
A more recent article on contraceptive services is available.
Am Fam Physician. 2015;91(9):625-633
Patient information: See related handout on birth control, written by the auhors of this article.
Author disclosure: No relevant financial affiliation.
The Centers for Disease Control and Prevention has released comprehensive recommendations for provision of family planning services. Contraceptive services may be addressed in five steps, and counseling may be provided in a tiered approach, whereby the most effective options are presented before less effective options. Clinicians should discuss all contraceptive methods that can be used safely by the patient, regardless of whether a method is available on site and even if the patient is an adolescent or a nulliparous woman. Physical assessment is usually limited to blood pressure evaluation before starting hormonal contraceptives or pelvic examination before placing an intrauterine device. Monitoring the patient's weight also may be helpful. If it is reasonably certain that the patient is not pregnant, any contraceptive may be started immediately. When hormonal contraceptives are selected, one year's supply should be prescribed to reduce barriers to use. Condoms should be made readily available. Documentation of visits for contraception should include patient understanding of use, benefits, and risks, plus an individualized follow-up plan. Bleeding irregularities generally are not harmful and may resolve with continued use of the contraceptive method. All patients—including adolescents; those who identify as lesbian, gay, bisexual, or transgender; and patients with disabilities or limited English proficiency—should receive high-quality care in an accommodating, nonjudgmental environment. The Centers for Disease Control and Prevention supports advance provision of emergency contraceptives. Because no test reliably verifies cessation of fertility, it is prudent to consider contraceptive use until menopause, or at least until 50 to 55 years of age.
Family physicians are uniquely suited to deliver comprehensive, unfragmented family planning services to patients of all ages.1 With access to an increasingly robust evidence base on ways to prevent or achieve pregnancy and treat sexually transmitted infections (STIs), clinicians are challenged to offer and manage the safest and most effective options available for patients with various medical conditions, social circumstances, adherence patterns, and financial barriers to care.
| Clinical recommendations | Evidence rating | References |
|---|---|---|
| Clinicians should consider a tiered approach to contraceptive counseling, whereby the most effective and appropriate options are presented before less effective options. | C | 4, 12–15 |
| Requiring prerequisite preventive services, such as cervical cytology; breast examination; or evaluation for sexually transmitted infections, diabetes mellitus, dyslipidemia, liver disease, or thrombophilia, can introduce unnecessary barriers to contraceptive care. | C | 4, 18, 19 |
| If a patient's pregnancy status is uncertain, clinicians may consider same-day start of a nonintrauterine method to provide immediate coverage, and should order follow-up pregnancy testing two to four weeks later. | C | 9, 17, 21, 22 |
| Prescription of hormonal contraceptives should preferentially cover one year's supply to decrease barriers to care. | C | 4 |
| Family planning services should be offered to adolescents with assurances of confidentiality, in the context of relevant law. | B | 4, 15, 32, 35–37 |
