
A more recent article on sodium disorders is available.
This is a corrected version of the article that appeared in print.
Am Fam Physician. 2015;91(5):299-307
Author disclosure: No relevant financial affiliations.
Hyponatremia and hypernatremia are common findings in the inpatient and outpatient settings. Sodium disorders are associated with an increased risk of morbidity and mortality. Plasma osmolality plays a critical role in the pathophysiology and treatment of sodium disorders. Hyponatremia and hypernatremia are classified based on volume status (hypovolemia, euvolemia, and hypervolemia). Sodium disorders are diagnosed by findings from the history, physical examination, laboratory studies, and evaluation of volume status. Treatment is based on symptoms and underlying causes. In general, hyponatremia is treated with fluid restriction (in the setting of euvolemia), isotonic saline (in hypovolemia), and diuresis (in hypervolemia). A combination of these therapies may be needed based on the presentation. Hypertonic saline is used to treat severe symptomatic hyponatremia. Medications such as vaptans may have a role in the treatment of euvolemic and hypervolemic hyponatremia. The treatment of hypernatremia involves correcting the underlying cause and correcting the free water deficit.
Hyponatremia is a common electrolyte disorder defined as a serum sodium level of less than 135 mEq per L.1–3 A Dutch systematic review of 53 studies showed that the prevalence of mild hyponatremia was 22.2% in geriatric hospital wards, 6.0% in nongeriatric wards, and 17.2% in the intensive care unit.2 The prevalence of severe hyponatremia (serum sodium level less than 125 mEq per L) was 4.5%, 0.8%, and 10.3%, respectively. It is estimated that hyponatremia occurs in 4% to 7% of the ambulatory population, with rates of 18.8% in nursing homes.2–4
| Clinical recommendation | Evidence rating | References | Comments |
|---|---|---|---|
| In patients with severe symptomatic hyponatremia, the rate of sodium correction should be 6 to 12 mEq per L in the first 24 hours and 18 mEq per L or less in 48 hours. | C | 13, 14 | Consensus guidelines based on systematic reviews |
| A bolus of 100 to 150 mL of hypertonic 3% saline can be given to correct severe hyponatremia. | C | 13, 14 | Consensus guidelines based on small studies |
| Vaptans appear to be safe for the treatment of severe hypervolemic and euvolemic hyponatremia but should not be used routinely. | C | 14 | Consensus guidelines based on observational studies |
| Chronic hypernatremia should be corrected at a rate of 0.5 mEq per L per hour, with a maximum change of 8 to 10 mEq per L in a 24-hour period. | C | 33 | Expert opinion |
Hyponatremia is associated with increased morbidity and mortality.1–6 In patients with heart failure who undergo cardiac surgery, hyponatremia increases rates of postoperative complications, length of hospital stay, and mortality.5,6 Mild hyponatremia in the ambulatory setting is associated with increased mortality (hazard ratio = 1.94) compared with normal sodium levels.3 Patients who develop hyponatremia during hospitalization have increased mortality rates compared with those who have hyponatremia on admission.7,8 It is unclear if hyponatremia is a marker for poor prognostic outcomes or merely a reflection of disease severity. Its presence suggests a worse prognosis in patients with liver cirrhosis, pulmonary hypertension, myocardial infarction, chronic kidney disease, hip fractures, and pulmonary embolism.1,8–10
Etiology and Pathophysiology
The most common classification system for hyponatremia is based on volume status: hypovolemic (decreased total body water with greater decrease in sodium level), euvolemic (increased total body water with normal sodium level), and hypervolemic (increased total body water compared with sodium).11
Plasma osmolality has a role in the pathophysiology of hyponatremia. Osmolality refers to the total concentration of solutes in water. Effective osmolality is the osmotic gradient created by solutes that do not cross the cell membrane. Effective osmolality determines the osmotic pressure and the flow of water.11 Plasma osmolality is maintained by strict regulation of the arginine vasopressin (also called antidiuretic hormone [ADH]) system and thirst. If plasma osmolality increases, ADH is secreted and water is retained by the kidneys, thus decreasing serum osmolality. If plasma osmolality decreases, ADH also decreases, resulting in diuresis of free water and a return to homeostasis.12,13
Diagnostic Approach to Hyponatremia
