Am Fam Physician. 2014;90(12):831-836
Author disclosure: No relevant financial affiliations.
Gout is characterized by painful joint inflammation, most commonly in the first metatarsophalangeal joint, resulting from precipitation of monosodium urate crystals in a joint space. Gout is typically diagnosed using clinical criteria from the American College of Rheumatology. Diagnosis may be confirmed by identification of monosodium urate crystals in synovial fluid of the affected joint. Acute gout may be treated with nonsteroidal anti-inflammatory drugs, corticosteroids, or colchicine. To reduce the likelihood of recurrent flares, patients should limit their consumption of certain purine-rich foods (e.g., organ meats, shellfish) and avoid alcoholic drinks (especially beer) and beverages sweetened with high-fructose corn syrup. Consumption of vegetables and low-fat or nonfat dairy products should be encouraged. The use of loop and thiazide diuretics can increase uric acid levels, whereas the use of the angiotensin receptor blocker losartan increases urinary excretion of uric acid. Reduction of uric acid levels is key to avoiding gout flares. Allopurinol and febuxostat are first-line medications for the prevention of recurrent gout, and colchicine and/or probenecid are reserved for patients who cannot tolerate first-line agents or in whom first-line agents are ineffective. Patients receiving urate-lowering medications should be treated concurrently with nonsteroidal anti-inflammatory drugs, colchicine, or low-dose corticosteroids to prevent flares. Treatment should continue for at least three months after uric acid levels fall below the target goal in those without tophi, and for six months in those with a history of tophi.
Gout is the most common inflammatory arthropathy, affecting more than 8 million Americans.1 Gout accounts for approximately 7 million ambulatory visits in the United States annually at a cost of nearly $1 billion.2 Risk factors include genetics, age, sex, and diet.2,3 These factors may contribute to a high serum uric acid level, which is currently defined as a value of at least 6.8 mg per dL (405 μmol per L).4,5
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Oral corticosteroids and nonsteroidal anti-inflammatory drugs are equally effective in the treatment of acute gout. | B | 20 |
| Febuxostat (Uloric) and allopurinol (Zyloprim) are equally effective in preventing recurrent gout. | B | 35, 36 |
| To prevent recurrent gout, patients should reduce their consumption of high-fructose corn syrup–sweetened soft drinks, fruit juices, and fructose-rich vegetables and fruits (e.g., applesauce, agave). Reducing consumption of meat and seafood, and increasing consumption of dairy products help reduce the frequency of gouty symptoms. Consumption of low-fat or nonfat dairy products may help reduce the frequency of flares. | C | 8, 12, 26 |
Pathophysiology and Risk Factors
Genetic mutations may be associated with overproduction—or more often underexcretion—of uric acid because of defects in the renal urate transporter system.6 The prevalence of gout increases with age and peaks at more than 12% in persons older than 80 years.1 Because female sex hormones increase urinary excretion of uric acid, pre-menopausal women have a substantially lower prevalence of gout compared with men (2.0% vs. 5.9%).6 Black persons have a higher risk.7 Consuming alcoholic drinks (particularly beer), meat (especially red meat, wild game, and organ meat), some seafood (e.g., shellfish, some large saltwater fish), fruit juice, and beverages sweetened with high-fructose corn syrup increases the risk of gout.8,9 Purine-rich foods such as nuts, oatmeal, asparagus, legumes, and mushrooms do not seem to increase the risk.10 Consumption of dairy products appears to confer slight protection from gout10 (Table 111,12 ).
