A more recent article on hyperthyroidism is available.
Am Fam Physician. 2016;93(5):363-370
Related letter: Hyperthyroidism Caused by Thyroid Hormone Therapy
Patient information: See related handout on overactive thyroid gland (hyperthyroidism), written by the author of this article.
Author disclosure: No relevant financial affiliations.
Hyperthyroidism is an excessive concentration of thyroid hormones in tissues caused by increased synthesis of thyroid hormones, excessive release of preformed thyroid hormones, or an endogenous or exogenous extrathyroidal source. The most common causes of an excessive production of thyroid hormones are Graves disease, toxic multinodular goiter, and toxic adenoma. The most common cause of an excessive passive release of thyroid hormones is painless (silent) thyroiditis, although its clinical presentation is the same as with other causes. Hyperthyroidism caused by overproduction of thyroid hormones can be treated with antithyroid medications (methimazole and propylthiouracil), radioactive iodine ablation of the thyroid gland, or surgical thyroidectomy. Radioactive iodine ablation is the most widely used treatment in the United States. The choice of treatment depends on the underlying diagnosis, the presence of contraindications to a particular treatment modality, the severity of hyperthyroidism, and the patient's preference.
Hyperthyroidism is an excessive concentration of thyroid hormones in tissues causing a characteristic clinical state. In the United States, the overall prevalence of hyperthyroidism is 1.2%, and the prevalences of overt hyperthyroidism and subclinical hyperthyroidism are 0.5% and 0.7%, respectively.1
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| The choice of treatment modality for hyperthyroidism caused by overproduction of thyroid hormones depends on the patient's age, symptoms, comorbidities, and preference. | C | 25, 26 |
| The diagnostic workup for hyperthyroidism includes measuring thyroid-stimulating hormone, free thyroxine (T4), and total triiodothyronine (T3) levels to determine the presence and severity of the condition, as well as radioactive iodine uptake and scan of the thyroid gland to determine the cause. | C | 20, 21 |
| Methimazole (Tapazole) is the preferred antithyroid medication except in the first trimester of pregnancy and in patients with an adverse reaction to the medication. | B | 26 |
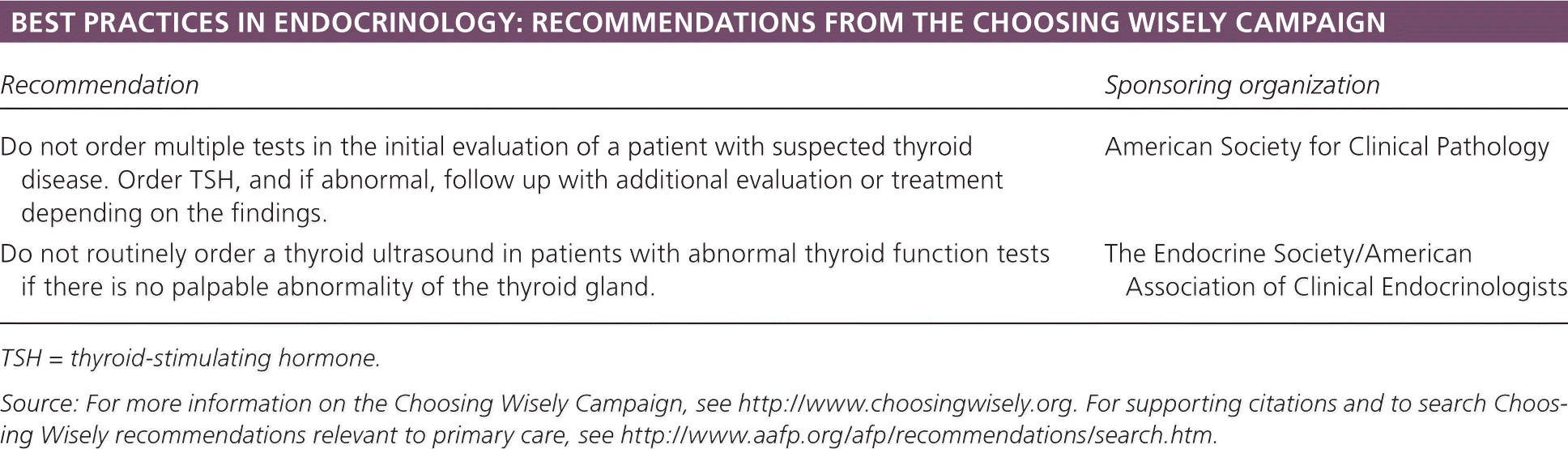
| Recommendation | Sponsoring organization |
|---|---|
