconclusive.
More than 1.5 million persons develop pleural effusions each year in the United States.1 Many of the disease processes commonly seen in primary care are associated with pleural effusion, which requires family physicians to be familiar with its causes, diagnosis, and management.
| Clinical recommendation | Evidence rating | References | Comments |
|---|---|---|---|
| Thoracentesis should be performed with ultrasound guidance. | A | 10, 20, 24 | Ultrasonography increases the likelihood of successful aspiration, decreases the risk of organ puncture (odds ratio for pneumothorax with ultrasonography = 0.3 to 0.8), and is associated with lower hospital costs. |
| Light's criteria should be used to differentiate transudative from exudative effusions. | C | 10, 27, 28 | Light's criteria have a diagnostic accuracy of 93% to 96%. |
| In patients with a pleural effusion classified as exudative by Light's criteria in which a cardiac etiology is suspected, N-terminal pro-brain natriuretic peptide can help differentiate cardiac from noncardiac conditions. | C | 10, 11 | — |
Etiology and Pathogenesis
The visceral and parietal pleural membranes border a potential space within the thoracic cavity. Normally, a small physiologic amount of pleural fluid (0.1 mL per kg) rests within this space. Oncotic and hydrostatic pressures regulate fluid movement between the pleura, which adapt to a range of pressures to maintain the amount of fluid within a preset range. Abnormally high capillary and interstitial hydrostatic pressures can cause an abnormal accumulation of pleural fluid (e.g., in heart failure), as can an abnormally decreased capillary oncotic pressure (e.g., in nephrotic syndrome). Fluid that accumulates as a