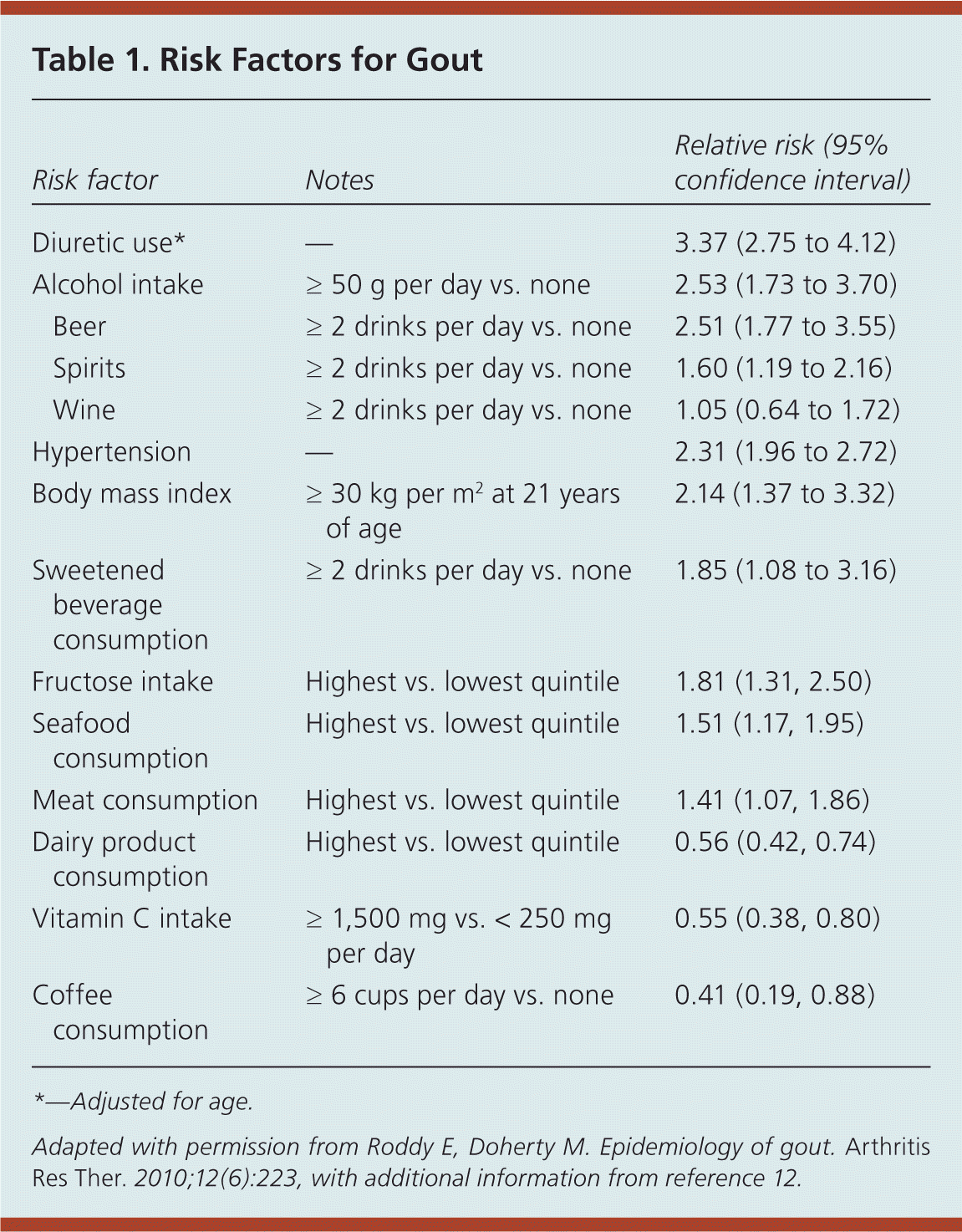
| Risk factor | Notes | Relative risk (95% confidence interval) | |
|---|---|---|---|
| Diuretic use* | — | 3.37 (2.75 to 4.12) | |
| Alcohol intake | ≥ 50 g per day vs. none | 2.53 (1.73 to 3.70) | |
| Beer | ≥ 2 drinks per day vs. none | 2.51 (1.77 to 3.55) | |
| Spirits | ≥ 2 drinks per day vs. none | 1.60 (1.19 to 2.16) | |
| Wine | ≥ 2 drinks per day vs. none | 1.05 (0.64 to 1.72) | |
| Hypertension | — | 2.31 (1.96 to 2.72) | |
| Body mass index | ≥ 30 kg per m2 at 21 years of age | 2.14 (1.37 to 3.32) | |
| Sweetened beverage consumption | ≥ 2 drinks per day vs. none | 1.85 (1.08 to 3.16) | |
| Fructose intake | Highest vs. lowest quintile | 1.81 (1.31, 2.50) | |
| Seafood consumption | Highest vs. lowest quintile | 1.51 (1.17, 1.95) | |
| Meat consumption | Highest vs. lowest quintile | 1.41 (1.07, 1.86) | |
| Dairy product consumption | Highest vs. lowest quintile | 0.56 (0.42, 0.74) | |
| Vitamin C intake | ≥ 1,500 mg vs. < 250 mg per day | 0.55 (0.38, 0.80) | |
| Coffee consumption | ≥ 6 cups per day vs. none | 0.41 (0.19, 0.88) | |
Gout results from the precipitation of monosodium urate crystals in a joint space. Crystal deposition then triggers immune activation with the release of several inflammatory cytokines and neutrophil recruitment.13 Over time, the joint space can be irreversibly damaged, leading to chronic pain and disability with grossly deformed joints. Tophi (i.e., subcutaneous nodules comprised of monosodium urate crystals in a matrix of lipids, protein, and mucopolysaccharides) may also form at the