| Do not order multiple tests in the initial evaluation of a patient with suspected thyroid disease. Order TSH, and if abnormal, follow up with additional evaluation or treatment depending on the findings. | American Society for Clinical Pathology |
| Do not routinely order a thyroid ultrasound in patients with abnormal thyroid function tests if there is no palpable abnormality of the thyroid gland. | The Endocrine Society/American Association of Clinical Endocrinologists |
Etiology and Pathogenesis
The common endogenous causes of hyperthyroidism are Graves disease, toxic multinodular goiter, toxic adenoma, and painless thyroiditis (Table 1).1–9 Graves disease, the most common cause of hyperthyroidism in the United States,2 is an autoimmune disorder in which thyroid-stimulating antibodies activate thyroid-stimulating hormone (TSH) receptors, triggering thyroid hormone synthesis. Risk factors for Graves disease include female sex and personal or family history of an autoimmune disorder.2,3
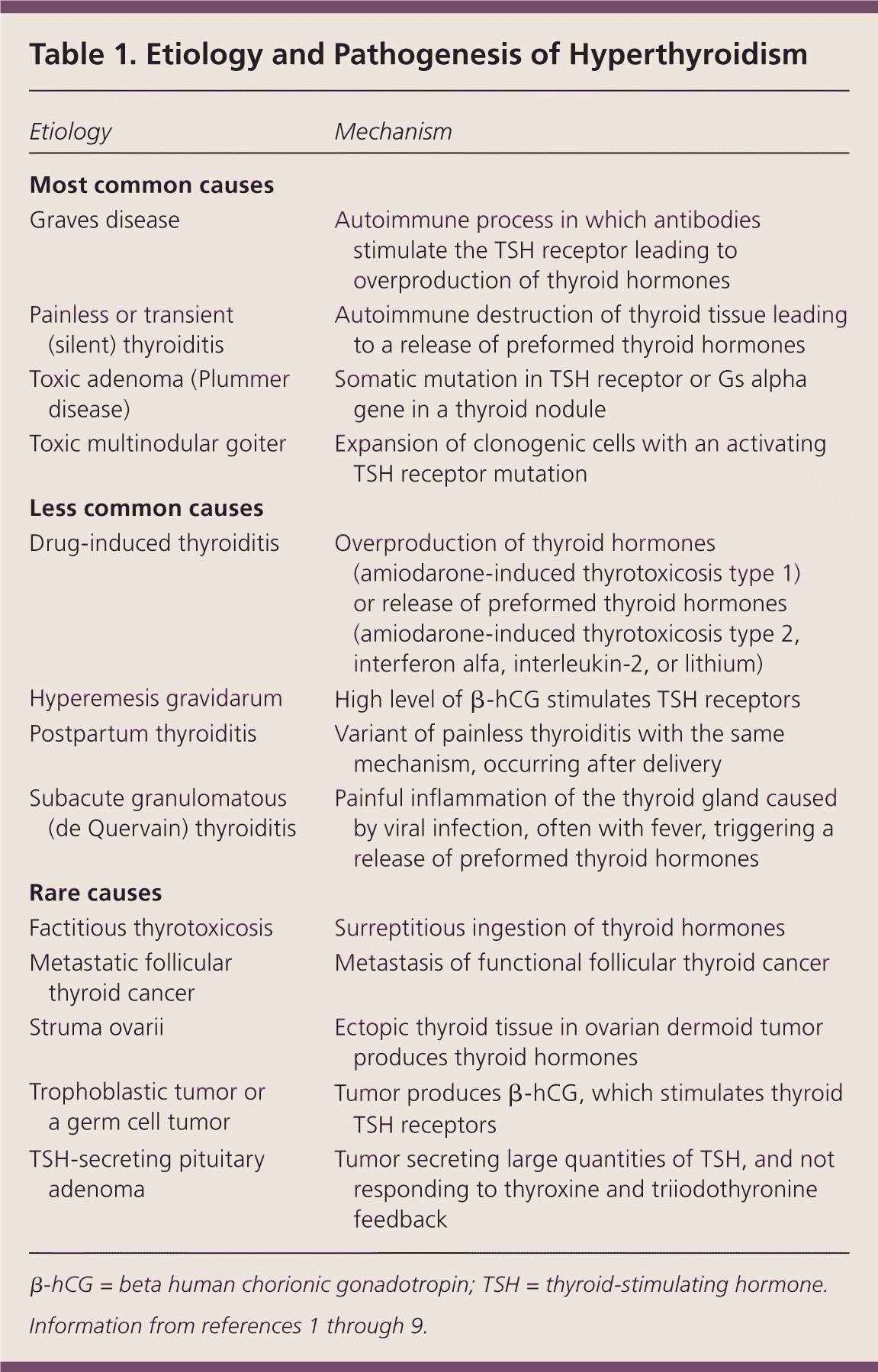
| Etiology | Mechanism |
|---|---|
| Most common causes | |
| Graves disease | Autoimmune process in which antibodies stimulate the TSH receptor leading to overproduction of thyroid hormones |
| Painless or transient (silent) thyroiditis | Autoimmune destruction of thyroid tissue leading to a release of preformed thyroid hormones |
| Toxic adenoma (Plummer disease) | Somatic mutation in TSH receptor or Gs alpha gene in a thyroid nodule |
| Toxic multinodular goiter | Expansion of clonogenic cells with an activating TSH receptor mutation |
| Less common causes | |
| Drug-induced thyroiditis | Overproduction of thyroid hormones (amiodarone-induced thyrotoxicosis type 1) or release of preformed thyroid hormones (amiodarone-induced thyrotoxicosis type 2, interferon alfa, interleukin-2, or lithium) |
| Hyperemesis gravidarum | High level of β-hCG stimulates TSH receptors |
| Postpartum thyroiditis | Variant of painless thyroiditis with the same mechanism, occurring after delivery |
| Subacute granulomatous (de Quervain) thyroiditis | Painful inflammation of the thyroid gland caused by viral infection, often with fever, triggering a release of preformed thyroid hormones |
| Rare causes | |
| Factitious thyrotoxicosis | Surreptitious ingestion of thyroid hormones |
| Metastatic follicular thyroid cancer | Metastasis of functional follicular thyroid cancer |
| Struma ovarii | Ectopic thyroid tissue in ovarian dermoid tumor produces thyroid hormones |