result of an imbalance in these forces produces transudative effusions. Additionally, inflammatory and malignant processes can promote local capillary and pleural membrane permeability or lymphatic blockage, which allows for the accumulation of exudative pleural fluid (i.e., fluid that is higher in protein and lactate dehydrogenase than transudative fluid).2 Furthermore, an interruption in diaphragmatic integrity can allow fluid to enter the pleural space.3
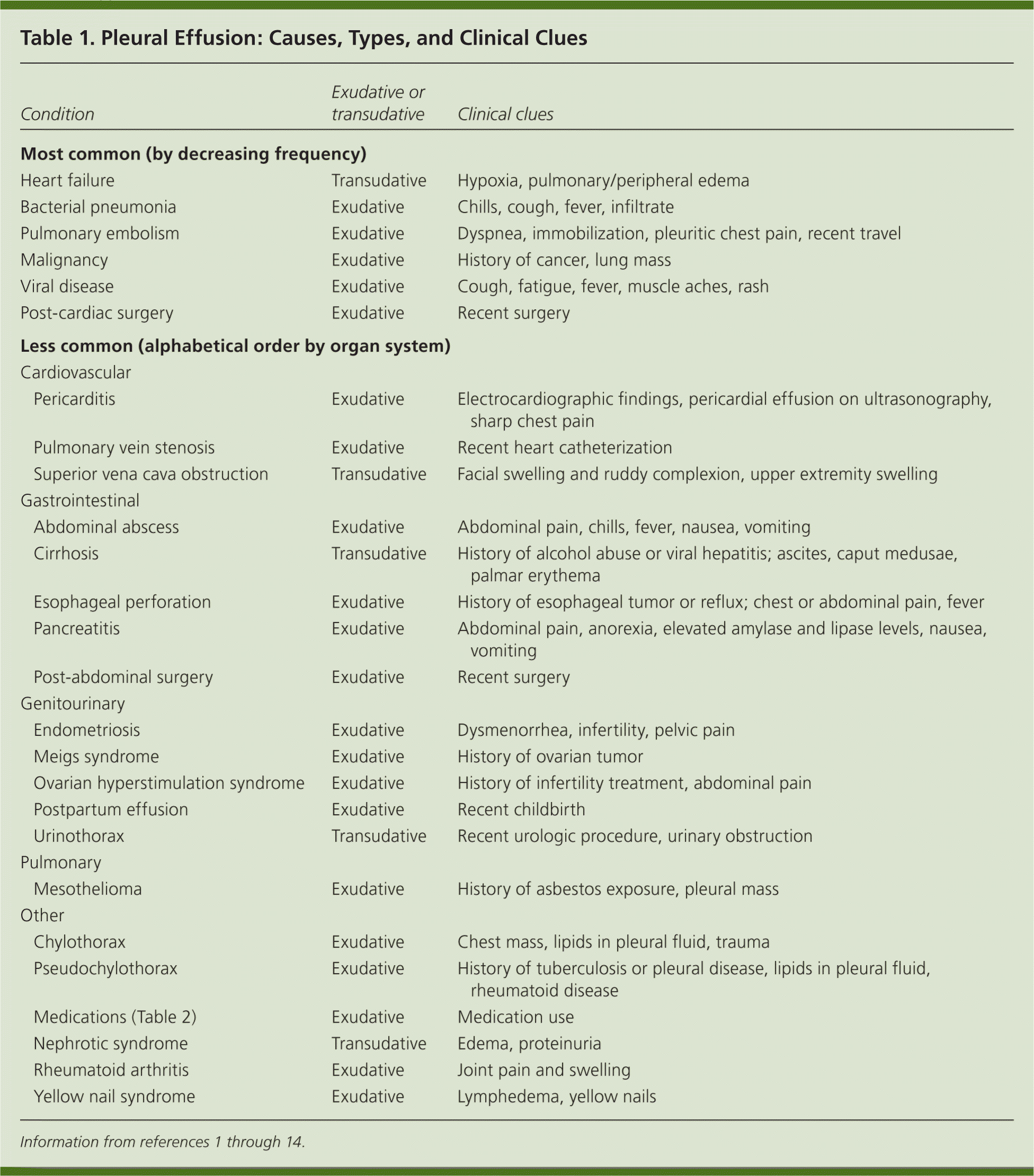
| Condition | Exudative or transudative | Clinical clues | |
|---|---|---|---|
| Most common (by decreasing frequency) | |||
| Heart failure | Transudative | Hypoxia, pulmonary/peripheral edema | |
| Bacterial pneumonia | Exudative | Chills, cough, fever, infiltrate | |
| Pulmonary embolism | Exudative | Dyspnea, immobilization, pleuritic chest pain, recent travel | |
| Malignancy | Exudative | History of cancer, lung mass | |
| Viral disease | Exudative | Cough, fatigue, fever, muscle aches, rash | |
| Post-cardiac surgery | Exudative | Recent surgery | |
| Less common (alphabetical order by organ system) | |||
| Cardiovascular | |||
| Pericarditis | Exudative | Electrocardiographic findings, pericardial effusion on ultrasonography, sharp chest pain | |
| Pulmonary vein stenosis | Exudative | Recent heart catheterization | |
| Superior vena cava obstruction | Transudative | Facial swelling and ruddy complexion, upper extremity swelling | |
| Gastrointestinal | |||
| Abdominal abscess | Exudative | Abdominal pain, chills, fever, nausea, vomiting | |
| Cirrhosis | Transudative | History of alcohol abuse or viral hepatitis; ascites, caput medusae, palmar erythema | |