joint space14 (Figure 1). The first metatarsophalangeal joint is most commonly affected. Other common sites include the midtarsal joints, ankles, knees, fingers (Figure 2), wrists, and elbows. Urate crystals may also be deposited throughout the body (e.g., vertebrae, skin, soft tissues), mimicking other disease states.15
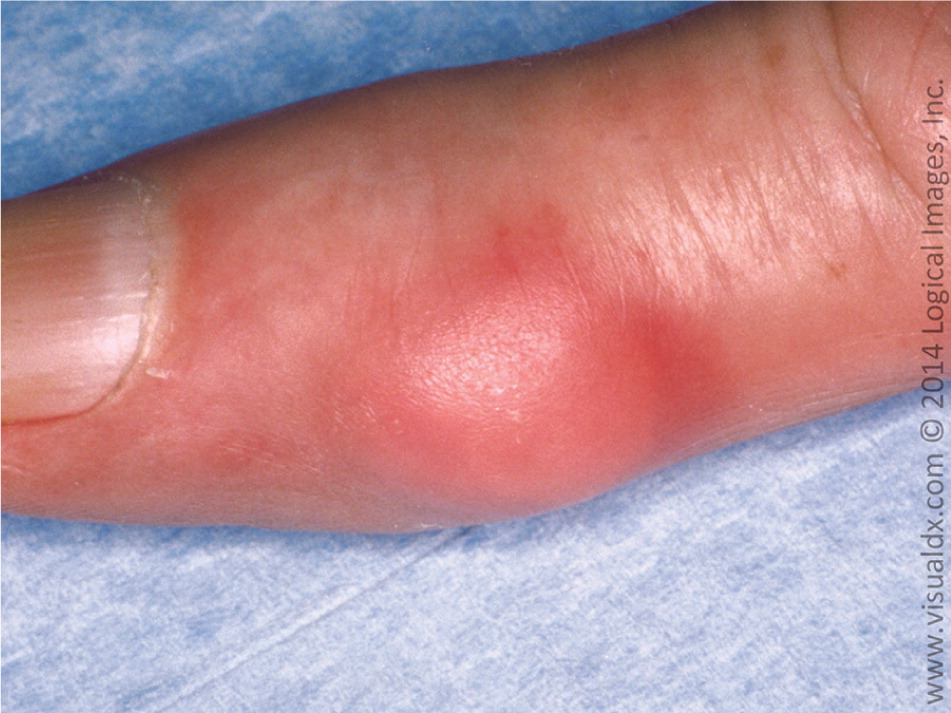
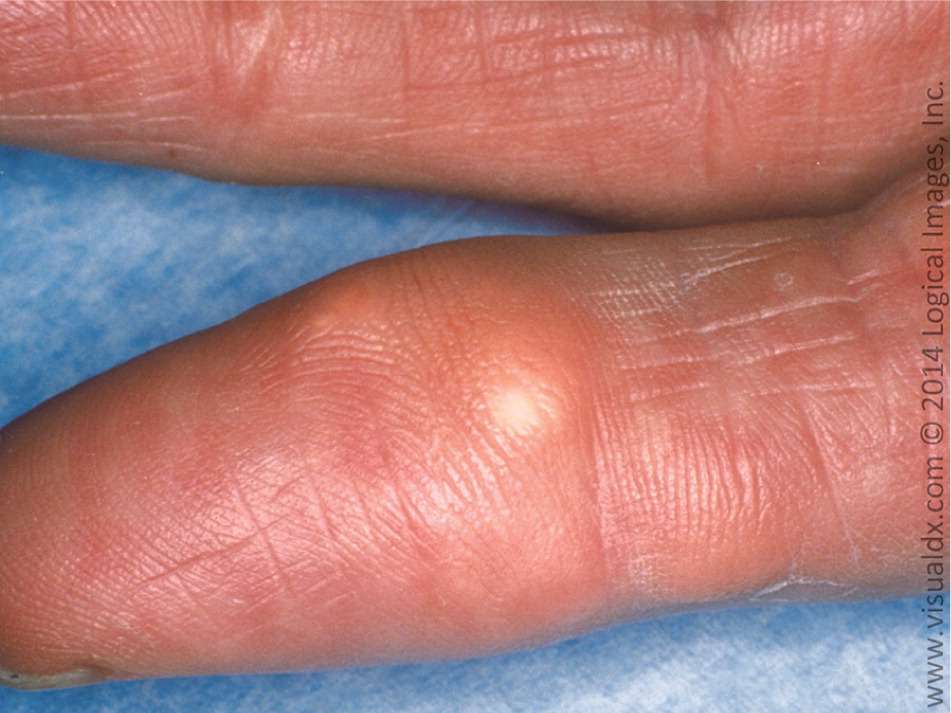
Clinical Presentation
Gout is typically diagnosed clinically based on the rapid development of monoarticular arthritis marked by swelling and redness usually involving the first metatarsophalangeal joint. The American College of Rheumatology criteria are the most widely used for diagnosis of gout (Table 2).16 An online risk calculator is also available (http://www.gp-training.net/rheum/gout_calc.htm); it uses sex, uric acid level, and five findings from the history and physical examination to predict the likelihood of an acute gout flare.17

| Presence of characteristic urate crystals in the joint fluid | |
| or | |
| Presence of a tophus proven to contain urate crystals by chemical means or polarized light microscopy | |
| or | |
| Presence of six or more of the following clinical, laboratory, or radiologic findings: | |
| Asymmetric swelling within a joint on radiography | |
| Attack of monoarticular arthritis | |
| Culture of joint fluid negative for microorganisms during attack of joint inflammation | |
| Development of maximal inflammation within one day | |
| Hyperuricemia | |
| Joint redness | |
| More than one attack of acute arthritis | |