| Trophoblastic tumor or a germ cell tumor | Tumor produces β-hCG, which stimulates thyroid TSH receptors |
| TSH-secreting pituitary adenoma | Tumor secreting large quantities of TSH, and not responding to thyroxine and triiodothyronine feedback |
Toxic multinodular goiter is the second most common cause of hyperthyroidism in the United States and the most common cause in older persons living in iodine-deficient areas.2 Over time, nodules arise from the frequent replication of clonogenic cells that leads to a somatic activating mutation of TSH receptors.4 A single nodule is called a toxic adenoma (Plummer disease).
In contrast with these three disorders, painless or transient (silent) thyroiditis causes a destruction of thyroid follicles via an autoimmune mechanism and a release of preformed thyroid hormones into the circulation.5 Its clinical presentation is the same as with other causes. In a Danish study, its prevalence among patients with thyrotoxicosis was 0.5%, as evaluated by scintigraphy.6 Painless thyroiditis can be triggered by childbirth (postpartum thyroiditis) or by use of medications such as lithium, interferon alfa, interleukin-2, and amiodarone.7
Gestational hyperthyroidism develops in the first trimester of pregnancy as a result of the stimulatory action of placental beta human chorionic gonadotropin (β-hCG), which shares structural features with TSH, on the thyroid gland.8 β-hCG-mediated hyperthyroidism can occasionally be caused by hyperemesis gravidarum and, rarely, by a gestational trophoblastic tumor.8
Other rare causes of hyperthyroidism are TSH-secreting pituitary adenoma, metastatic follicular thyroid cancer, and struma ovarii.9
Clinical Manifestations
The clinical presentation of hyperthyroidism ranges from asymptomatic to thyroid storm (Table 2).10–18 Elevated thyroid hormone levels amplify catecholamine signaling through increased numbers of cell surface beta-adrenergic receptors. The resulting adrenergic symptoms (e.g., palpitations, heat intolerance, diaphoresis, tremor, stare [an appearance of a fixed look due to retraction of eyelids], lid lag, hyperdefecation) are the most common manifestations of hyperthyroidism.10 Hypermetabolism induces weight loss despite an increased appetite. Neuromuscular symptoms include weakness of proximal muscles.11 Psychiatric symptoms range from anxiety to frank psychosis.12 Patients with long-standing untreated hyperthyroidism may develop atrial fibrillation (10% to 15% of patients13,14) or heart failure (5.8% of patients15).