| Esophageal perforation | Exudative | History of esophageal tumor or reflux; chest or abdominal pain, fever | |
| Pancreatitis | Exudative | Abdominal pain, anorexia, elevated amylase and lipase levels, nausea, vomiting | |
| Post-abdominal surgery | Exudative | Recent surgery | |
| Genitourinary | |||
| Endometriosis | Exudative | Dysmenorrhea, infertility, pelvic pain | |
| Meigs syndrome | Exudative | History of ovarian tumor | |
| Ovarian hyperstimulation syndrome | Exudative | History of infertility treatment, abdominal pain | |
| Postpartum effusion | Exudative | Recent childbirth | |
| Urinothorax | Transudative | Recent urologic procedure, urinary obstruction | |
| Pulmonary | |||
| Mesothelioma | Exudative | History of asbestos exposure, pleural mass | |
| Other | |||
| Chylothorax | Exudative | Chest mass, lipids in pleural fluid, trauma | |
| Pseudochylothorax | Exudative | History of tuberculosis or pleural disease, lipids in pleural fluid, rheumatoid disease | |
| Medications (Table 2) | Exudative | Medication use | |
| Nephrotic syndrome | Transudative | Edema, proteinuria | |
| Rheumatoid arthritis | Exudative | Joint pain and swelling | |
| Yellow nail syndrome | Exudative | Lymphedema, yellow nails | |
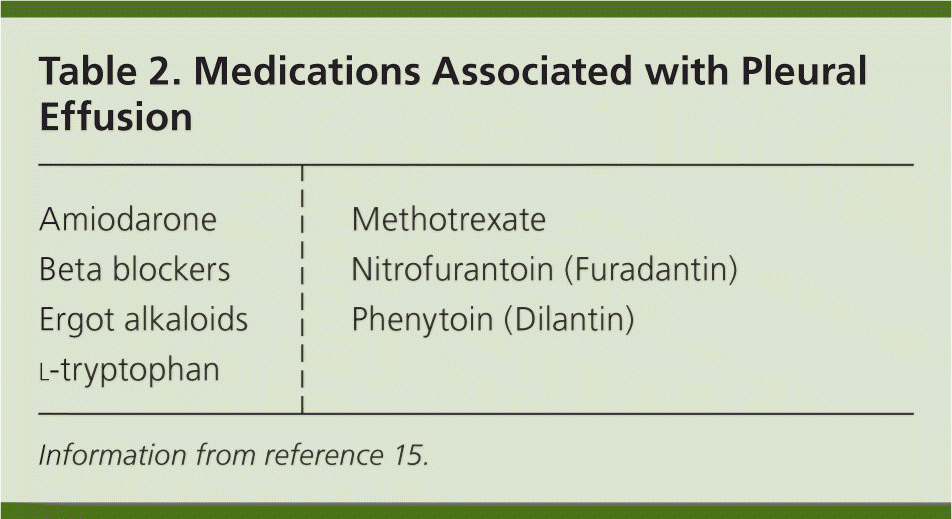
| Amiodarone |
| Beta blockers |
| Ergot alkaloids |
| l-tryptophan |
| Methotrexate |
| Nitrofurantoin (Furadantin) |
| Phenytoin (Dilantin) |
Clinical Presentation
Patients with pleural effusion can be asymptomatic or can present with dyspnea, cough, or pleuritic chest pain. The history and physical examination can narrow the diagnostic considerations (Table 31–10,13 and Table 417 ). The history should focus on differentiating pulmonary etiologies from cardiovascular and other causes of effusion. A