| Pain or redness in the first metatarsophalangeal joint | |
| Subcortical cyst without erosions on radiography | |
| Suspected tophus | |
| Unilateral attack involving first metatarsophalangeal joint | |
| Unilateral attack involving tarsal joint | |
Microscopy of joint fluid is used less often, primarily in equivocal cases. In these situations, the diagnosis is established by aspiration of a joint or tophus and identification of needle-shaped monosodium urate crystals, preferably intracellular, with bright, negative birefringence on compensated polarized light microscopy. Ultrasonography, magnetic resonance imaging, and computed tomography are typically not necessary for diagnosis.
The differential diagnosis for acute monoarticular joint swelling includes pseudogout, infection, and trauma. Pseudogout, or calcium pyrophosphate deposition disease, can mimic gout in clinical appearance and may respond to nonsteroidal anti-inflammatory drugs (NSAIDs). Findings of calcium pyrophosphate crystals and normal serum uric acid levels on joint fluid analysis can differentiate pseudogout from gout. Septic arthritis may present without a fever or elevated white blood cell count; arthrocentesis is required to distinguish this condition from acute gout. Gout and septic arthritis can occur concomitantly, but this is rare.18 Trauma-associated joint swelling is typically identified by the history; however, trauma may result in an acute gout flare caused by increased concentrations of synovial urate.19 Imaging may be necessary to rule out fracture in a patient with gout-like symptoms after a joint injury.