| Intrauterine devices and contraceptive implants are safe and effective for postmenarchal adolescents. | B | 4, 9, 15, 17, 39–43 |
| Estrogen-containing contraceptives should be deferred until at least three or up to six weeks postpartum, partly because of the risk of venous thromboembolism. | C | 10, 52 |
| Contraceptive use for unintended pregnancy prevention should be considered until menopause, or at least until 50 to 55 years of age. | C | 17, 54 |
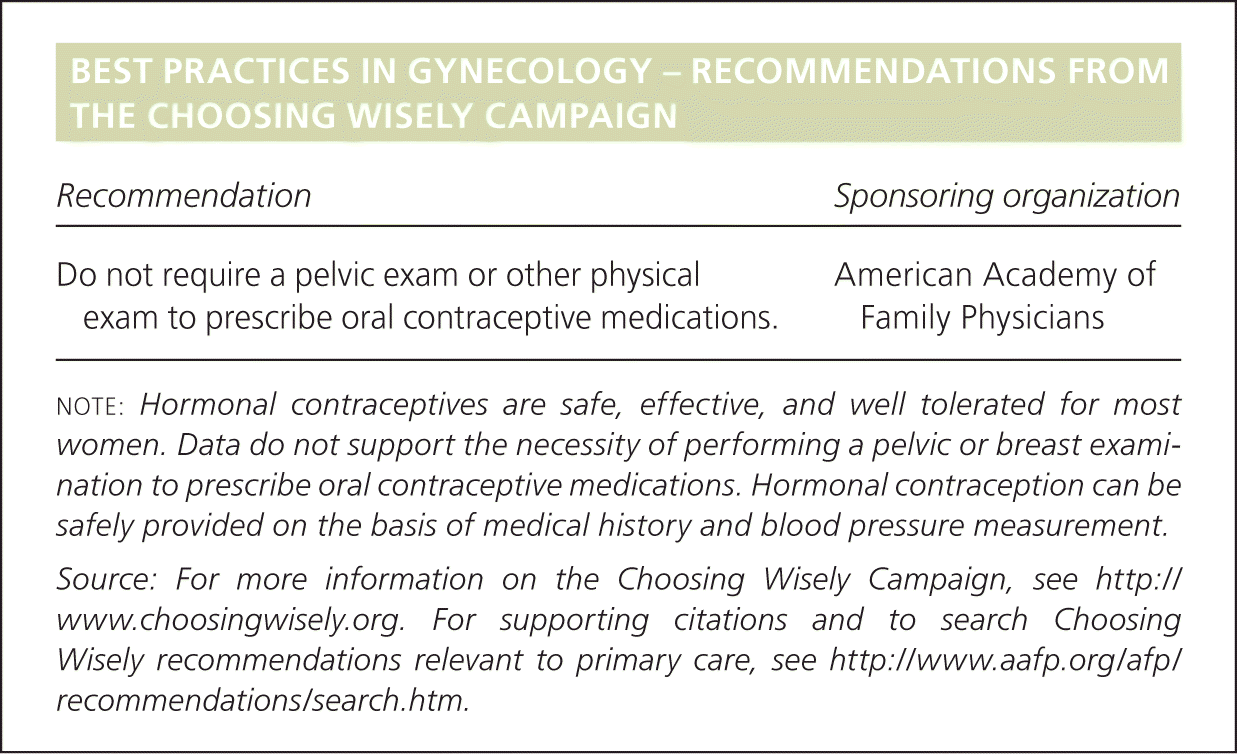
| Recommendation | Sponsoring organization |
|---|---|
| Do not require a pelvic exam or other physical exam to prescribe oral contraceptive medications. | American Academy of Family Physicians |
Approximately one-half of the 6.6 million pregnancies each year in the United States are unintended, and the rates are highest among young, poor, and minority women.2 In 2008, women 15 to 44 years of age with incomes at or below the federal poverty level were five times more likely than women at the highest income level to become pregnant unintentionally.2 Women who use contraception consistently and correctly account for only 5% of unintended pregnancies.3
To help clinicians provide optimal sexual and reproductive health services, the Centers for Disease Control and Prevention (CDC) has released comprehensive, evidence-based recommendations that have been methodically and scientifically established by technical and expert panels4 (eTable A). Recommendations pertaining to contraceptive management are addressed in this review. At every health care visit, clinicians should discuss family planning needs with patients of reproductive age and provide requested services as appropriate.4

| Clinical question | Resource | Content | Website |
|---|---|---|---|
| Which services should be offered in a family planning visit? | Providing Quality Family Planning Services: Recommendations of the CDC and the U.S. Office of Population Affairs (2014) |
| http://www.cdc.gov/reproductivehealth/UnintendedPregnancy/QFP.htm |
| What are the best ways to initiate and manage specific contraceptive methods? | U.S. Selected Practice Recommendations (US SPR) for Contraceptive Use, 2013 (adapted from the World Health Organization Selected Practice Recommendations for Contraceptive Use, 2nd ed.) |
| http://www.cdc.gov/reproductivehealth/UnintendedPregnancy/USSPR.htm |
| Which contraceptive methods are safe for women with specific characteristics and medical conditions? | U.S. Medical Eligibility Criteria (US MEC) for Contraceptive Use, 2010 (updated for use during postpartum period, and for those with, or at high risk of, human immunodeficiency virus infection) |
| http://www.cdc.gov/reproductivehealth/UnintendedPregnancy/USMEC.htm |
| What is the appropriate treatment of persons who have or are at risk of sexually transmitted infections? | Sexually Transmitted Diseases Treatment Guidelines, 2010 (updated for drug resistance patterns) |
| http://www.cdc.gov/std/treatment/2010/default.htm |
| What information is available for patients who are considering various contraceptive options? | CDC patient resource on contraception |
| http://www.cdc.gov/reproductivehealth/unintendedpregnancy/contraception.htm |
Provision of Contraception
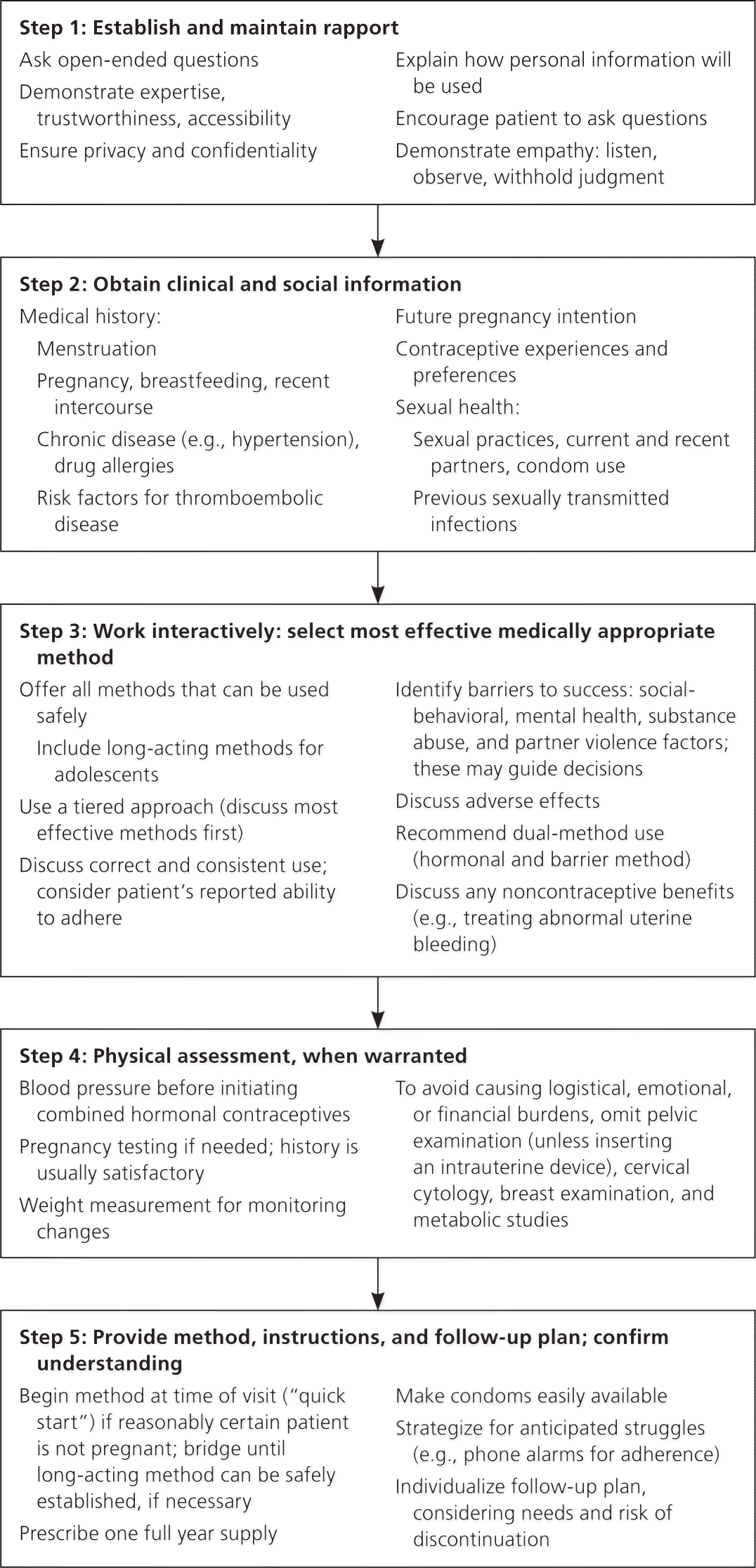
STEP 1: ESTABLISH RAPPORT
Family planning discussions can be inherently sensitive; therefore, establishing and maintaining rapport is critical for success.4–8 Clinicians should ask open-ended questions; demonstrate expertise, trustworthiness, accessibility, and empathy; ensure privacy and confidentiality; explain how personal information will be used; and encourage patients to ask questions.