thorough chest examination should be performed, with particular attention to dullness to percussion because it is sensitive and specific for diagnosing effusion.17 Figure 1 outlines an approach to evaluating and diagnosing the cause of pleural effusion.10,17–20
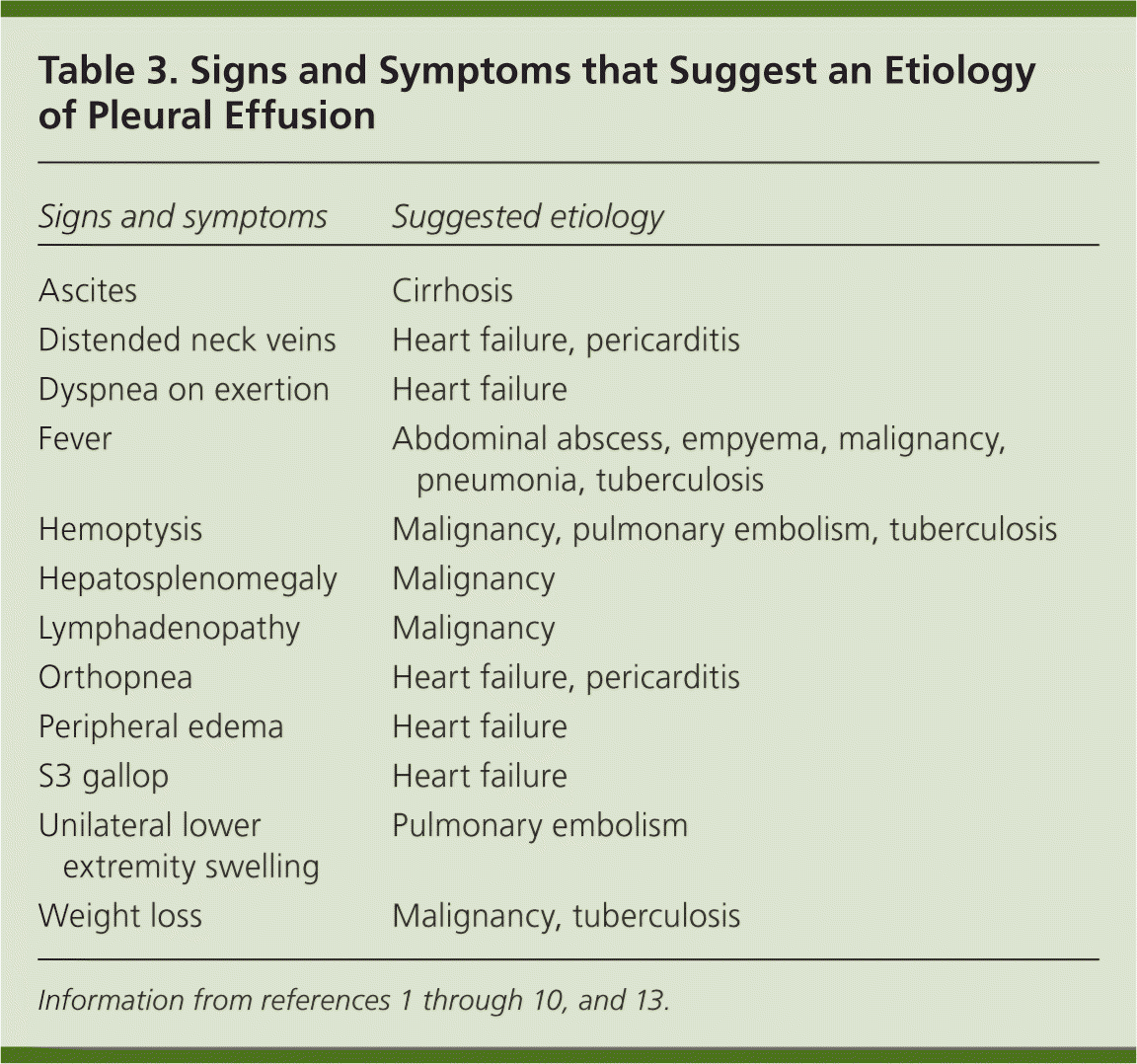
| Signs and symptoms | Suggested etiology |
|---|---|
| Ascites | Cirrhosis |
| Distended neck veins | Heart failure, pericarditis |
| Dyspnea on exertion | Heart failure |
| Fever | Abdominal abscess, empyema, malignancy, pneumonia, tuberculosis |
| Hemoptysis | Malignancy, pulmonary embolism, tuberculosis |
| Hepatosplenomegaly | Malignancy |
| Lymphadenopathy | Malignancy |
| Orthopnea | Heart failure, pericarditis |
| Peripheral edema | Heart failure |
| S3 gallop | Heart failure |
| Unilateral lower extremity swelling | Pulmonary embolism |
| Weight loss | Malignancy, tuberculosis |
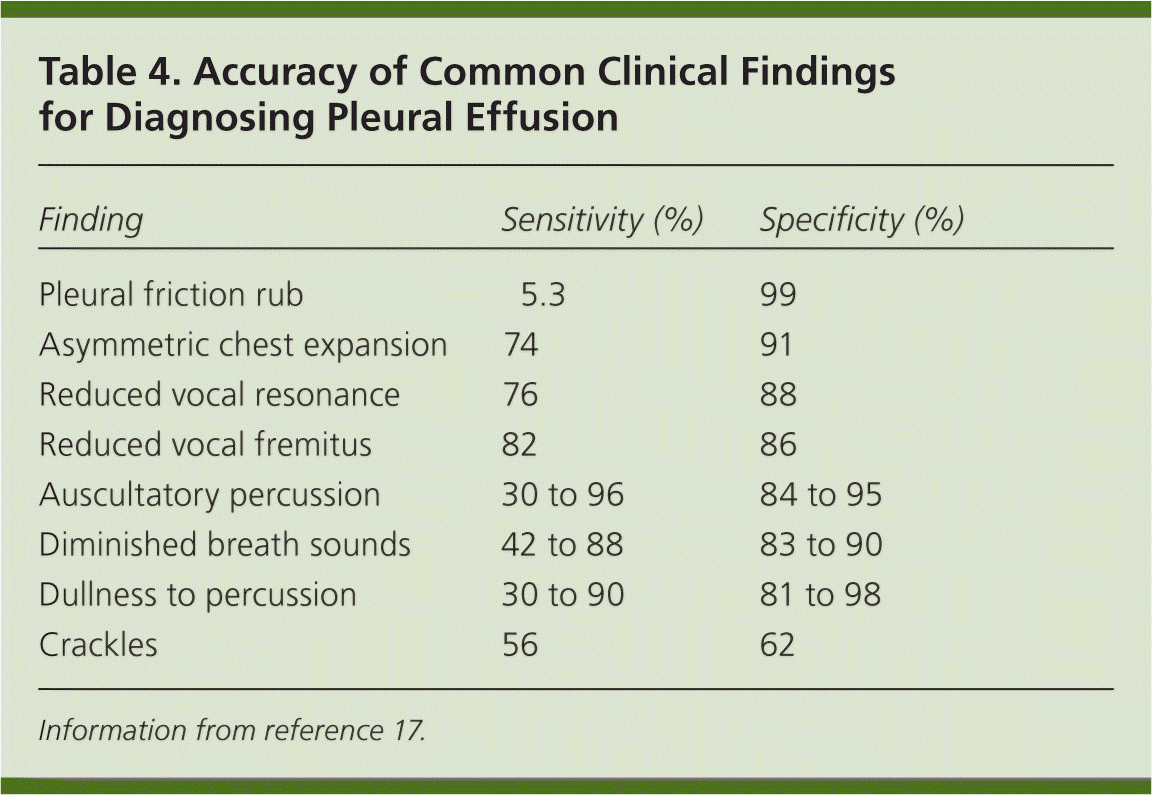
| Finding | Sensitivity (%) | Specificity (%) |
|---|---|---|
| Pleural friction rub | 5.3 | 99 |
| Asymmetric chest expansion | 74 | 91 |
| Reduced vocal resonance | 76 | 88 |
| Reduced vocal fremitus | 82 | 86 |
| Auscultatory percussion | 30 to 96 | 84 to 95 |
| Diminished breath sounds | 42 to 88 | 83 to 90 |
| Dullness to percussion | 30 to 90 | 81 to 98 |
| Crackles | 56 | 62 |
Imaging
When pleural effusion is suspected, chest radiography should be performed to confirm the diagnosis. Abnormal findings can be detected on posteroanterior radiography in the presence of 200 mL of fluid, and on lateral radiography with as little as 50 mL of fluid.10 Lateral decubitus radiography may be obtained to help determine the size of the effusion and whether it is free-flowing or loculated.