Treatment
To achieve rapid and complete resolution of symptoms, treatment of acute gout should commence within 24 hours of symptom onset20 (Table 321 ). Oral corticosteroids, intravenous corticosteroids, NSAIDs, and colchicine are equally effective in treating acute flares of gout.20 NSAIDs are the first-line treatment. Indomethacin (Indocin) has historically been the preferred choice; however, there is no evidence it is more effective than any other NSAID. Intramuscular ketorolac appears to have similar effectiveness.21 Any oral NSAID may be given at the maximal dosage and continued for one to two days after relief of symptoms.
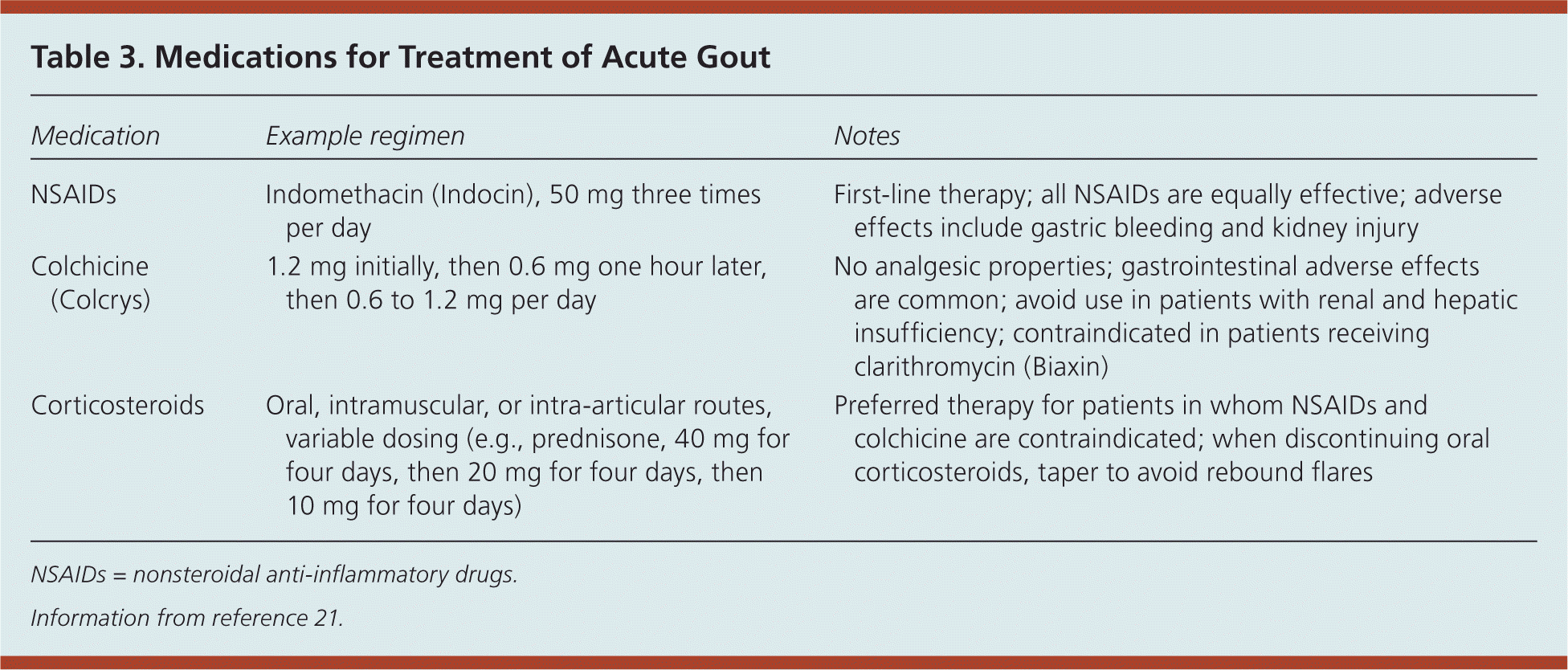
| Medication | Example regimen | Notes |
|---|---|---|
| NSAIDs | Indomethacin (Indocin), 50 mg three times per day | First-line therapy; all NSAIDs are equally effective; adverse effects include gastric bleeding and kidney injury |
| Colchicine (Colcrys) | 1.2 mg initially, then 0.6 mg one hour later, then 0.6 to 1.2 mg per day | No analgesic properties; gastrointestinal adverse effects are common; avoid use in patients with renal and hepatic insufficiency; contraindicated in patients receiving clarithromycin (Biaxin) |
| Corticosteroids | Oral, intramuscular, or intra-articular routes, variable dosing (e.g., prednisone, 40 mg for four days, then 20 mg for four days, then 10 mg for four days) | Preferred therapy for patients in whom NSAIDs and colchicine are contraindicated; when discontinuing oral corticosteroids, taper to avoid rebound flares |
Corticosteroids are an appropriate alternative for patients who cannot tolerate NSAIDs or colchicine.22 Patients with diabetes mellitus can be given corticosteroids for short-term use with appropriate monitoring for hyperglycemia. When gout is limited to a single joint, intra-articular corticosteroid injections may be preferable to systemic corticosteroids because of their lower adverse effect profile.23 Rebound flares are common after discontinuation of corticosteroid therapy for acute gout. To reduce the risk of a rebound flare, preventive treatment and initiation of a tapered course of corticosteroids over 10 to 14 days is recommended after resolution of symptoms.