| Adrenergic |
| Palpitations, tachycardia, anxiety, tremor, jitteriness, diaphoresis, heat intolerance, stare, lid lag, hyperdefecation (not diarrhea) |
| Cardiovascular |
| Tachycardia, irregular pulse (in atrial fibrillation), dyspnea, orthopnea and peripheral edema (in heart failure) |
| Cutaneous |
| Onycholysis (Plummer nails), patchy or generalized hyperpigmentation (especially of the face and neck) |
| Symptoms pathognomonic for Graves disease: pretibial myxedema (thyroid dermopathy) and thyroid acropachy (clubbing of fingers and toes accompanied by soft-tissue swelling of the hands and feet) |
| Patchy vitiligo can also be observed in Graves disease |
| Hypermetabolism |
| Weight loss in spite of increased appetite, fever (in thyroid storm) |
| Neuromuscular |
| Brisk peripheral reflexes with accelerated relaxation phase and weakness of proximal muscles |
| Neuropsychiatric |
| Anxiety, rapid and pressured speech, insomnia, psychosis (if hyperthyroidism is severe) |
| Ocular |
| Increased lacrimation, incomplete closure of the eyes when sleeping reported by the patient's partner, photophobia, increased eye sensitivity to wind or smoke, grittiness or sensation of a foreign body or sand in the eyes |
| Symptoms pathognomonic for Graves disease: exophthalmos, periorbital edema, diplopia, blurred vision, reduced color perception |
Signs that are pathognomonic for Graves disease include orbitopathy, pretibial myxedema (thyroid dermopathy), and thyroid acropachy, which occur in 25%, 1.5%, and 0.3% of patients, respectively.16 Goiters that develop in Graves disease are usually smooth and may have a thrill on palpation or a bruit on auscultation. Single or multiple nodules on palpation raise suspicion for a toxic adenoma or a toxic multinodular goiter, although nonfunctioning thyroid nodules may coexist with a goiter in Graves disease.16