STEP 2: OBTAIN CLINICAL AND SOCIAL INFORMATION
Contraceptive recommendations should be personalized, focusing on the patient's safety and reproductive life plan.4 The interview should elicit, at minimum, menstrual, gynecologic, and obstetric history; medication allergies; infectious or chronic health conditions; and tobacco use. These may alter the patient's medical eligibility for a particular method9,10 (eTable B).

| Condition | Associated risk | Comment | |
|---|---|---|---|
| Category 4: unacceptable health risk if method is used | |||
| Breast cancer | Theoretical concern for worse prognosis of disease | Category 3 if in complete remission for 5 years | |
| Deep venous thrombosis or pulmonary embolism | Thromboembolic disease; stroke | Category 3 if no risk factors for recurrence and if condition is resolved or patient is on anticoagulation therapy for ≥ 3 months | |
| Hypertension (systolic ≥ 160 mm Hg or diastolic ≥ 100 mm Hg) | Acute MI and stroke | — | |
| Ischemic heart disease | Acute MI | Current or history of ischemic heart disease | |
| Known thrombogenic mutations | Thromboembolic disease; stroke | Routine screening is not appropriate | |
| Liver disease (severe cirrhosis; hepatoma or hepatocellular adenoma) | May affect estrogen metabolism and place additional burden on decompensated liver | — | |
| Major surgery with prolonged immobilization | Thromboembolic disease; stroke | — | |
| Migraine with aura | Stroke | — | |
| Smoking, age ≥ 35 years | Cardiovascular disease including acute MI | Category 3 if age ≥ 35 years and < 15 cigarettes per day | |
| Solid organ transplantation | Theoretical concerns for worse prognosis, cardiovascular disease, and decreased contraceptive effectiveness with immunosuppressive therapy | Only if complicated | |
| Stroke | Recurrence or worsening of disease | Any history of cerebrovascular accident | |
| Systemic lupus erythematosus (with antiphospholipid antibodies) | Arterial and venous thrombosis | Only if positive (or unknown) antiphospholipid antibodies | |
| Valvular heart disease | Arterial thrombosis | Only if complicated | |
| Vascular disease | Acute MI and stroke | Other than superficial disease | |
| Category 3: method is theoretical or proven risks usually outweigh its advantages | |||
| Acute viral hepatitis | May affect estrogen metabolism and place additional burden on decompensated liver | Initiation of method only; category 4 if severe | |
| Bariatric surgery | May decrease contraceptive effectiveness of oral contraceptives (not patch or ring) | Only malabsorptive procedures (e.g., Roux-en-Y gastric bypass); not restrictive procedures (e.g., gastric banding) | |
| Diabetes with complications (or > 20 years duration) | May worsen control of diabetes, which may worsen diabetic complications | Category 4 if severe | |
| Hypertension (systolic = 140 to 159 mm Hg or diastolic = 90 to 99 mm Hg) | Acute MI and stroke | — | |
| Inflammatory bowel disease | Thromboembolic disease; stroke | Active or complicated disease only (treatment or sequelae may predispose patient to thromboembolism) | |
| Medication interactions | |||
| Lamotrigine (Lamictal) monotherapy | Seizure activity may increase | Lamotrigine levels may decrease | |
| Rifampicin or rifabutin (Mycobutin) therapy | Reduces contraceptive effectiveness | This interaction does not apply to most broad-spectrum antibiotics, antifungals, and antiparasitics | |
| Specific anticonvulsants (e.g., topiramate [Topamax], phenytoin [Dilantin]) | Reduces contraceptive effectiveness | Consider other contraceptive options and ethinyl estradiol dose of at least 30 mcg | |
| Specific antiretrovirals | Reduces contraceptive effectiveness | Recommend condom use and ethinyl estradiol dose of at least 30 mcg | |
| Migraine without aura and age ≥ 35 years | Stroke | Age < 35 years, if migraine without aura develops during method use | |
Knowing the patient's contraceptive experience and intentions regarding pregnancy is useful. A sexual health assessment—including practices, partners, STI status, condom use, and recent intercourse—may aid in selecting and safely initiating appropriate contraception.4
STEP 3: WORK INTERACTIVELY TO HELP THE PATIENT SELECT THE MOST EFFECTIVE AND APPROPRIATE METHOD
Clinicians should discuss all available contraceptive methods that the patient can use safely, regardless of which are available on site.4,11 The CDC encourages a tiered approach to counseling, whereby the most effective and medically appropriate options are presented before less effective options4,12–15 (eFigure A).