Symptoms of hyponatremia depend on its severity and on the rate of sodium decline. Gradual decreases in sodium usually result in minimal symptoms, whereas rapid decreases can result in severe symptoms. Polydipsia, muscle cramps, headaches, falls, confusion, altered mental status, obtundation, coma, and status epilepticus may indicate the need for acute intervention. Most patients with hyponatremia are asymptomatic, and hyponatremia is noted incidentally. Volume status should be assessed to help determine the underlying cause11,13 (Figure 111–16 [corrected]).
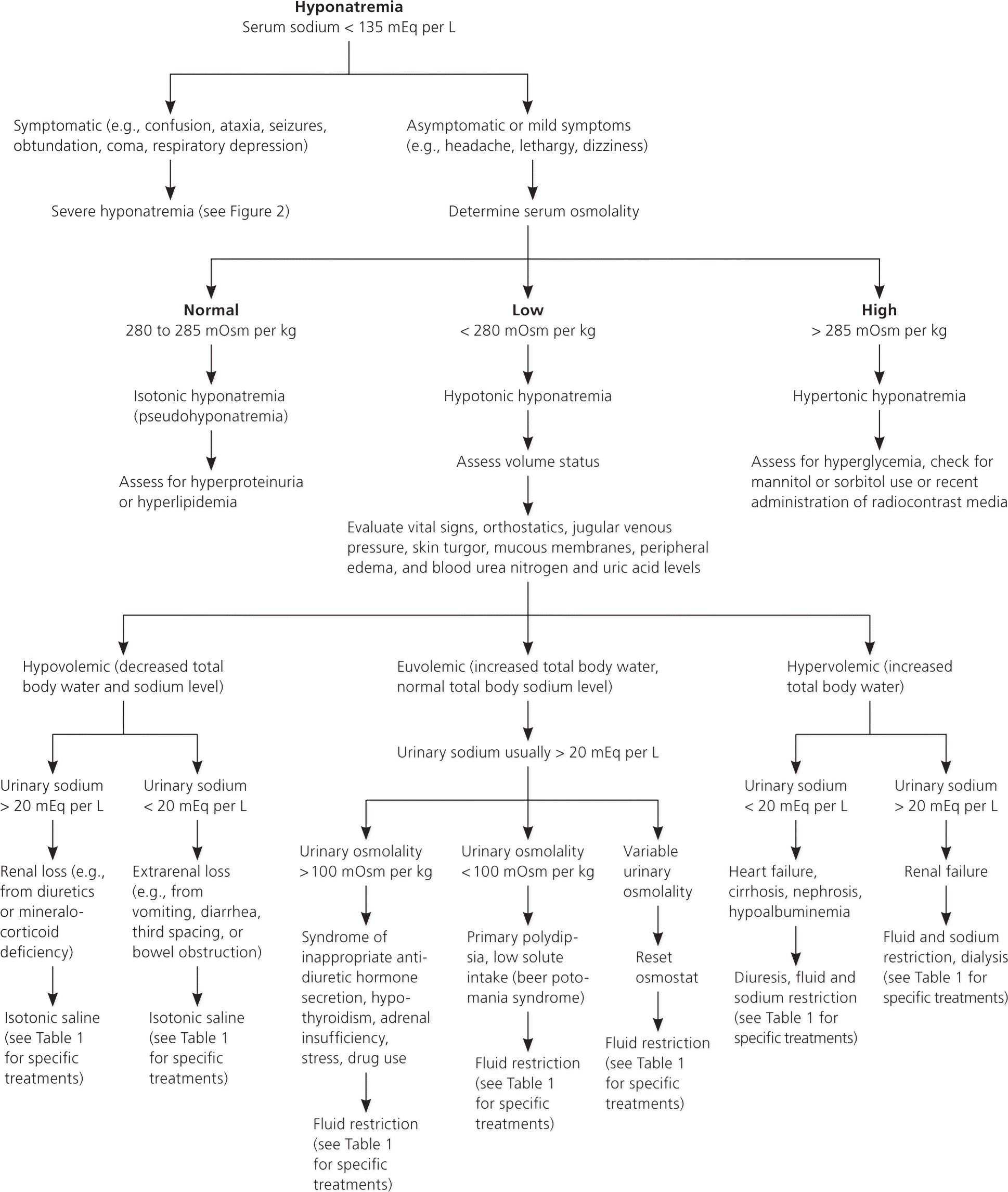
The diagnostic workup should include a history and physical examination with specific attention to cardiac, cancer, pulmonary, surgical, endocrine, gastrointestinal, neurologic, and renal histories (Table 1).11–13 Diuretics, carbamazepine (Tegretol), and selective serotonin reuptake inhibitors can cause hypovolemia; therefore, medications should be reviewed. Alcohol and illicit drug use (especially beer and 3,4-methylenedioxymethamphetamine [“Ecstasy”]) can cause hyponatremia.11–13 Athletes should be asked about training regimens because high endurance activities can lead to hyponatremia.
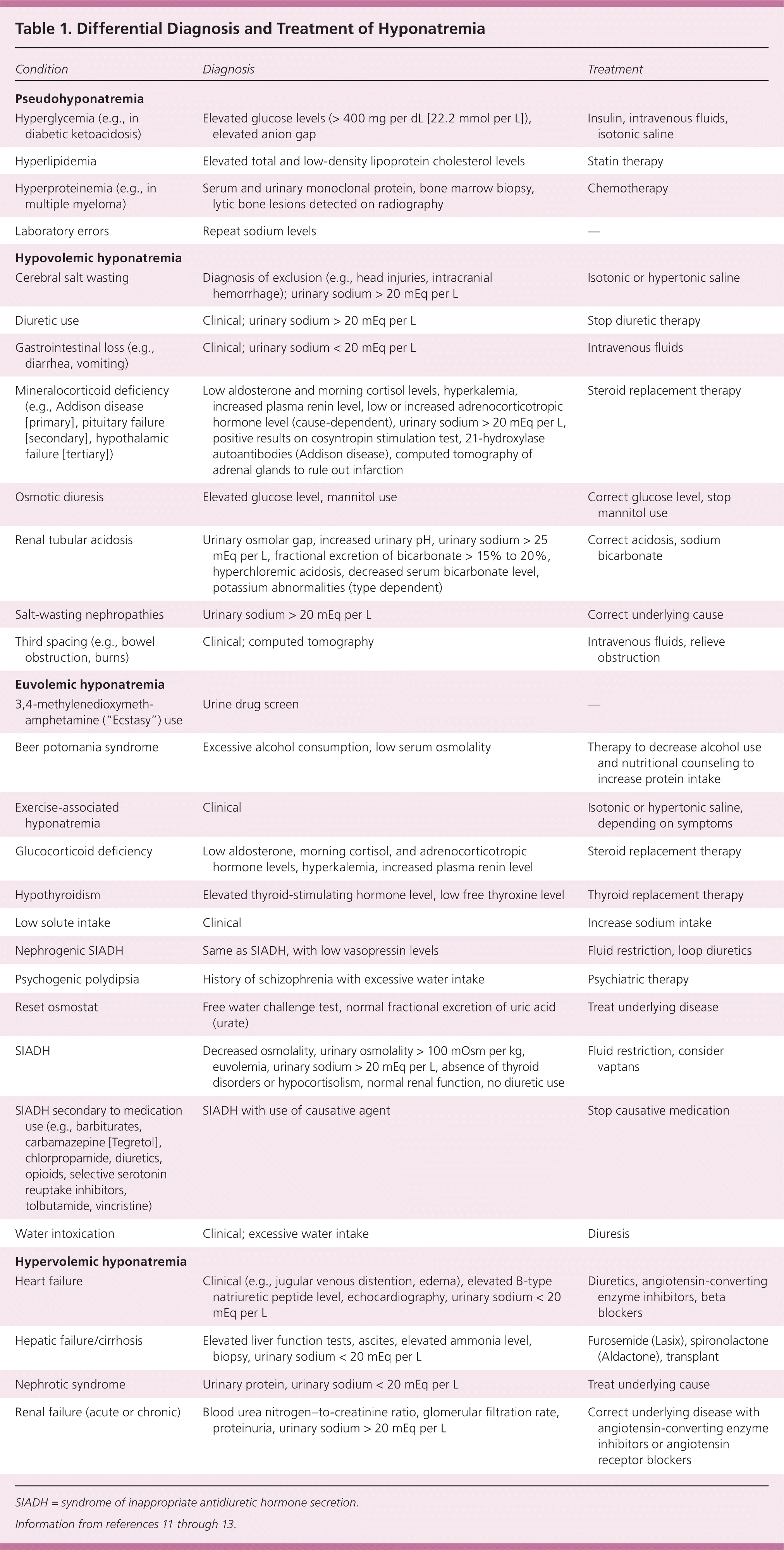
| Condition | Diagnosis | Treatment |
|---|---|---|
| Pseudohyponatremia | ||
| Hyperglycemia (e.g., in diabetic ketoacidosis) | Elevated glucose levels (> 400 mg per dL [22.2 mmol per L]), elevated anion gap | Insulin, intravenous fluids, isotonic saline |
| Hyperlipidemia | Elevated total and low-density lipoprotein cholesterol levels | Statin therapy |
| Hyperproteinemia (e.g., in multiple myeloma) | Serum and urinary monoclonal protein, bone marrow biopsy, lytic bone lesions detected on radiography | Chemotherapy |
| Laboratory errors | Repeat sodium levels | — |
| Hypovolemic hyponatremia | ||
| Cerebral salt wasting | Diagnosis of exclusion (e.g., head injuries, intracranial hemorrhage); urinary sodium > 20 mEq per L | Isotonic or hypertonic saline |
| Diuretic use | Clinical; urinary sodium > 20 mEq per L | Stop diuretic therapy |
| Gastrointestinal loss (e.g., diarrhea, vomiting) | Clinical; urinary sodium < 20 mEq per L | Intravenous fluids |
| Mineralocorticoid deficiency (e.g., Addison disease [primary], pituitary failure [secondary], hypothalamic failure [tertiary]) | Low aldosterone and morning cortisol levels, hyperkalemia, increased plasma renin level, low or increased adrenocorticotropic hormone level (cause-dependent), urinary sodium > 20 mEq per L, positive results on cosyntropin stimulation test, 21-hydroxylase autoantibodies (Addison disease), computed tomography of adrenal glands to rule out infarction | Steroid replacement therapy |
| Osmotic diuresis | Elevated glucose level, mannitol use | Correct glucose level, stop mannitol use |
| Renal tubular acidosis | Urinary osmolar gap, increased urinary pH, urinary sodium > 25 mEq per L, fractional excretion of bicarbonate > 15% to 20%, hyperchloremic acidosis, decreased serum bicarbonate level, potassium abnormalities (type dependent) | Correct acidosis, sodium bicarbonate |
| Salt-wasting nephropathies | Urinary sodium > 20 mEq per L | Correct underlying cause |
| Third spacing (e.g., bowel obstruction, burns) | Clinical; computed tomography | Intravenous fluids, relieve obstruction |
| Euvolemic hyponatremia | ||
| 3,4-methylenedioxymeth-amphetamine (“Ecstasy”) use | Urine drug screen | — |
| Beer potomania syndrome | Excessive alcohol consumption, low serum osmolality | Therapy to decrease alcohol use and nutritional counseling to increase protein intake |
| Exercise-associated hyponatremia | Clinical | Isotonic or hypertonic saline, depending on symptoms |
| Glucocorticoid deficiency | Low aldosterone, morning cortisol, and adrenocorticotropic hormone levels, hyperkalemia, increased plasma renin level | Steroid replacement therapy |
| Hypothyroidism | Elevated thyroid-stimulating hormone level, low free thyroxine level | Thyroid replacement therapy |
| Low solute intake | Clinical | Increase sodium intake |
| Nephrogenic SIADH | Same as SIADH, with low vasopressin levels | Fluid restriction, loop diuretics |
| Psychogenic polydipsia | History of schizophrenia with excessive water intake | Psychiatric therapy |
| Reset osmostat | Free water challenge test, normal fractional excretion of uric acid (urate) | Treat underlying disease |
| SIADH | Decreased osmolality, urinary osmolality > 100 mOsm per kg, euvolemia, urinary sodium > 20 mEq per L, absence of thyroid disorders or hypocortisolism, normal renal function, no diuretic use | Fluid restriction, consider vaptans |
| SIADH secondary to medication use (e.g., barbiturates, carbamazepine [Tegretol], chlorpropamide, diuretics, opioids, selective serotonin reuptake inhibitors, tolbutamide, vincristine) | SIADH with use of causative agent | Stop causative medication |