If chest radiography is inconclusive, computed tomography and ultrasonography may be useful.21,22 Computed tomography can detect effusions not apparent on plain radiography, distinguish between pleural fluid and pleural thickening, and provide clues to the underlying etiology.22 Ultrasonography is more accurate than auscultation or chest radiography in detecting pleural effusion in the critical care setting, and is more sensitive than computed tomography in detecting pleural fluid septations.10,21
Thoracentesis
INDICATIONS AND PROCEDURE
Diagnostic small-volume aspiration of pleural fluid (50 to 60 mL) is indicated when the underlying cause of effusion is unknown. Large-volume aspiration is reserved for treatment of effusion-related symptoms, such as dyspnea.10,23 Emergent thoracentesis and/or chest tube placement is necessary in patients with pleural effusion and significant respiratory or cardiac decompensation.
Chest radiography can help guide patient selection. Aspiration is required in an undiagnosed patient with an effusion larger than 1 cm on a decubitus film.18 Likewise, an effusion larger than 5 cm on a lateral radiograph in a patient with pneumonia warrants diagnostic aspiration, because parapneumonic effusions and empyema can cause nonresponse to treatment.19 Patients with suspected transudative bilateral effusions should not undergo thoracentesis unless they have atypical features (e.g., fever, pleuritic chest pain, effusions of disparate size) or do not respond to treatment.10,18 Guidelines recommend that, when possible, thoracentesis be performed with ultrasound guidance; this increases the likelihood of successful aspiration, decreases the risk of organ puncture (odds ratio for pneumothorax with ultrasonography = 0.3 to 0.8), and is associated with lower hospital