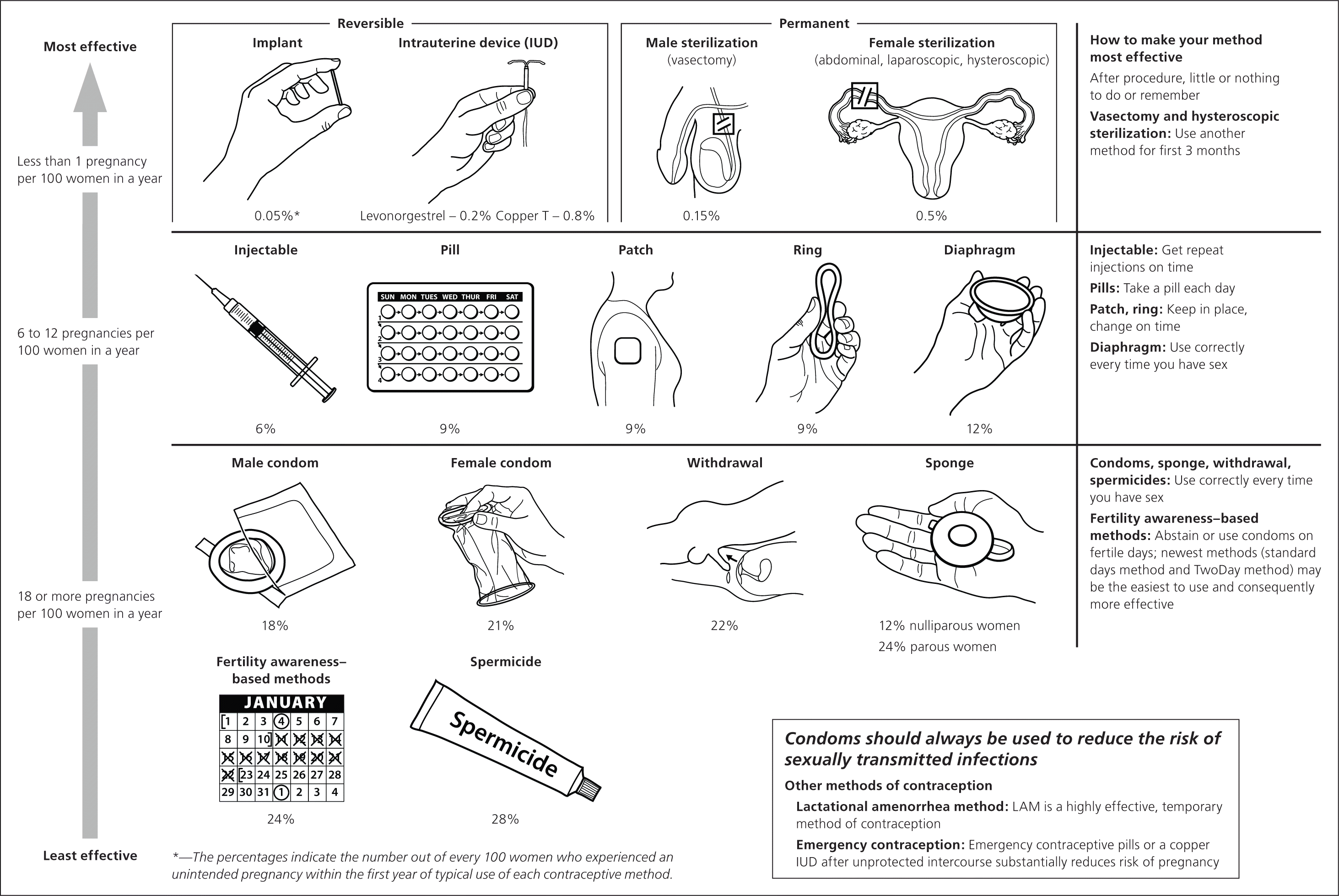
Other factors that may be important to the patient should be considered, such as her ability to use the method correctly and consistently, and possible adverse effects.4 Information on noncontraceptive benefits of various methods, such as a potential reduction in dysmenorrhea, abnormal uterine bleeding, or acne, may help patients select and adhere to medication regimens.4 For patients with or at risk of an STI, dual-method contraception, which combines condoms and another method, should be discussed.4,16
Medication adherence concerns apply to patients with substance use, mental illness, difficulties with medication regimens, and sociobehavioral factors.4 For example, if intimate partner violence is suspected, in addition to providing appropriate safety referrals (e.g., to law enforcement and violence support groups), clinicians may offer long-acting reversible contraception to decrease barriers to contraception adherence.4
STEP 4: CONDUCT PHYSICAL ASSESSMENT
Clinicians should determine the patient's pregnancy status before initiating a contraceptive method; conduct a blood pressure evaluation before prescribing hormonal contraception; and perform a bimanual examination and cervical inspection before inserting an intrauterine device (IUD) or fitting a cervical cap.4,17 A bimanual examination is needed before diaphragm fitting.4,17 In patients with cervicitis or pelvic inflammatory disease, IUD placement should be deferred until appropriate treatment has occurred.4,9,17
Requiring other preventive services (e.g., cervical cytology; breast examination; evaluation for STI, diabetes mellitus, dyslipidemia, liver disease, thrombophilia) can introduce barriers to contraceptive care; therefore, such services should be offered as optional adjuncts and not as prerequisites.4,18,19 Testing for STIs can be performed at the time of IUD placement in asymptomatic patients, and should be performed if routine screening has not occurred according to CDC guidelines.