Graves orbitopathy manifests as exophthalmos or periorbital edema, and it can trigger photophobia, excessive lacrimation, increased eye sensitivity to wind or smoke, or a sensation of a foreign body in the eyes. In severe cases, blurred vision, diplopia, or reduced color perception may develop.16 Smoking increases the risk of developing Graves orbitopathy (odds ratio = 3.7).17
Pretibial myxedema, a less common finding, develops from fibroblast activation and manifests as swelling over the tibiae with the skin assuming a peau d'orange (orange peel) appearance.18 Thyroid acropachy, an uncommon sign, is the clubbing of fingers and toes with soft-tissue swelling of the hands and feet.19 Other skin manifestations of Graves disease include patchy hyperpigmentation and vitiligo.19
Laboratory and Radiographic Assessment
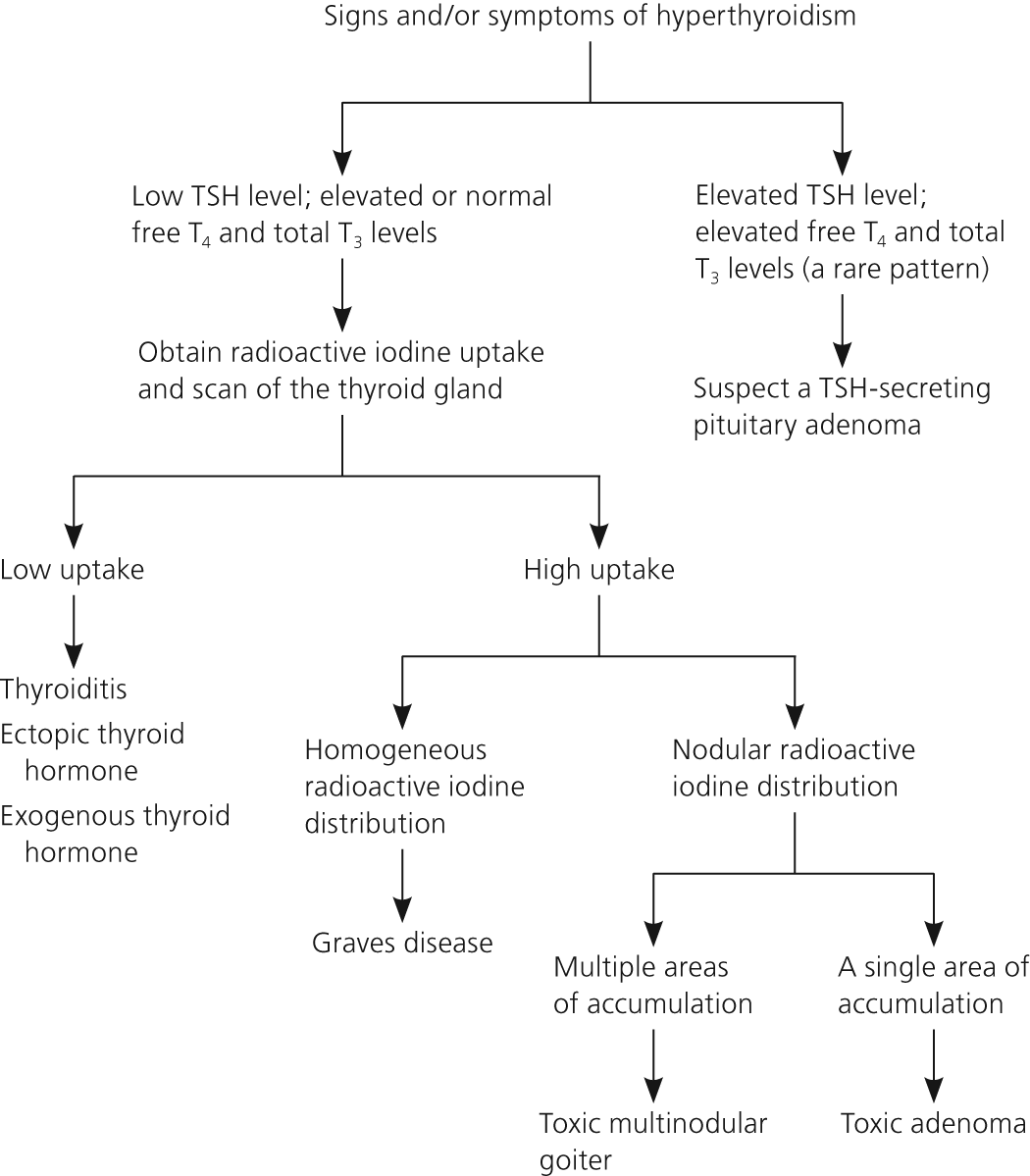
Clinical suspicion of hyperthyroidism should prompt laboratory testing (Figure 120–23 ). Some physicians first order a TSH test, which has the highest sensitivity and specificity for hyperthyroidism, and then subsequently obtain free thyroxine (T4) and total triiodothyronine (T3) levels (free T3 assays are poorly validated1) if the TSH level is low. Others prefer to order all three tests if hyperthyroidism is suspected to make the diagnosis more efficiently. Many laboratories perform reflex free T4 testing if TSH is suppressed. Table 3 lists patterns of thyroid function tests in hyperthyroidism.20,21 The serum level of thyroid-stimulating immunoglobulins or TSH-receptor antibodies helps distinguish Graves disease from other causes of hyperthyroidism in patients who lack signs pathognomonic of Graves disease and have a contraindication to radioactive iodine uptake and scan.