Colchicine is another treatment option for acute gout. Generic colchicine, which has been used for decades, did not undergo formal review by the U.S. Food and Drug Administration (FDA) for this indication until 2009, when branded colchicine (Colcrys) was approved. However, Colcrys is expensive, and generic colchicine is no longer available. In addition, colchicine does not have analgesic properties and may be less effective in treating acute flares when given beyond 72 to 96 hours after symptom onset. Common adverse effects include nausea, vomiting, and diarrhea.21,23 Colchicine should be used with caution in patients with hepatic or renal impairment.22
Prevention
Serum urate–lowering therapy should be initiated to prevent recurrences in persons with a history of gout and any one of the following: at least two flares per year (one per year in persons with chronic kidney disease stage 2 or greater), tophi, or a history of nephrolithiasis.20
Serum urate should be lowered to a target of less than 5 to 6 mg per dL (297 to 357 μmol per L), depending on the crystal and tophaceous burden.20 Normal serum urate levels do not exclude the diagnosis of gout. They should be monitored periodically to assess preventive therapy in patients with recurrent gout and a history of elevated urate levels.22 Urate-lowering therapy should be continued for three to six months after a flare if there are no ongoing symptoms. Therapy should continue indefinitely if there are ongoing signs or symptoms (e.g., one or more tophi on examination).20
DIETARY MODIFICATIONS
Weight gain is a significant risk factor for gout in men, whereas weight loss reduces the risk.12 Intake of high-fructose corn syrup should be restricted25,26 because the fructose contributes to increased uric acid production as a byproduct of adenosine triphosphate catabolism.20,27 Patients with gout should limit their intake of purine-rich animal protein (e.g., organ meats, beef, lamb, pork, shellfish) and avoid alcohol (especially beer).20 Purine-rich vegetables do not increase the risk of gout.8,9 Consumption of vegetables9 and low-fat or nonfat dairy products9 should be encouraged.
PHARMACOLOGIC OPTIONS
Pharmacologic options for prevention of chronic gout are outlined in Table 4.21,28 Although avoidance of loop and thiazide diuretics has been recommended for patients with hypertension and gout because these agents can increase uric acid levels, a systematic review found only small increases in the risk of gouty flares.29 Calcium channel blockers and the angiotensin receptor blocker losartan (Cozaar) are associated with a decreased risk of incident gout.24,30 Losartan is the only angiotensin receptor blocker with this property.