STEP 5: INITIATE METHOD AND ESTABLISH PLAN
If it is reasonably certain that a patient is not pregnant (Table 14,17,21–24) and that she meets appropriate medical eligibility criteria, any contraceptive may be started immediately.4,9,10,17 If pregnancy status is uncertain, clinicians may consider same-day start of a nonintrauterine method to provide immediate coverage, and then order follow-up pregnancy testing two to four weeks later.9,17,21,22
A negative pregnancy test result is insufficient for definitively ruling out pregnancy because results can be affected by test characteristics, the time since most recent intercourse, and recent pregnancy. It may help confirm that pregnancy is unlikely in patients who meet the criteria in Table 14,17,21–24; accordingly, pregnancy testing is not universally required before initiating contraception.17,23,24

| A clinician can be reasonably certain that a woman is not pregnant if: | ||
| (1) The patient has no signs or symptoms of pregnancy | ||
| and | ||
| (2) The patient meets at least one of the following: | ||
| Is seven days or less after start of her normal menses | ||
| Has not had intercourse since the start of her last normal menses | ||
| Has been correctly and consistently using a reliable method of contraception | ||
| Is seven days or less after a spontaneous or induced abortion | ||
| Is within four weeks postpartum | ||
| Is fully or nearly fully breastfeeding (exclusively breastfeeding or most feeds [≥ 85%] are breastfeeds), amenorrheic, and less than six months postpartum | ||
To reduce barriers to care when prescribing combined contraceptives, the prescription for hormonal contraceptives should cover one year's supply, and condoms should be made readily available.4,17 If requested contraceptive services are unavailable, a temporary method should be established until the patient can be referred for further assessment.4 Recommendations for backup contraception during method initiation and switching are provided in Table 2.17

| Backup method | ||
|---|---|---|
| Contraceptive method | Initiation* | Method switching |
| Copper-containing IUD | None needed | None needed |
| Levonorgestrel-containing IUD | 7 days; only needed if > 7 days after starting menses | 7 days; only needed if > 7 days after starting menses† |
| Implant | 7 days; only needed if > 5 days after starting menses | 7 days; only needed if > 5 days after starting menses‡ |
| Injectable | 7 days; only needed if > 7 days after starting menses | 7 days; only needed if > 7 days after starting menses‡ |
| Combined hormonal contraceptives | 7 days; only needed if > 5 days after starting menses | 7 days; only needed if > 5 days after starting menses‡ |
| Progestin-only pill | 2 days; only needed if > 5 days after starting menses | 2 days; only needed if > 5 days after starting menses‡ |
Clinicians should reserve adequate time to counsel patients, particularly on common adverse effects (e.g., abnormal uterine bleeding, weight gain), to help reduce inconsistent use or early method discontinuation.4,17,20,25,26 Educational resources are presented in eTable A. Concern about forgetfulness may be mitigated by reminder systems (e.g., smartphone applications) or use of long-acting reversible contraception.4,17,26
Follow-Up Considerations
Patients should be advised to return at any time to discuss questions or concerns about contraceptive method use, but for most women, routine follow-up visits are not required.17 When patients present for other routine visits, clinicians should assess for satisfaction and concerns, changes in health status or medication use that might affect eligibility for continued use, and changes in blood pressure (if using a combined hormonal method).4,17 Patients who use methods inconsistently or incorrectly, or who experience vomiting or severe diarrhea (i.e., affecting absorption), should be counseled on appropriate backup contraception (Table 317 ).
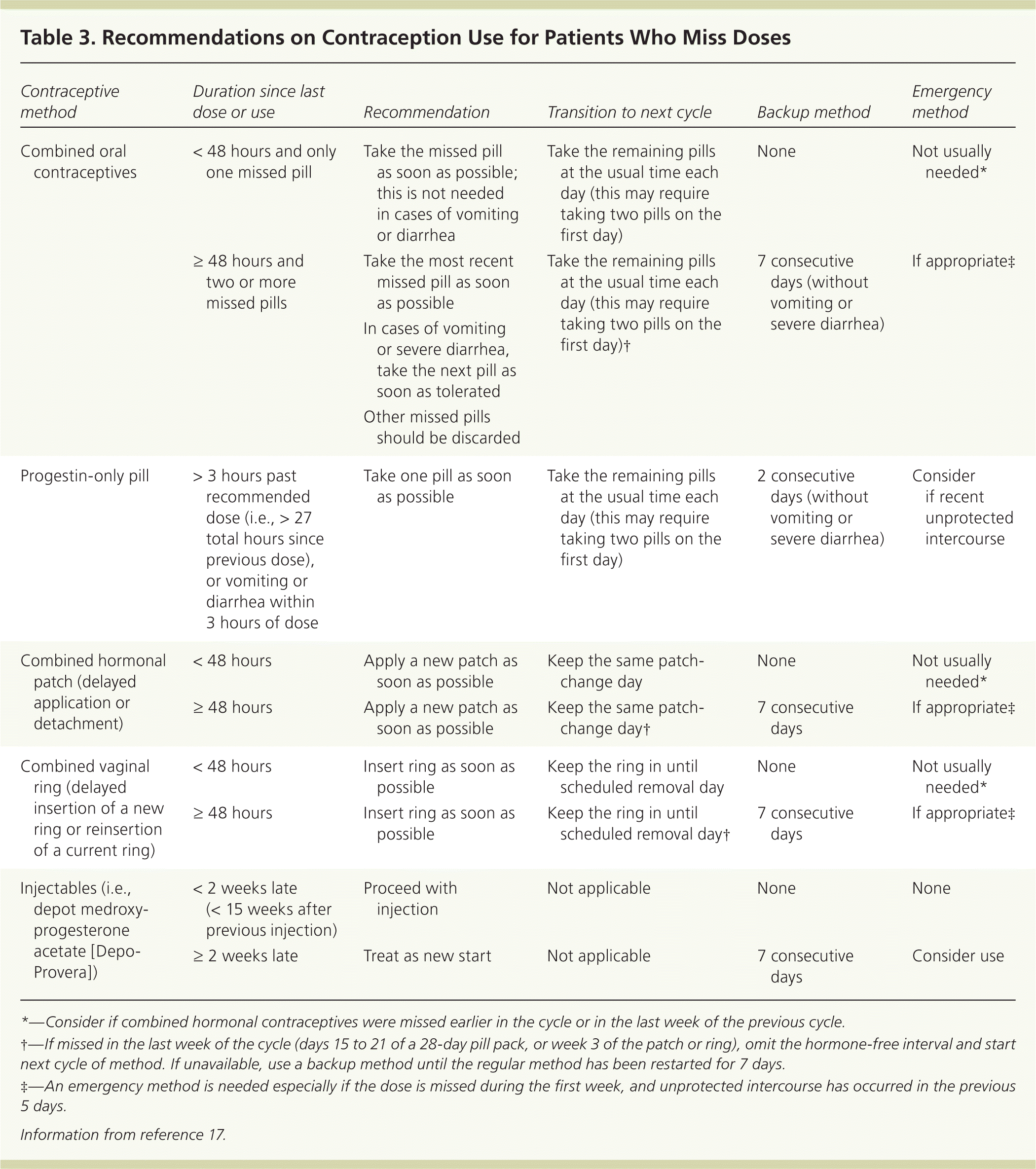
| Contraceptive method | Duration since last dose or use | Recommendation | Transition to next cycle | Backup method | Emergency method |
|---|---|---|---|---|---|
| Combined oral contraceptives | < 48 hours and only one missed pill | Take the missed pill as soon as possible; this is not needed in cases of vomiting or diarrhea | Take the remaining pills at the usual time each day (this may require taking two pills on the first day) | None | Not usually needed* |