costs.10,20,24 A recent systematic review, however, indicates no benefit from skin marking or ultrasound-guided needle insertion.25 Postprocedural chest radiography is not indicated unless symptoms develop.20,26 A video depicting thoracentesis is available at http://www.nejm.org/doi/full/10.1056/NEJMvcm053812 (subscription required).
FLUID ANALYSIS
Gross appearance of the pleural fluid can provide diagnostic clues (Table 5).10,11 Milky fluid may indicate a chylothorax or pseudochylothorax, whereas food particles suggest an esophageal perforation. Routine testing includes protein and lactate dehydrogenase levels, Gram staining and culture, cytology (malignant effusions can be diagnosed by cytology in 60% of cases), and pH level. Glucose levels may also be obtained.10,18
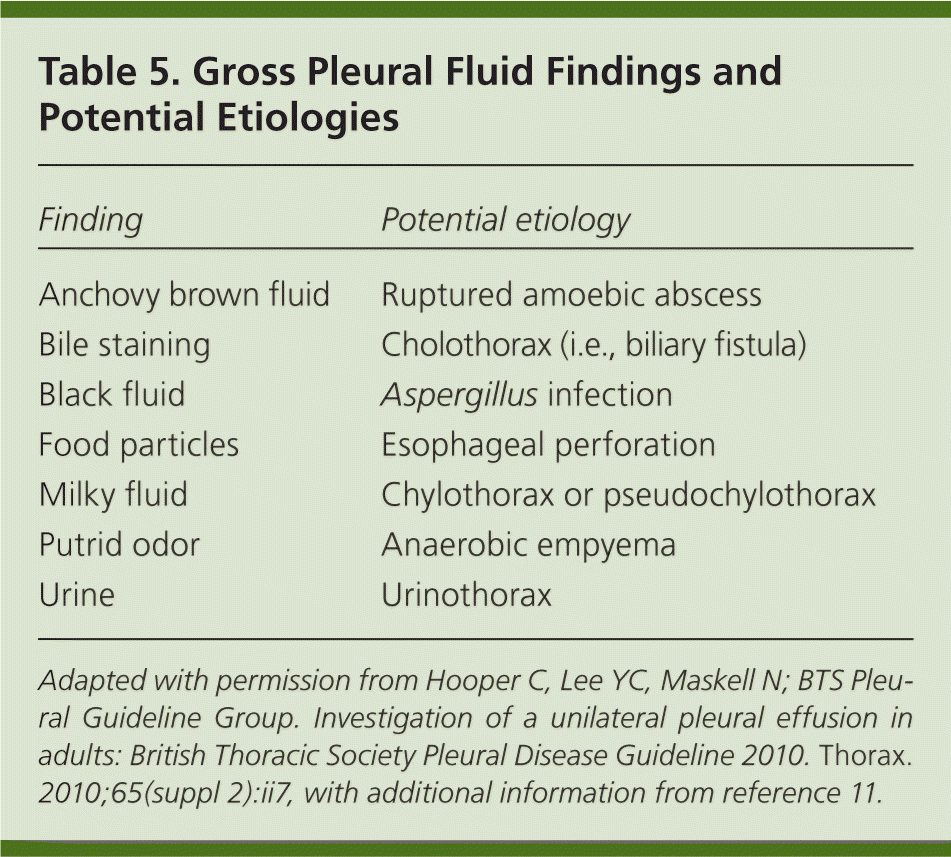
| Finding | Potential etiology |
|---|---|
| Anchovy brown fluid | Ruptured amoebic abscess |
| Bile staining | Cholothorax (i.e., biliary fistula) |
| Black fluid | Aspergillus infection |
| Food particles | Esophageal perforation |
| Milky fluid | Chylothorax or pseudochylothorax |
| Putrid odor | Anaerobic empyema |
| Urine | Urinothorax |
Protein and lactate dehydrogenase levels help determine whether collected pleural fluid represents a transudative or exudative effusion and are used to assess for Light's criteria (Figure 1).10,17–20 Light's criteria are 99.5% sensitive for diagnosing exudative effusion27 and differentiate exudative from transudative effusions in 93% to 96% of cases.10,28 In the absence of serum testing, pleural fluid protein and lactate hydrogenase levels have a 92% concordance with Light's criteria for differentiating between transudative and exudative effusions.29 A recent systematic review revealed that a pleural cholesterol level greater than 55 mg per dL (1.42 mmol per L), a pleural to serum cholesterol ratio greater than 0.3, and a pleural lactate dehydrogenase level greater than 200 units per L (3.3 μkat per L) were among the most specific findings for diagnosing an exudate.25