| Water intoxication | Clinical; excessive water intake | Diuresis |
| Hypervolemic hyponatremia | ||
| Heart failure | Clinical (e.g., jugular venous distention, edema), elevated B-type natriuretic peptide level, echocardiography, urinary sodium < 20 mEq per L | Diuretics, angiotensin-converting enzyme inhibitors, beta blockers |
| Hepatic failure/cirrhosis | Elevated liver function tests, ascites, elevated ammonia level, biopsy, urinary sodium < 20 mEq per L | Furosemide (Lasix), spironolactone (Aldactone), transplant |
| Nephrotic syndrome | Urinary protein, urinary sodium < 20 mEq per L | Treat underlying cause |
| Renal failure (acute or chronic) | Blood urea nitrogen–to-creatinine ratio, glomerular filtration rate, proteinuria, urinary sodium > 20 mEq per L | Correct underlying disease with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers |
Laboratory tests include a complete metabolic panel and urinary sodium and creatinine levels.11,13 Serum osmolality and fractional excretion of sodium should be calculated (eTable A). Measurement of thyroid-stimulating hormone, urinary uric acid, adrenocorticotropic hormone, plasma cortisol, and brain natriuretic peptide may be considered in select patients to rule out other causes.13 The diagnosis of reset osmostat (a variation of syndrome of inappropriate antidiuretic hormone secretion [SIADH] in which ADH secretion occurs despite low plasma osmolality) may be aided using fractional excretion of urate (uric acid) in nonedematous patients who have hyponatremia that does not respond to usual treatment.17

| Measurement | Equation | |
|---|---|---|
| Corrected sodium | Measured sodium + 0.024 × (serum glucose − 100)* | |
| or | ||
| Measured sodium + 0.016 × (serum glucose − 100) | ||
| Normal = 135 to 145 mEq per L | ||
| Online calculators available at http://www.mdcalc.com/sodium-correction-for-hyperglycemia and http://www.medcalc.com/correctna.html | ||
| Fractional excretion of sodium | ([Plasma creatinine × urinary sodium] / [plasma sodium × urinary creatinine]) × 100 | |
| Prerenal < 1%, intrinsic > 1%, and postrenal > 4% | ||
| Online calculators available at http://www.mdcalc.com/fractional-excretion-of-sodium-fena and http://www.medcalc.com/fena.html | ||
| Infusion rate of sodium | Online calculators for the rate of infusion and the concentration of sodium required are available at http://www.mdcalc.com/sodium-correction-rate-in-hyponatremia, http://www.medcalc.com/sodium.html, and http://www.nephromatic.com/sodium_correction.php | |
| Serum sodium correction should generally not proceed faster than 0.5 mEq per L per hour for the first 24 to 48 hours; however, in severely symptomatic patients, the rate can be 1.0 to 2.0 mEq per L per hour; these situations typically require use of 3% saline | ||
| The goal is to raise the serum sodium level not to exceed 10 to 12 mEq per L in the first 24 hours and 18 mEq per L in the first 48 hours | ||
| Isotonic saline contains 154 mEq of sodium per L, and 3% saline contains 513 mEq of sodium per L | ||
| Serum osmolality | (Sodium × 2) + (glucose / 18) + (blood urea nitrogen / 2.8) | |
| Normal = 280 to 295 mOsm per kg | ||
| In patients with hyperglycemia, uncorrected sodium should be used to calculate the osmolality | ||
| Online calculators available at http://www.mdcalc.com/serum-osmolality-osmolarity and http://www.medcalc.com/osmol.html | ||
| Sodium deficit | Total body water % × weight in kg × (desired sodium − actual sodium) | |
| For total body water %, use 0.6 for men and 0.5 for women | ||
| Example: for a 70-kg man with a serum sodium level of 120 mEq per L and a desired serum sodium level of 140 mEq per L, the calculation is 0.6 × 70 (140 − 120) = 42 × 20 = 840 mEq | ||
| Online calculator available at http://www.mdcalc.com/sodium-deficit-in-hyponatremia | ||
| Water deficit | Volume (L) = (total body water %) × weight in kg × [(sodium − 140) / 140] | |
| For total body water %, use 0.45 for women older than 65 years, 0.5 for women 65 years and younger and for men older than 65 years, and 0.6 for men 65 years and younger and for children | ||
| Example: for a 70-kg man with a serum sodium level of 120 mEq per L, the calculation is 0.6 × 70 × ([120 − 140] / 140) = 42 × (−20 / 140) = 42 × (−1 / 7) = −6 L | ||
| Online calculators available at http://www.mdcalc.com/free-water-deficit-in-hypernatremia and http://www.medcalc.com/freewater.html | ||
Pseudohyponatremia
Pseudohyponatremia occurs when seemingly low sodium levels are actually normal. Causes include hyperglycemia, hyperproteinemia, mannitol use, or laboratory errors. Osmolality remains unchanged, and patients are usually euvolemic.12,13 A corrected sodium calculation is needed in the setting of hyperglycemia (eTable A).