| ≥ 48 hours and two or more missed pills |
| Take the remaining pills at the usual time each day (this may require taking two pills on the first day)† | 7 consecutive days (without vomiting or severe diarrhea) | If appropriate‡ | |
| Progestin-only pill | > 3 hours past recommended dose (i.e., > 27 total hours since previous dose), or vomiting or diarrhea within 3 hours of dose | Take one pill as soon as possible | Take the remaining pills at the usual time each day (this may require taking two pills on the first day) | 2 consecutive days (without vomiting or severe diarrhea) | Consider if recent unprotected intercourse |
| Combined hormonal patch (delayed application or detachment) | < 48 hours | Apply a new patch as soon as possible | Keep the same patch-change day | None | Not usually needed* |
| ≥ 48 hours | Apply a new patch as soon as possible | Keep the same patch-change day† | 7 consecutive days | If appropriate‡ | |
| Combined vaginal ring (delayed insertion of a new ring or reinsertion of a current ring) | < 48 hours | Insert ring as soon as possible | Keep the ring in until scheduled removal day | None | Not usually needed* |
| ≥ 48 hours | Insert ring as soon as possible | Keep the ring in until scheduled removal day† | 7 consecutive days | If appropriate‡ | |
| Injectables (i.e., depot medroxy-progesteroneacetate [Depo-Provera]) | < 2 weeks late (< 15 weeks after previous injection) | Proceed with injection | Not applicable | None | None |
| ≥ 2 weeks late | Treat as new start | Not applicable | 7 consecutive days | Consider use |
Irregular bleeding with contraceptives is generally not harmful, and may decrease with continued method use.17,27 Other reasons for bleeding may be considered, including inconsistent use, medication interactions, infection, pregnancy, and pathologic uterine conditions.17,27 If patients request treatment for bleeding, five to seven days of nonsteroidal anti-inflammatory drugs may be offered to those using the copper-containing IUD (Paragard), etonogestrel implant (Nexplanon), or depot medroxyprogesterone acetate (Depo-Provera). Alternatively, patients who use an implant or depot medroxyprogesterone acetate may benefit from low-dose combined oral contraceptives or estrogen for 10 to 20 days.17 Bleeding with the levonorgestrel-containing IUD (Mirena) tends to resolve in three to six months without treatment.17 Patients who use combined hormonal methods without hormone-free intervals (e.g., continuous use) may skip contraceptive pills for three to four consecutive days to temporarily induce bleeding and endometrial thinning, although not more often than monthly or before the first 21 days of use.17
If irregular bleeding persists and is unacceptable to the patient, the clinician should assist with choosing another contraceptive method.17,27 Women who use intrauterine contraception and who are diagnosed with pelvic inflammatory disease should be treated according to the CDC's STI treatment guidelines; however, the clinician may leave the device in place for two to three days and then reassess the patient for disposition.17,28 If the device is removed, emergency contraception should be offered if residual sperm are potentially present.
Special Populations
Clinicians should ensure that all patients—including special populations, such as those who have disabilities or limited English proficiency, or who identify as lesbian, gay, bisexual, or transgender—receive high-quality care in a nonjudgmental, accommodating environment. Sexual behaviors and pregnancy risk should not be assumed.4,15,29–31
When evaluating males, clinicians should remember to ascertain the patient's sexual health care needs, including contraceptive, preconception, and STI services.4 Condoms should be made available without additional evaluation, and the principles of the five contraceptive care steps should be applied.4
Adolescents should be given the opportunity to privately discuss their family planning needs and to subsequently receive care in the context of relevant law.4,15,32–34 Without assurances of confidentiality, adolescents are significantly less likely to use family planning services; however, parent-patient communication about sexual and reproductive health may be encouraged to foster support, access to services, contraceptive use, and healthy development.4,15,34–38 Some adolescents may initially prefer to limit patient-parent discussion to the noncontraceptive uses of hormonal methods.