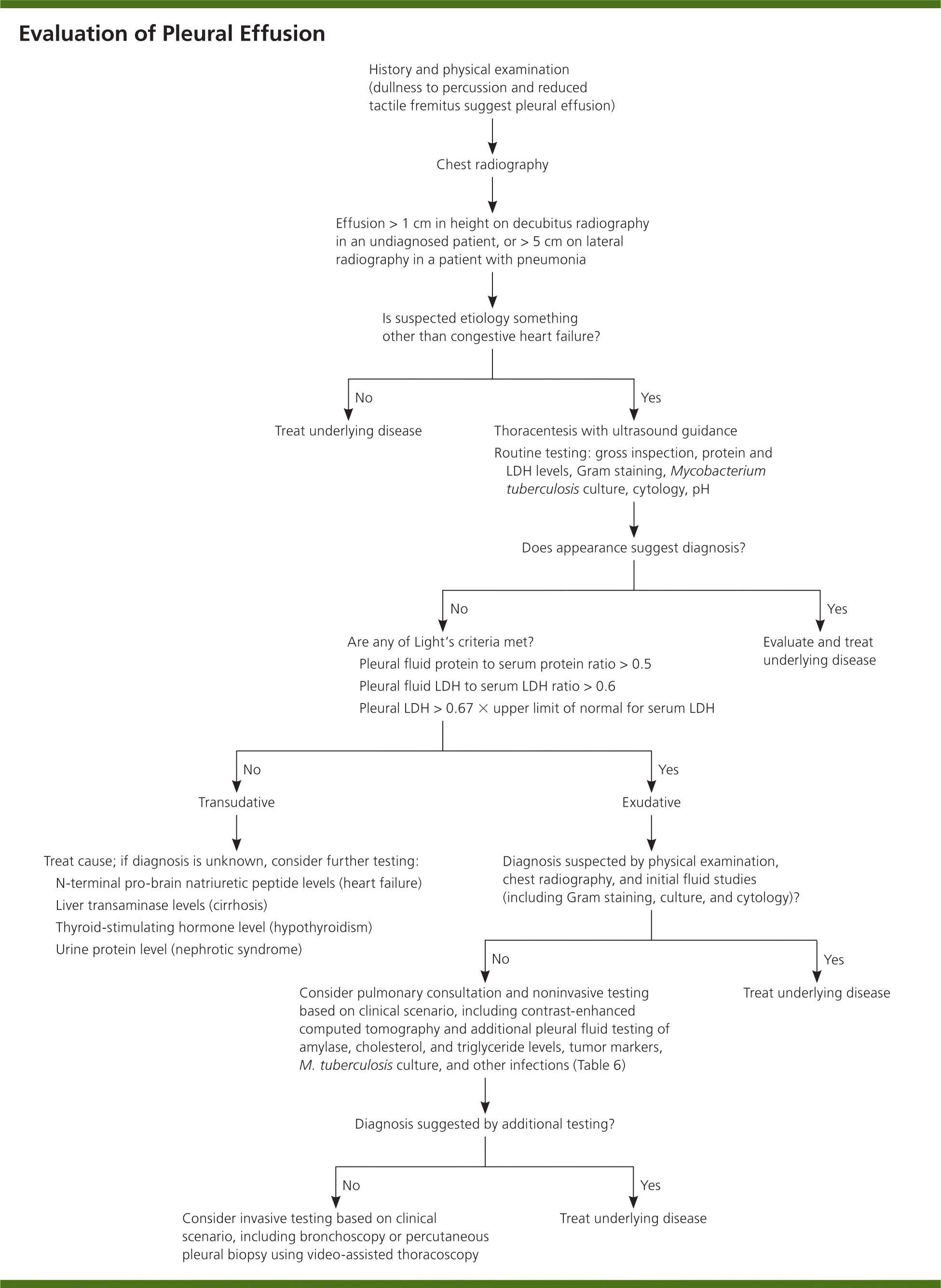
Gram staining may help identify a causative pathogen. A cell count may also reveal an underlying etiology. Neutrophil predominance tends to indicate an acute process, such as a parapneumonic effusion or pulmonary embolism, whereas lymphocyte predominance may be noted in longstanding effusions, heart failure, malignancy, tuberculosis, and thoracic duct injury.10,30 Pleural fluid pH less than 7.30 may indicate a malignant effusion, connective tissue disease, or esophageal perforation; a value less than 7.20 indicates the need for tube drainage in patients with parapneumonic effusions, especially in the setting of an elevated lactate dehydrogenase level and a glucose level less than 60 mg per dL (3.3 mmol per L).10 Further tests are guided by clinical suspicion and may include acid-fast bacillus testing (including adenosine deaminase) for tuberculosis and measurement of triglyceride, cholesterol, amylase, hematocrit, and N-terminal pro-brain natriuretic peptide levels (Table 6).6,10,11,18,30,31 Tumor markers are not routinely obtained.
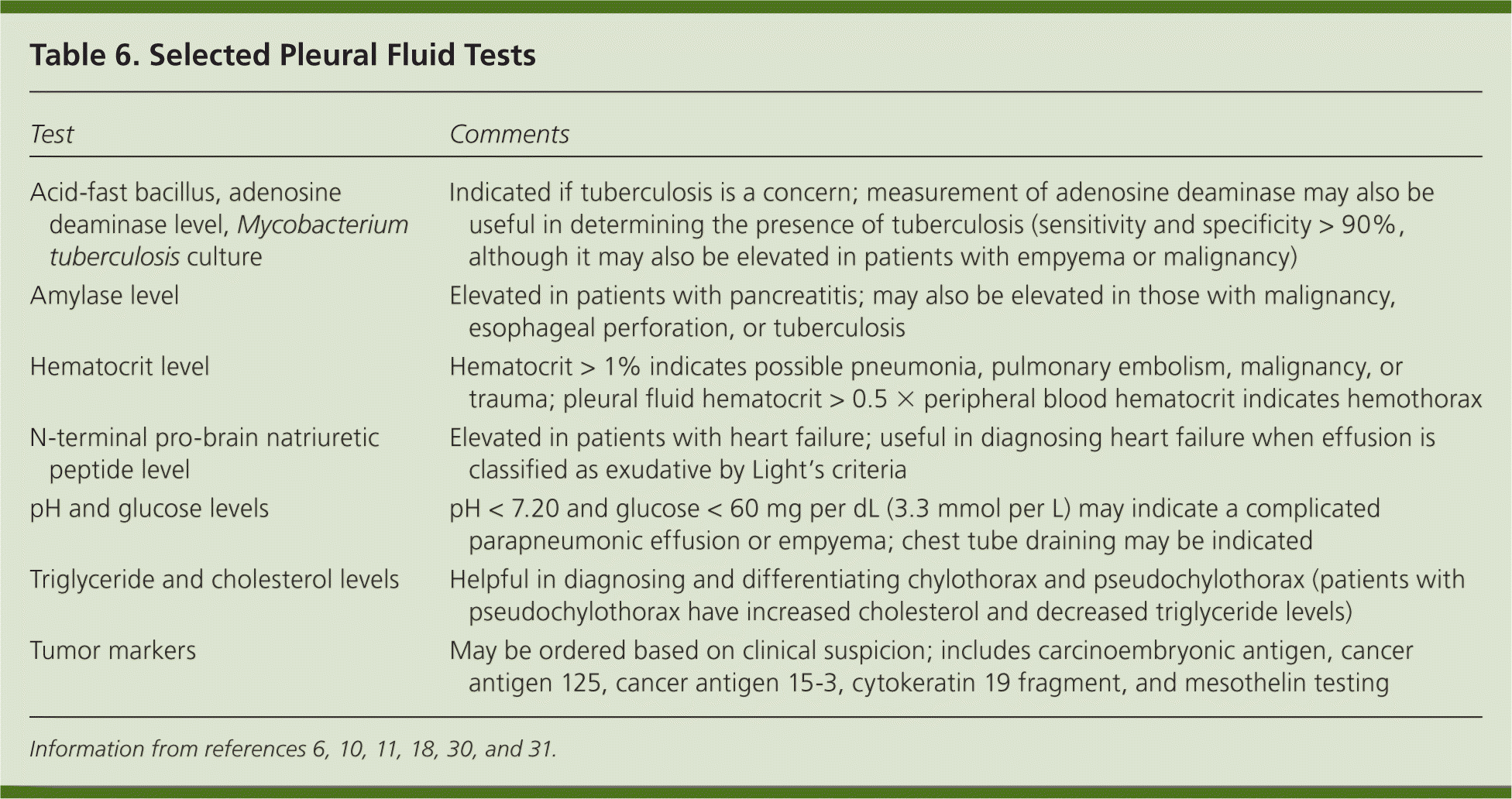
| Test | Comments |
|---|---|
| Acid-fast bacillus, adenosine deaminase level, Mycobacterium tuberculosis culture | Indicated if tuberculosis is a concern; measurement of adenosine deaminase may also be useful in determining the presence of tuberculosis (sensitivity and specificity > 90%, although it may also be elevated in patients with empyema or malignancy) |
| Amylase level | Elevated in patients with pancreatitis; may also be elevated in those with malignancy, esophageal perforation, or tuberculosis |