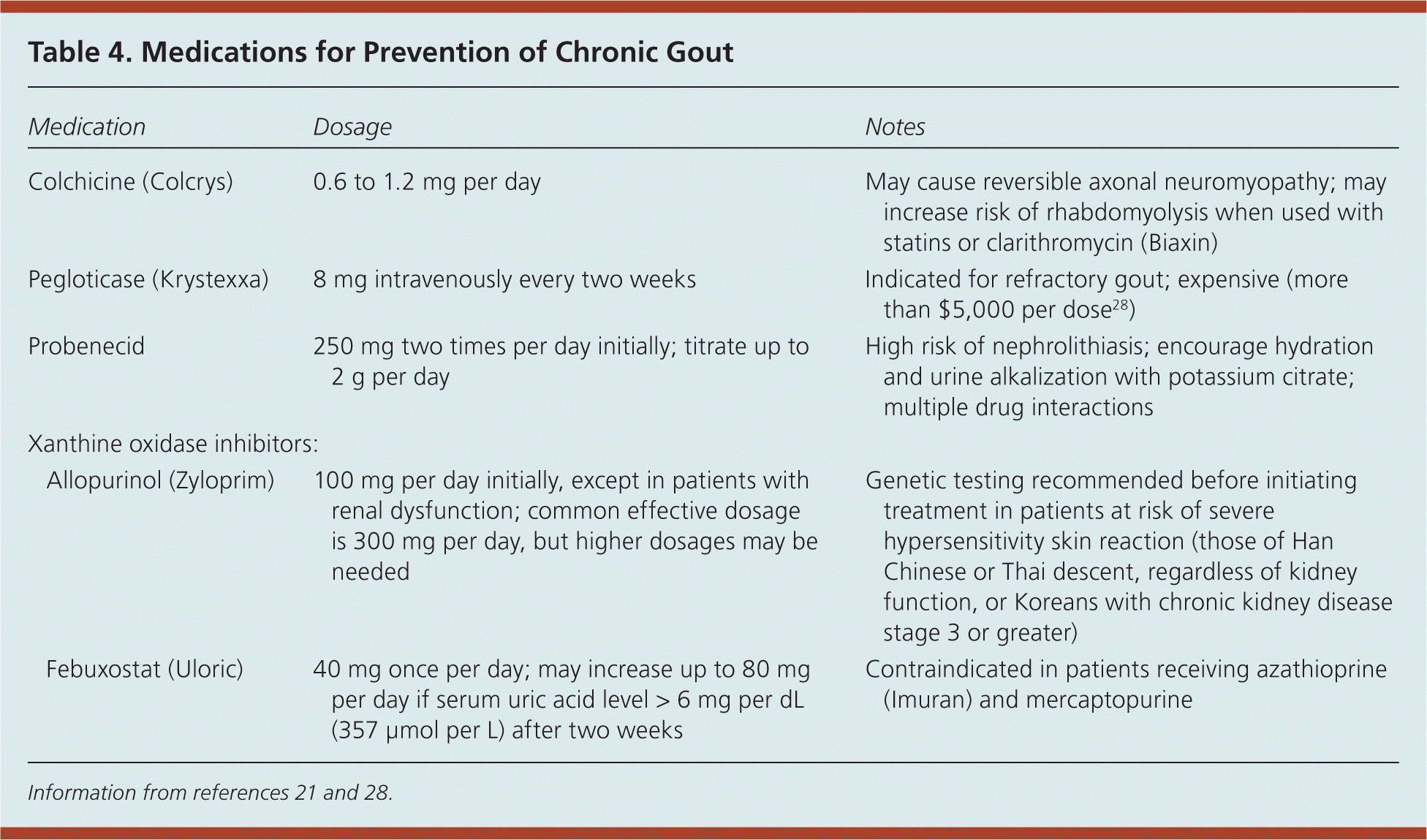
| Medication | Dosage | Notes | |
|---|---|---|---|
| Colchicine (Colcrys) | 0.6 to 1.2 mg per day | May cause reversible axonal neuromyopathy; may increase risk of rhabdomyolysis when used with statins or clarithromycin (Biaxin) | |
| Pegloticase (Krystexxa) | 8 mg intravenously every two weeks | Indicated for refractory gout; expensive (more than $5,000 per dose28) | |
| Probenecid | 250 mg two times per day initially; titrate up to 2 g per day | High risk of nephrolithiasis; encourage hydration and urine alkalization with potassium citrate; multiple drug interactions | |
| Xanthine oxidase inhibitors: | |||
| Allopurinol (Zyloprim) | 100 mg per day initially, except in patients with renal dysfunction; common effective dosage is 300 mg per day, but higher dosages may be needed | Genetic testing recommended before initiating treatment in patients at risk of severe hypersensitivity skin reaction (those of Han Chinese or Thai descent, regardless of kidney function, or Koreans with chronic kidney disease stage 3 or greater) | |
| Febuxostat (Uloric) | 40 mg once per day; may increase up to 80 mg per day if serum uric acid level > 6 mg per dL (357 μmol per L) after two weeks | Contraindicated in patients receiving azathioprine (Imuran) and mercaptopurine | |
Historically, urate-lowering medication was thought to worsen acute gout flares, but recent evidence suggests that allopurinol (Zyloprim) can be started during an acute flare if it is used in conjunction with an NSAID and colchicine.31 Patients receiving a urate-lowering medication should be treated concurrently with an NSAID, colchicine, or low-dose corticosteroid to prevent a flare. Treatment should continue for at least three months after uric acid levels fall below the target goal in those without tophi, or for six months in those with a history of tophi.20 NSAIDs and corticosteroids should not be used for long periods without a urate-lowering medication because uric acid crystals continue to accumulate and damage the joint, despite a lack of pain or clinical signs of inflammation.22 If a patient has a gout flare while receiving a urate-lowering agent, the medication should be continued while the flare is treated acutely.20
Allopurinol. Allopurinol, a xanthine oxidase inhibitor, is a first-line agent to prevent recurrent gout.9 In patients with gout and chronic kidney disease or congestive heart failure, allopurinol has the added benefit of preventing chronic disease progression.31,32 The starting dosage is 100 mg per day, and 300 mg per day is a common maintenance dosage. Dosing is guided by the target serum uric acid level.9,22 In patients with chronic kidney disease, low initial doses are recommended with slow titration to achieve target uric acid levels.33 Dosages higher than 300 mg may be