IUDs and implants are safe and effective for postmenarcheal adolescents,4,9,17,39–41 and may be considered first-line options regardless of parity.15,42,43 Of 1,099 patients 14 to 19 years of age in the Contraceptive CHOICE Project, a large prospective contraception study, more than 80% of long-acting reversible contraceptive users continued their method over 12 months vs. only one-half of those using short-acting methods.44
Emergency Contraception
Emergency contraceptives have been reviewed in American Family Physician, including oral methods and the copper-containing IUD received within five days of unprotected intercourse to reduce unintended pregnancy risk.45 Notably, the CDC highlights that ulipristal (Ella) may be more effective than levonorgestrel formulations after the first 72 hours and for women who are overweight or obese.17,46,47 The CDC also supports advance provision of emergency contraceptive pills.17,48
Any contraceptive method may be started after completion of emergency contraceptive pills. The patient should use a barrier method or abstain from intercourse for seven days (14 days after ulipristal use), and should take a pregnancy test if she does not experience withdrawal bleeding within three weeks.17 Patients who vomit within three hours after using emergency contraceptive pills should take another dose, at which point an antiemetic may be offered.17 As of 2013, Plan B One-Step is available over the counter to patients of any age.49
Postpregnancy Contraceptive Options
Clinicians should emphasize postpregnancy contraception counseling. Almost one-half of abortions are repeat abortions, and nearly one in five births to teenagers is a repeat birth.50,51 The CDC specifies time frames for postpregnancy contraception (Table 4).9,10,17 Estrogen-containing methods should be deferred until at least three or up to six weeks postpartum, partly because of the risk of venous thromboembolism.10,52 Progestin-only methods can be safely started immediately postpartum.10
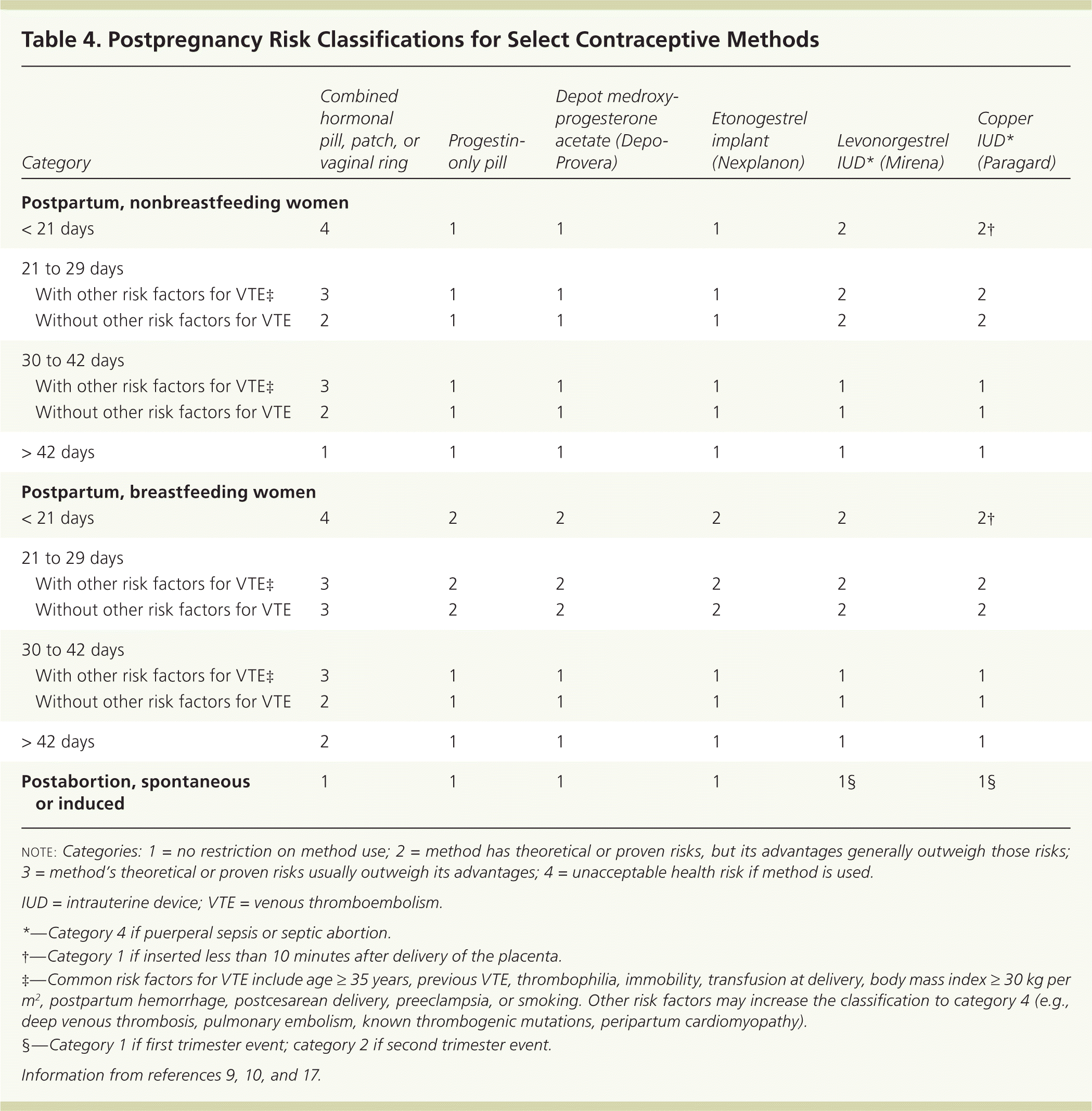
| Category | Combined hormonal pill, patch, or vaginal ring | Progestin-only pill | Depot medroxy-progesterone acetate (Depo-Provera) | Etonogestrel implant (Nexplanon) | Levonorgestrel IUD* (Mirena) | Copper IUD* (Paragard) | |
|---|---|---|---|---|---|---|---|
| Postpartum, nonbreastfeeding women | |||||||
| < 21 days | 4 | 1 | 1 | 1 | 2 | 2† | |
| 21 to 29 days | |||||||
| With other risk factors for VTE‡ | 3 | 1 | 1 | 1 | 2 | 2 | |
| Without other risk factors for VTE | 2 | 1 | 1 | 1 | 2 | 2 | |
| 30 to 42 days | |||||||
| With other risk factors for VTE‡ | 3 | 1 | 1 | 1 | 1 | 1 | |
| Without other risk factors for VTE | 2 | 1 | 1 | 1 | 1 | 1 | |
| > 42 days | 1 | 1 | 1 | 1 | 1 | 1 | |
| Postpartum, breastfeeding women | |||||||
| < 21 days | 4 | 2 | 2 | 2 | 2 | 2† | |
| 21 to 29 days | |||||||
| With other risk factors for VTE‡ | 3 | 2 | 2 | 2 | 2 | 2 | |
| Without other risk factors for VTE | 3 | 2 | 2 | 2 | 2 | 2 | |
| 30 to 42 days | |||||||
| With other risk factors for VTE‡ | 3 | 1 | 1 | 1 | 1 | 1 | |
| Without other risk factors for VTE | 2 | 1 | 1 | 1 | 1 | 1 | |