Hypovolemic Hyponatremia
There are numerous causes of hypovolemic hyponatremia (Table 1).11–13 Patients typically have signs and symptoms associated with volume depletion (e.g., vomiting, diarrhea, tachycardia, elevated blood urea nitrogen–to-creatinine ratio). Urinary sodium levels are typically less than 20 mEq per L unless the kidney is the site of sodium loss. Fractional excretion of sodium is often inaccurately elevated in patients receiving diuretics because of diuretic-induced natriuresis; fractional excretion of urea can be utilized in these patients instead. Fractional excretion of urea less than 35% is more sensitive and specific for diagnosing prerenal azotemia in this setting.18 Treatment generally consists of volume repletion with isotonic (0.9%) saline, occasional use of salt tablets, and treatment of the underlying condition.13,14 Monitoring of urine output is recommended because output of more than 100 mL per hour can be a warning sign of overcorrection.14
Euvolemic Hyponatremia
Euvolemic hyponatremia is most commonly caused by SIADH, but can also be caused by hypothyroidism and glucocorticoid deficiency. Euvolemia is diagnosed by findings from the history and physical examination, low serum uric acid levels, a normal blood urea nitrogen–to-creatinine ratio, and spot urinary sodium greater than 20 mEq per L. Diuretic therapy can artificially elevate urinary sodium, whereas a low-salt diet can artificially lower urinary sodium, thus clouding the diagnosis of hypovolemia vs. euvolemia. Treatment generally consists of fluid restriction and correcting the underlying cause. Fluid restriction should be limited to 500 mL less than the daily urinary volume.13 Salt and protein intake should not be restricted. Predictors of failure with fluid restriction include urinary osmolality greater than 500 mOsm per kg, 24-hour urinary volume less than 1.5 L, an increase in the serum sodium level of less than 2 mEq per L within 24 to 48 hours, and a serum sodium level less than the sum of the urinary sodium and potassium levels.13 Volume status can be difficult to determine; therefore, a trial of intravenous fluids may be warranted.11 Sodium levels in patients with SIADH will decrease further with intravenous fluid administration. The use of demeclocycline (Declomycin) and lithium is not recommended because of an increased risk of harm.14
Hypervolemic Hyponatremia
Hypervolemic hyponatremia occurs when the kidneys cannot excrete water efficiently. In volume overload states, the effective arterial blood volume is decreased compared with venous volume, resulting in excess ADH secretion. The most common causes of hypervolemic hyponatremia are heart failure, cirrhosis, and kidney injury. Treatment consists of correcting the underlying cause, sodium and fluid restriction, and diuretic therapy to increase excretion of solute-free water.13,14 A randomized controlled trial of 46 patients with heart failure showed that restricting fluid intake to 1 L per day improved quality of life 60 days after discharge.19
Severe Symptomatic Hyponatremia
Severe symptomatic hyponatremia occurs when sodium levels decrease over less than 24 hours. Severe symptoms (e.g., coma, seizures) typically occur when the sodium level falls below 120 mEq per L, but can occur at less than 125 mEq per L. Severe symptomatic hyponatremia must be corrected promptly because it can lead to cerebral edema, irreversible neurologic damage, respiratory arrest, brainstem herniation, and death. Treatment includes the use of hypertonic 3% saline infused at a rate of 0.5 to 2 mL per kg per hour until symptoms resolve. At this time, vaptans have no role in the treatment of symptomatic hyponatremia because of the potential for overcorrection of sodium and variable sodium fluctuations.13 Loop diuretics may be needed in patients with concurrent symptomatic hyponatremia and volume overload. The rate of sodium correction should be 6 to 12 mEq per L in the first 24 hours and 18 mEq per L or less in 48 hours.12–14 An increase of 4 to 6 mEq per L is usually sufficient to reduce symptoms of acute hyponatremia.20 Rapid correction of sodium can result in osmotic demyelination (previously called central pontine myelinolysis). Overcorrection is common and is typically caused by rapid diuresis secondary to decreasing ADH levels. Every attempt should be made not to overcorrect sodium levels. One study of 25 patients with severe symptoms and sodium levels less than 120 mEq per L showed that concurrent treatment with a weight-based dose of 3% saline and 1 to 2 mcg of desmopressin every six to eight hours resulted in a rate of correction of 3 to 7 mEq per L per hour without causing overcorrection.21 Another study used a 100-mL bolus of 3% saline infused over 10 minutes in marathon runners; symptoms improved without over-correcting. This method increased sodium levels by 1.5 to 2.0 mEq per L per hour.13,22,23 Guidelines from the European Society of Endocrinology recommend infusing one dose of 150 mL of 3% saline over 20 minutes, with sodium monitoring every 20 minutes until symptoms resolve.14 This regimen may be repeated if the patient remains symptomatic or until the goal sodium target of 5 mEq per L is achieved (Figure 213,14,20–23[ corrected]).