used—even in those with renal impairment—as long as patients are closely monitored for adverse effects.9 Certain ethnic groups have a higher risk of a severe hypersensitivity skin reaction when starting allopurinol therapy. Screening for human leukocyte antigen-B*5801 genotype is recommended before initiating treatment in patients of Han Chinese or Thai descent, regardless of kidney function, or in Koreans with chronic kidney disease stage 3 or greater.34
Febuxostat. Febuxostat (Uloric) is a xanthine oxidase inhibitor that was approved by the FDA in 2009. Although febuxostat is superior to 300 mg allopurinol at lowering serum uric acid levels, it is not more effective at reducing the frequency of gout flares.35,36 Febuxostat is considered a first-line agent to prevent recurrent gout,9 but it is considerably more expensive than allopurinol.
Colchicine. Colchicine prevents gout flares at a dosage of 0.6 to 1.2 mg per day. The dose should be adjusted in patients with chronic kidney disease and when used with cytochrome P450 3A4 or P-glycoprotein inhibitors. The long-term adverse effects of colchicine include reversible axonal neuromyopathy (less than 1%). Patients should be advised to stop taking colchicine and tell their physician if they experience leg weakness or pain. Treatment should be discontinued if any signs or symptoms of nerve or muscle damage are present. The rare risk of rhabdomyolysis is increased when colchicine is used concomitantly with statins or clarithromycin (Biaxin), especially in older adults or those with chronic kidney disease; therefore, close monitoring is recommended.37
Probenecid. Probenecid increases urinary excretion of uric acid and is typically used as a second-line treatment because of numerous drug interactions. Of particular concern, probenecid increases blood levels of methotrexate and ketorolac, which may result in severe toxicity. Probenecid may be used in combination with allopurinol or febuxostat when one drug does not independently lower serum uric acid to target levels. Nephrolithiasis is a common adverse effect that may be avoided by high fluid intake and urine alkalization with potassium citrate.9
Pegloticase. Pegloticase (Krystexxa) is an intravenous uricase approved by the FDA in 2010. The mechanism of action involves metabolism of uric acid to allantoin. It is a third-line agent and is indicated for treatment of refractory gout. It is usually administered by a rheumatologist and is given every two weeks at a cost of more than $5,000 per dose.28
Data Sources: We searched PubMed, the Cochrane database, Essential Evidence Plus, and the National Guideline Clearinghouse. The search included expert consensus statements, clinical reviews, and clinical trials. Search terms included gout, gouty arthritis, gout prevention, and gout therapy. Search date: May 2014.