THYROID FUNCTION TEST RESULTS SIMULATING HYPERTHYROIDISM
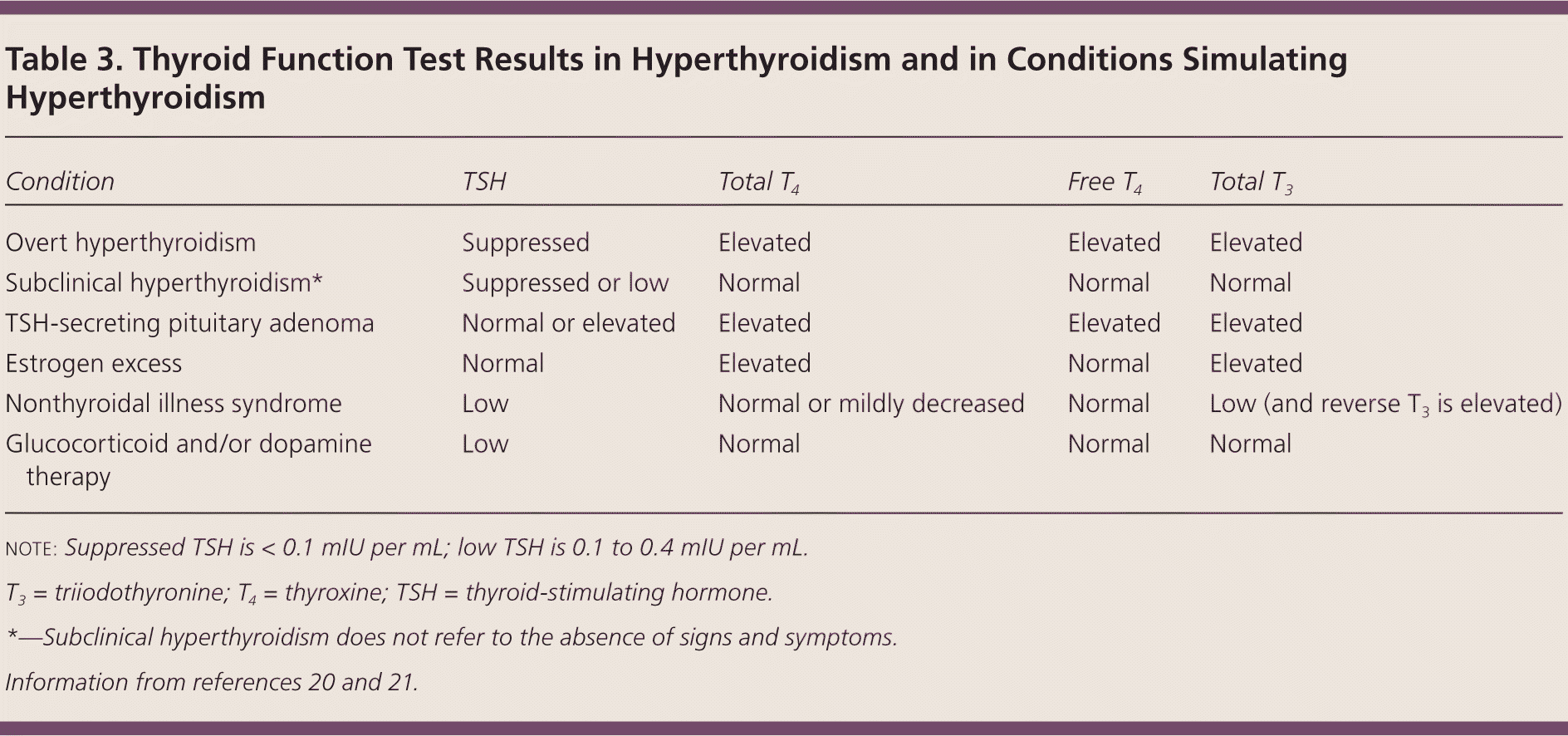
| Condition | TSH | Total T4 | Free T4 | Total T3 |
|---|---|---|---|---|
| Overt hyperthyroidism | Suppressed | Elevated | Elevated | Elevated |
| Subclinical hyperthyroidism* | Suppressed or low | Normal | Normal | Normal |
| TSH-secreting pituitary adenoma | Normal or elevated | Elevated | Elevated | Elevated |
| Estrogen excess | Normal | Elevated | Normal | Elevated |
| Nonthyroidal illness syndrome | Low | Normal or mildly decreased | Normal | Low (and reverse T3 is elevated) |
| Glucocorticoid and/or dopamine therapy | Low | Normal | Normal | Normal |
Patients with critical or acute illness often develop the nonthyroidal illness syndrome manifesting as mildly decreased TSH levels (0.1 to 0.4 mIU per mL) and normal or mildly decreased T4 levels. In contrast to subclinical hyperthyroidism, the T3 level is usually low and the reverse T3 level is elevated. This condition resolves spontaneously when the patient recovers from the acute illness.21
Exogenous glucocorticoids or dopamine may cause a mild decrease of TSH levels, a situation often occurring in the intensive care unit. Total T4, total T3, and free T4 levels remain normal. TSH levels return to normal after these medications are discontinued.21
RADIOACTIVE IODINE UPTAKE AND THYROID SCAN