| Hematocrit level | Hematocrit > 1% indicates possible pneumonia, pulmonary embolism, malignancy, or trauma; pleural fluid hematocrit > 0.5 × peripheral blood hematocrit indicates hemothorax |
| N-terminal pro-brain natriuretic peptide level | Elevated in patients with heart failure; useful in diagnosing heart failure when effusion is classified as exudative by Light's criteria |
| pH and glucose levels | pH < 7.20 and glucose < 60 mg per dL (3.3 mmol per L) may indicate a complicated parapneumonic effusion or empyema; chest tube draining may be indicated |
| Triglyceride and cholesterol levels | Helpful in diagnosing and differentiating chylothorax and pseudochylothorax (patients with pseudochylothorax have increased cholesterol and decreased triglyceride levels) |
| Tumor markers | May be ordered based on clinical suspicion; includes carcinoembryonic antigen, cancer antigen 125, cancer antigen 15-3, cytokeratin 19 fragment, and mesothelin testing |
If thoracentesis is unsuccessful or the results of fluid analysis are unclear, pulmonary consultation and additional testing can be helpful. Percutaneous pleural biopsy or thoracoscopy may be indicated if malignancy is suspected. Bronchoscopy may be warranted if hemoptysis or bronchial obstruction is present.10
Data Sources: A PubMed search was completed using the keyword and medical subject headings pleural effusion and thoracentesis. The search included randomized controlled trials, meta-analyses, clinical trials, systematic reviews, clinical practice guidelines, and review articles. Also searched were Essential Evidence Plus, the National Guideline Clearinghouse, and the Cochrane Database of Systematic Reviews. Search dates: January 2012 through April 2014.