| > 42 days | 2 | 1 | 1 | 1 | 1 | 1 | |
| Postabortion, spontaneous or induced | 1 | 1 | 1 | 1 | 1§ | 1§ | |
Contraception in Women Approaching Menopause
In the United States, the median age of menopause is 51 years, but the normal range is from 40 to 60 years.53,54 Because there is no reliable test to verify permanent cessation of fertility, it is prudent to consider contraceptive use to prevent unintended pregnancy until menopause, or at least until 50 to 55 years of age.17,54
All contraceptive methods are considered U.S. Medical Eligibility Criteria category 1 or 2 (i.e., no restriction, or advantages generally outweigh theoretical or proven risks, respectively) based on the patient's age alone. However, clinicians must balance risks of pregnancy at advanced maternal age against risks of contraceptives—particularly those containing estrogen, which may precipitate acute cardiovascular and thromboembolic events.9,17 Patients should be screened for preexisting conditions that affect medical eligibility criteria.9,17
Sterilization
Several permanent sterilization options are available in the United States, including abdominal, laparoscopic, or hysteroscopic tubal ligation for women and vasectomy for men17,55–57 (eTable C). Patients should be counseled about the intended irreversibility of these procedures and the availability of long-acting reversible contraception.9,17
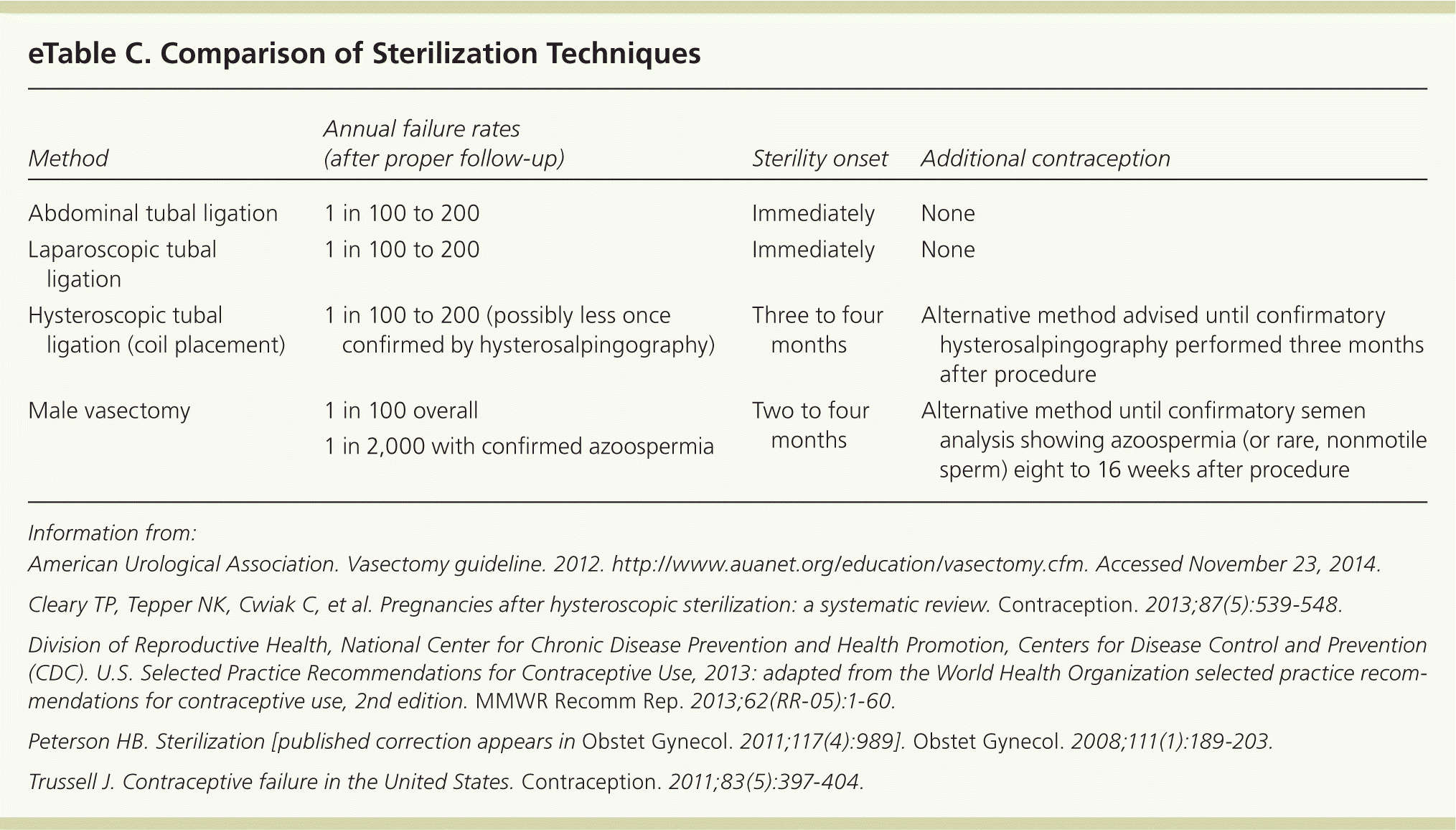
| Method | Annual failure rates (after proper follow-up) | Sterility onset | Additional contraception |
|---|---|---|---|
| Abdominal tubal ligation | 1 in 100 to 200 | Immediately | None |
| Laparoscopic tubal ligation | 1 in 100 to 200 | Immediately | None |
| Hysteroscopic tubal ligation (coil placement) | 1 in 100 to 200 (possibly less once confirmed by hysterosalpingography) | Three to four months | Alternative method advised until confirmatory hysterosalpingography performed three months after procedure |
| Male vasectomy | 1 in 100 overall | Two to four months | Alternative method until confirmatory semen analysis showing azoospermia (or rare, nonmotile sperm) eight to 16 weeks after procedure |
| 1 in 2,000 with confirmed azoospermia |
Data Sources: A PubMed search was completed using the MeSH function with the key phrase contraception combined with at least one of the following terms: effectiveness, safety, recommendations, guidelines, adolescent, female, or male. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. Also searched were Essential Evidence Plus, the Cochrane Database of Systematic Reviews, and the U.S. Preventive Services Task Force website. The references of the relevant CDC resources were reviewed, as were the references from the systematic reviews generated to create the highlighted CDC resources. Search dates: May 1 to July 1, 2014, and November 23, 2014.
The authors thank Ms. Margaret Freiberg for her editorial assistance.
The opinions and assertions contained herein are the personal views of the authors and are not to be construed as official or as reflecting the views of the U.S. armed services or any of their medical departments.