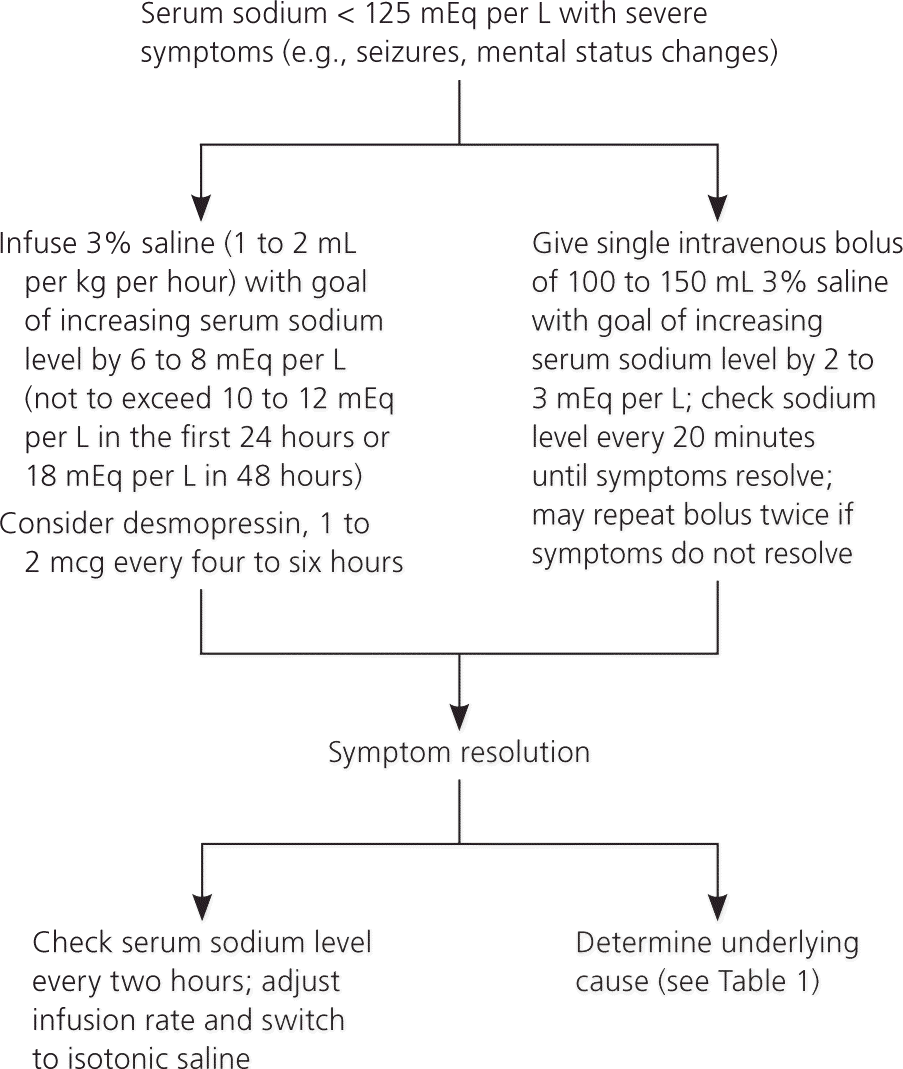
Vaptans
Vaptans (conivaptan [Vaprisol] and tolvaptan [Samsca]) are vasopressin-receptor antagonists approved for the treatment of hospitalized patients with severe hypervolemic and euvolemic hyponatremia (eTable B). However, their use in the management of hyponatremia is controversial. Several trials have demonstrated that vaptans increase sodium levels in patients with cirrhosis and heart failure.24 In the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan, patients with hyponatremia and heart failure who received tolvaptan had an associated reduction in cardiovascular morbidity and mortality, although there were several confounding variables, and further study is needed.25 The Study of Ascending Levels of Tolvaptan in Hyponatremia (SALT) trials demonstrated increased sodium levels with tolvaptan in patients with SIADH, cirrhosis, and heart failure.26 An extension of these studies, the SALTWATER trial, showed that long-term use of tolvaptan is safe and effective in increasing sodium levels, although this study did not specify subgroups.27,28 Regardless of their effectiveness in increasing sodium levels, vaptans—specifically tolvaptan—should not be used in patients with hepatic impairment because they may worsen liver function.29,30 The European Society of Endocrinology recommends against the routine use of vaptans, citing a lack of reduction in overall mortality rates and increased risk of rapid overcorrection.14 Further study is needed to clarifiy the role of vaptans.
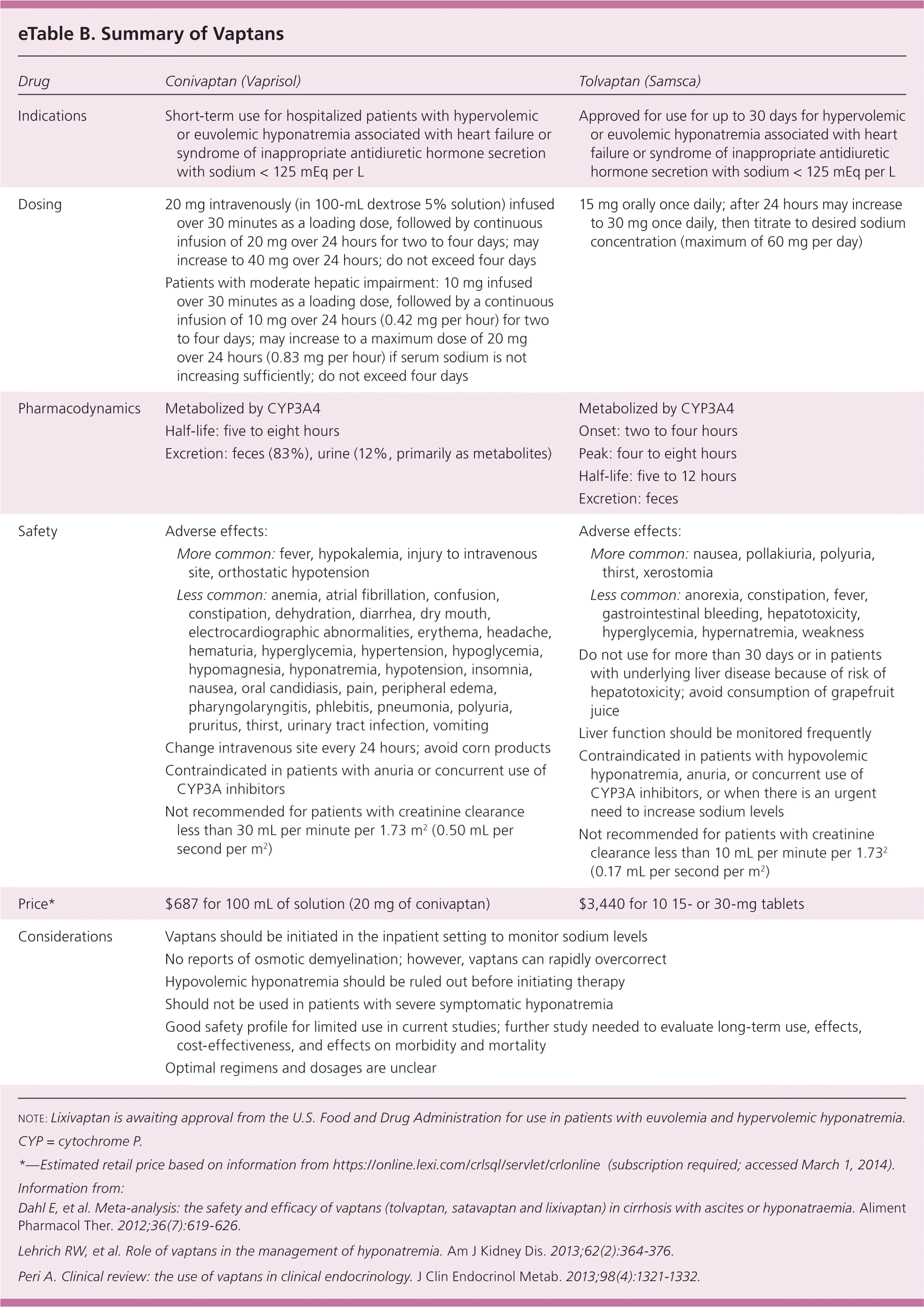
| Drug | Conivaptan (Vaprisol) | Tolvaptan (Samsca) | ||
|---|---|---|---|---|
| Indications | Short-term use for hospitalized patients with hypervolemic or euvolemic hyponatremia associated with heart failure or syndrome of inappropriate antidiuretic hormone secretion with sodium < 125 mEq per L | Approved for use for up to 30 days for hypervolemic or euvolemic hyponatremia associated with heart failure or syndrome of inappropriate antidiuretic hormone secretion with sodium < 125 mEq per L | ||
| Dosing | 20 mg intravenously (in 100-mL dextrose 5% solution) infused over 30 minutes as a loading dose, followed by continuous infusion of 20 mg over 24 hours for two to four days; may increase to 40 mg over 24 hours; do not exceed four days | 15 mg orally once daily; after 24 hours may increase to 30 mg once daily, then titrate to desired sodium concentration (maximum of 60 mg per day) | ||
| Patients with moderate hepatic impairment: 10 mg infused over 30 minutes as a loading dose, followed by a continuous infusion of 10 mg over 24 hours (0.42 mg per hour) for two to four days; may increase to a maximum dose of 20 mg over 24 hours (0.83 mg per hour) if serum sodium is not increasing sufficiently; do not exceed four days | ||||
| Pharmacodynamics | Metabolized by CYP3A4 | Metabolized by CYP3A4 | ||
| Half-life: five to eight hours | Onset: two to four hours | |||
| Excretion: feces (83%), urine (12%, primarily as metabolites) | Peak: four to eight hours | |||
| Half-life: five to 12 hours | ||||
| Excretion: feces | ||||
| Safety | Adverse effects: | Adverse effects: | ||
| More common: fever, hypokalemia, injury to intravenous site, orthostatic hypotension | More common: nausea, pollakiuria, polyuria, thirst, xerostomia | |||
| Less common: anemia, atrial fibrillation, confusion, constipation, dehydration, diarrhea, dry mouth, electrocardiographic abnormalities, erythema, headache, hematuria, hyperglycemia, hypertension, hypoglycemia, hypomagnesia, hyponatremia, hypotension, insomnia, nausea, oral candidiasis, pain, peripheral edema, pharyngolaryngitis, phlebitis, pneumonia, polyuria, pruritus, thirst, urinary tract infection, vomiting | Less common: anorexia, constipation, fever, gastrointestinal bleeding, hepatotoxicity, hyperglycemia, hypernatremia, weakness | |||
| Change intravenous site every 24 hours; avoid corn products | Do not use for more than 30 days or in patients with underlying liver disease because of risk of hepatotoxicity; avoid consumption of grapefruit juice | |||
| Contraindicated in patients with anuria or concurrent use of CYP3A inhibitors | Liver function should be monitored frequently | |||
| Not recommended for patients with creatinine clearance less than 30 mL per minute per 1.73 m2 (0.50 mL per second per m2) | Contraindicated in patients with hypovolemic hyponatremia, anuria, or concurrent use of CYP3A inhibitors, or when there is an urgent need to increase sodium levels | |||
| Not recommended for patients with creatinine clearance less than 10 mL per minute per 1.732 (0.17 mL per second per m2) | ||||
| Price* | $687 for 100 mL of solution (20 mg of conivaptan) | $3,440 for 10 15- or 30-mg tablets | ||
| Considerations | Vaptans should be initiated in the inpatient setting to monitor sodium levels | |||
| No reports of osmotic demyelination; however, vaptans can rapidly overcorrect | ||||
| Hypovolemic hyponatremia should be ruled out before initiating therapy | ||||
| Should not be used in patients with severe symptomatic hyponatremia | ||||
| Good safety profile for limited use in current studies; further study needed to evaluate long-term use, effects, cost-effectiveness, and effects on morbidity and mortality | ||||
| Optimal regimens and dosages are unclear | ||||
Hypernatremia
Hypernatremia is defined as a serum sodium level greater than 145 mEq per L. It is associated with increased morbidity and mortality in the inpatient setting.31,32 Hypernatremia is caused by net water loss (increased loss or decreased intake) or, rarely, sodium gain. Patients at increased risk include those with an impaired thirst mechanism or restricted access to water (e.g., those with altered mental status, intubated patients, infants, older adults).
Symptoms of hypernatremia in infants can include tachypnea, muscle weakness, restlessness, a high-pitched cry, insomnia, lethargy, and coma. Seizures usually occur only in cases of inadvertent sodium loading or rapid rehydration. In adults, symptoms tend to be mild and may include anorexia, muscle weakness, restlessness, nausea, and vomiting. Severe symptoms are likely to occur with acute increases in plasma sodium levels or at concentrations greater than 160 mEq per L. Hypernatremia can cause brain shrinkage, resulting in vascular rupture and intracranial bleeding.33
DIAGNOSTIC APPROACH
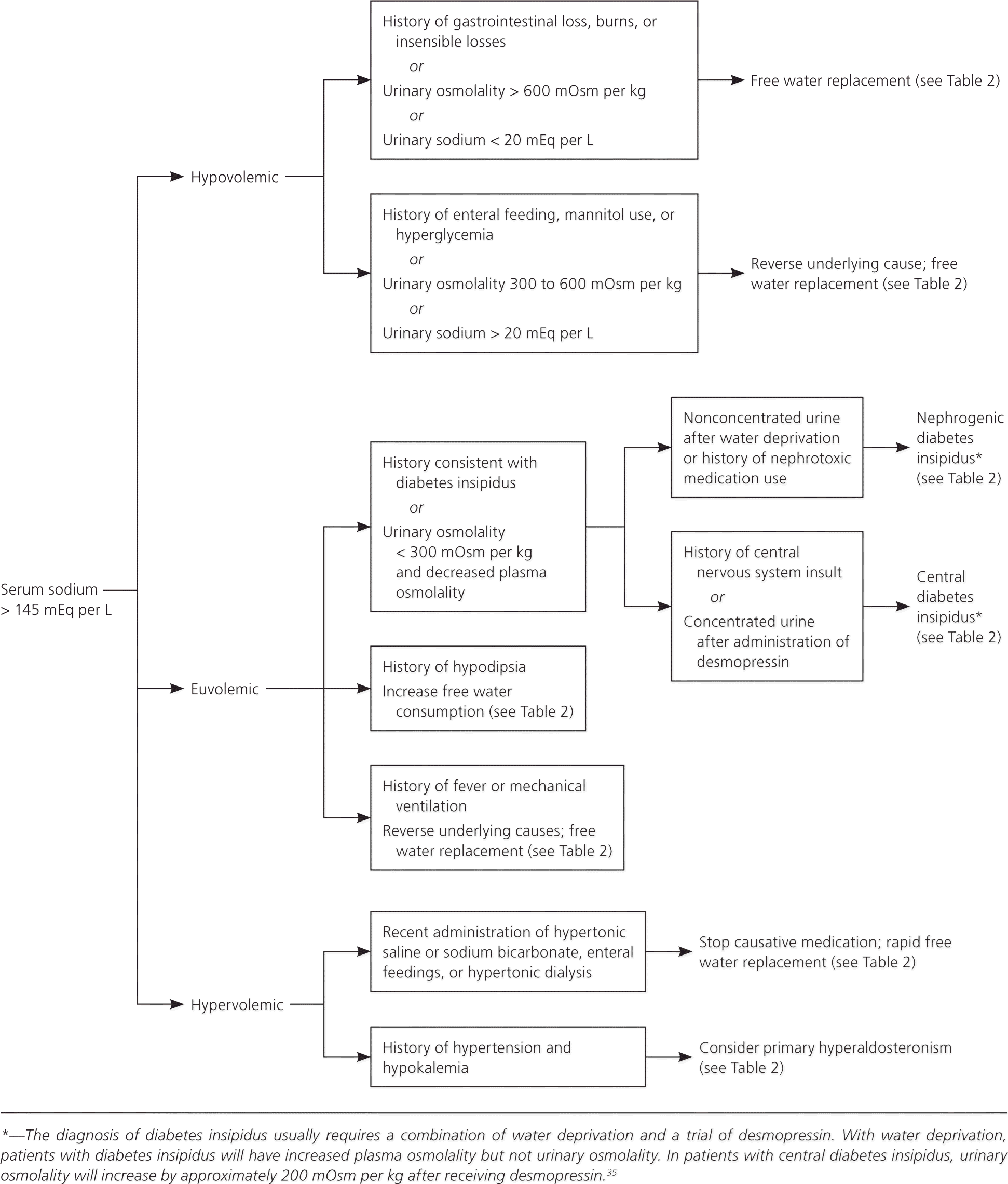
The cause of hypernatremia is usually evident from the history and physical examination, and is typically water loss (e.g., gastrointestinal loss, restricted access to water) or sodium gain (Table 2).3,12,33,34 Patients are often asymptomatic but can present with irritability, nausea, weakness, altered mental status, or coma. Water loss can be pure water loss (e.g., in diabetes insipidus) or hypotonic fluid loss (e.g., renal, gastrointestinal, or cutaneous losses). Sodium gain is usually iatrogenic from the infusion of hypertonic solutions. Laboratory studies are not necessary if the cause is apparent from the history, but frequent electrolyte checks are recommended during correction. When the cause is not clear, laboratory studies should be guided by the history12 (Figure 335 ).
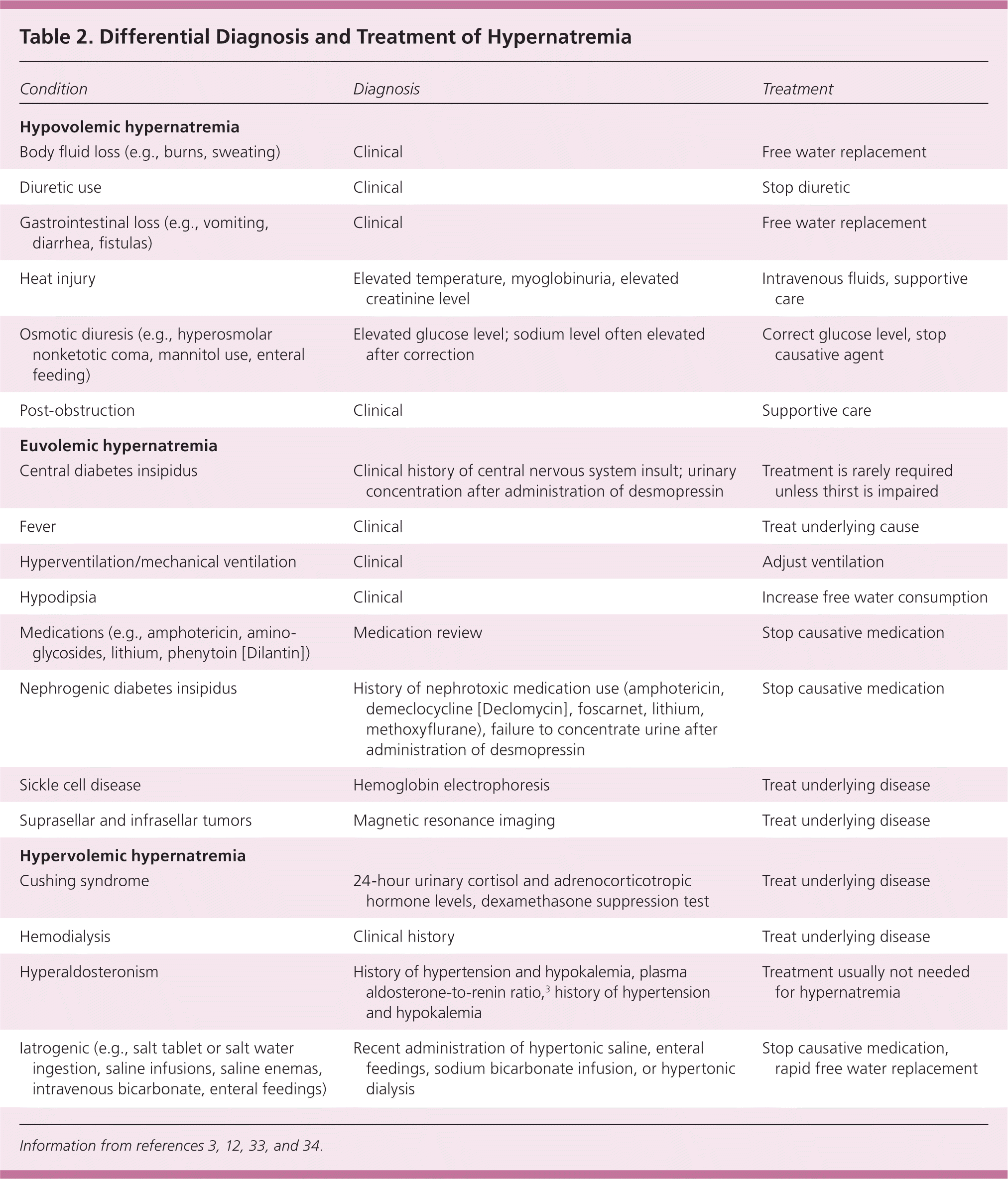
| Condition | Diagnosis | Treatment |
|---|---|---|
| Hypovolemic hypernatremia | ||
| Body fluid loss (e.g., burns, sweating) | Clinical | Free water replacement |
| Diuretic use | Clinical | Stop diuretic |
| Gastrointestinal loss (e.g., vomiting, diarrhea, fistulas) | Clinical | Free water replacement |
| Heat injury | Elevated temperature, myoglobinuria, elevated creatinine level | Intravenous fluids, supportive care |
| Osmotic diuresis (e.g., hyperosmolar nonketotic coma, mannitol use, enteral feeding) | Elevated glucose level; sodium level often elevated after correction | Correct glucose level, stop causative agent |
| Post-obstruction | Clinical | Supportive care |
| Euvolemic hypernatremia | ||
| Central diabetes insipidus | Clinical history of central nervous system insult; urinary concentration after administration of desmopressin | Treatment is rarely required unless thirst is impaired |
| Fever | Clinical | Treat underlying cause |
| Hyperventilation/mechanical ventilation | Clinical | Adjust ventilation |
| Hypodipsia | Clinical | Increase free water consumption |
| Medications (e.g., amphotericin, aminoglycosides, lithium, phenytoin [Dilantin]) | Medication review | Stop causative medication |
| Nephrogenic diabetes insipidus | History of nephrotoxic medication use (amphotericin, demeclocycline [Declomycin], foscarnet, lithium, methoxyflurane), failure to concentrate urine after administration of desmopressin | Stop causative medication |
| Sickle cell disease | Hemoglobin electrophoresis | Treat underlying disease |
| Suprasellar and infrasellar tumors | Magnetic resonance imaging | Treat underlying disease |
| Hypervolemic hypernatremia | ||
| Cushing syndrome | 24-hour urinary cortisol and adrenocorticotropic hormone levels, dexamethasone suppression test | Treat underlying disease |
| Hemodialysis | Clinical history | Treat underlying disease |
| Hyperaldosteronism | History of hypertension and hypokalemia, plasma aldosterone-to-renin ratio,3 history of hypertension and hypokalemia | Treatment usually not needed for hypernatremia |
| Iatrogenic (e.g., salt tablet or salt water ingestion, saline infusions, saline enemas, intravenous bicarbonate, enteral feedings) | Recent administration of hypertonic saline, enteral feedings, sodium bicarbonate infusion, or hypertonic dialysis | Stop causative medication, rapid free water replacement |
Diabetes insipidus is caused by a defect in ADH, either at the level of the central nervous system (central diabetes insipidus) or kidneys (nephrogenic diabetes insipidus). Inappropriately dilute urine (osmolality less than 300 mOsm per kg) in the setting of hypernatremia suggests diabetes insipidus. Hyperaldosteronism can cause mild hypernatremia but is rarely clinically relevant. Hyperglycemia can also cause hypernatremia, even after correction of glucose levels.36
TREATMENT
The treatment of hypernatremia involves treating the underlying cause and correcting the water deficit. Determining volume status and calculating the total body water deficit are important(eTable A). When correcting the total body water deficit, oral or enteral free water should be used whenever possible. When intravenous fluids are required, hypotonic solutions should be used. Rapid over-correction can result in cerebral edema; therefore, the least amount of fluid possible should be used.33 eTable C lists the sodium content of various intravenous fluids.
In patients with rapid development of hypernatremia, sodium can be corrected quickly with isotonic saline or water without increasing the risk of cerebral edema. A correction rate of 1 mEq per L per hour is considered safe in these patients.12,36 In patients with hypernatremia that developed over a longer period, the sodium level should be corrected at a rate of 0.5 mEq per L per hour, with no more than an 8 to 10 mEq per L decrease over 24 hours.33,36,37 The target sodium level should be 145 mEq per L.33
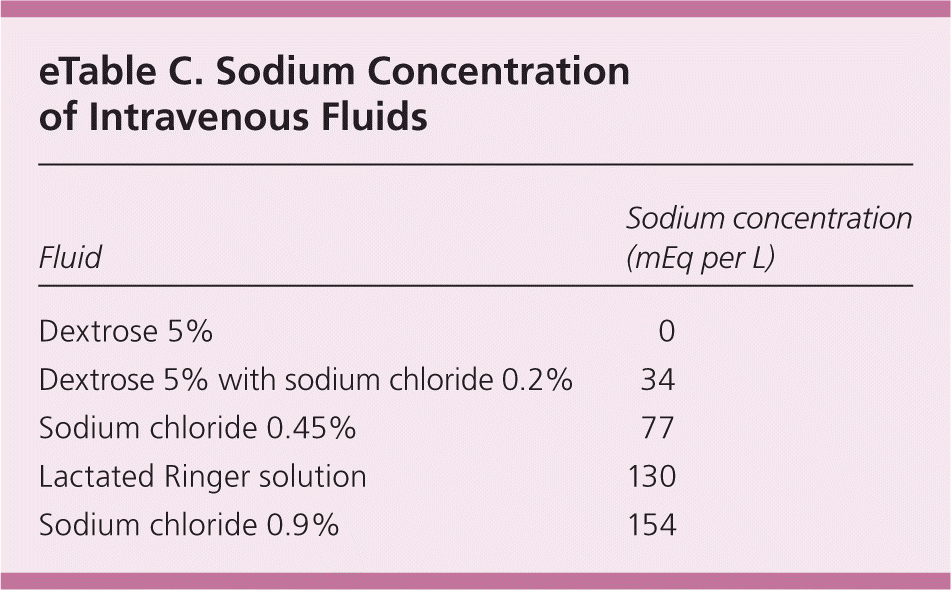
| Fluid | Sodium concentration (mEq per L) |
|---|---|
| Dextrose 5% | 0 |
| Dextrose 5% with sodium chloride 0.2% | 34 |
| Sodium chloride 0.45% | 77 |
| Lactated Ringer solution | 130 |
| Sodium chloride 0.9% | 154 |
Data Sources: We searched the Cochrane database, Dynamed, PubMed, PEPID, Clinical Evidence, the National Guideline Clearinghouse, UpToDate, and OVID using the key terms hyponatremia, hypernatremia, vaptans, diagnosis, and treatment. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. Search dates: November 15, 2013; March 1, 2014; and October 5, 2014.
The views expressed are those of the authors and do not reflect the official policy of the Department of the Army, the Department of Defense, or the U.S. government.