A radioactive iodine uptake test and thyroid scan help determine the cause of hyperthyroidism (Table 4). Uptake is the percentage of an iodine 123 (I-123) tracer dose taken up by the thyroid gland, ranging from 15% to 25% at 24 hours. The uptake is very low (0% to 2%) in patients with thyroiditis and high in patients with Graves disease, a toxic adenoma, or a toxic multinodular goiter.23,24
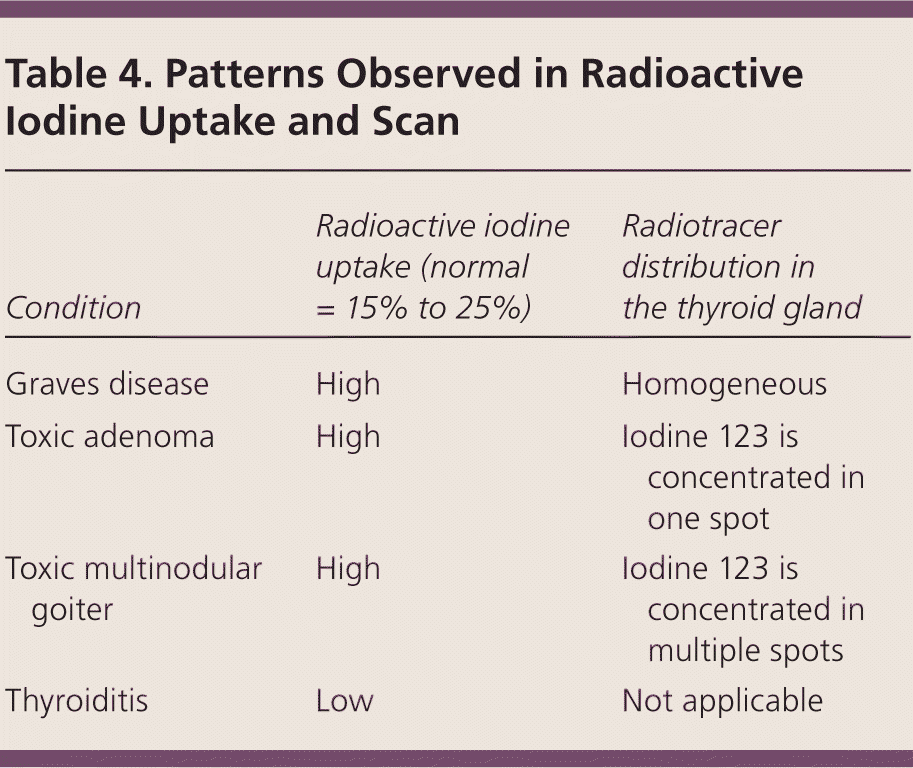
| Condition | Radioactive iodine uptake (normal = 15% to 25%) | Radiotracer distribution in the thyroid gland |
|---|---|---|
| Graves disease | High | Homogeneous |
| Toxic adenoma | High | Iodine 123 is concentrated in one spot |
| Toxic multinodular goiter | High | Iodine 123 is concentrated in multiple spots |
| Thyroiditis | Low | Not applicable |

Ultrasonography is sometimes used as a cost-effective and safe alternative to radioactive iodine uptake and scan. It is the primary imaging modality used during pregnancy, lactation, and in amiodarone-induced thyrotoxicosis.24
Treatment
Regardless of the cause of hyperthyroidism, the adrenergic symptoms are controlled by beta blockers (Table 5).25–28 Propranolol has the theoretical advantage of also inhibiting 5′-monodeiodinase, thus blocking peripheral conversion of T4 to T3.25 The choice of treatment modality for hyperthyroidism caused by overproduction of thyroid hormones depends on the patient's age, symptoms, comorbidities, and preference.25,26
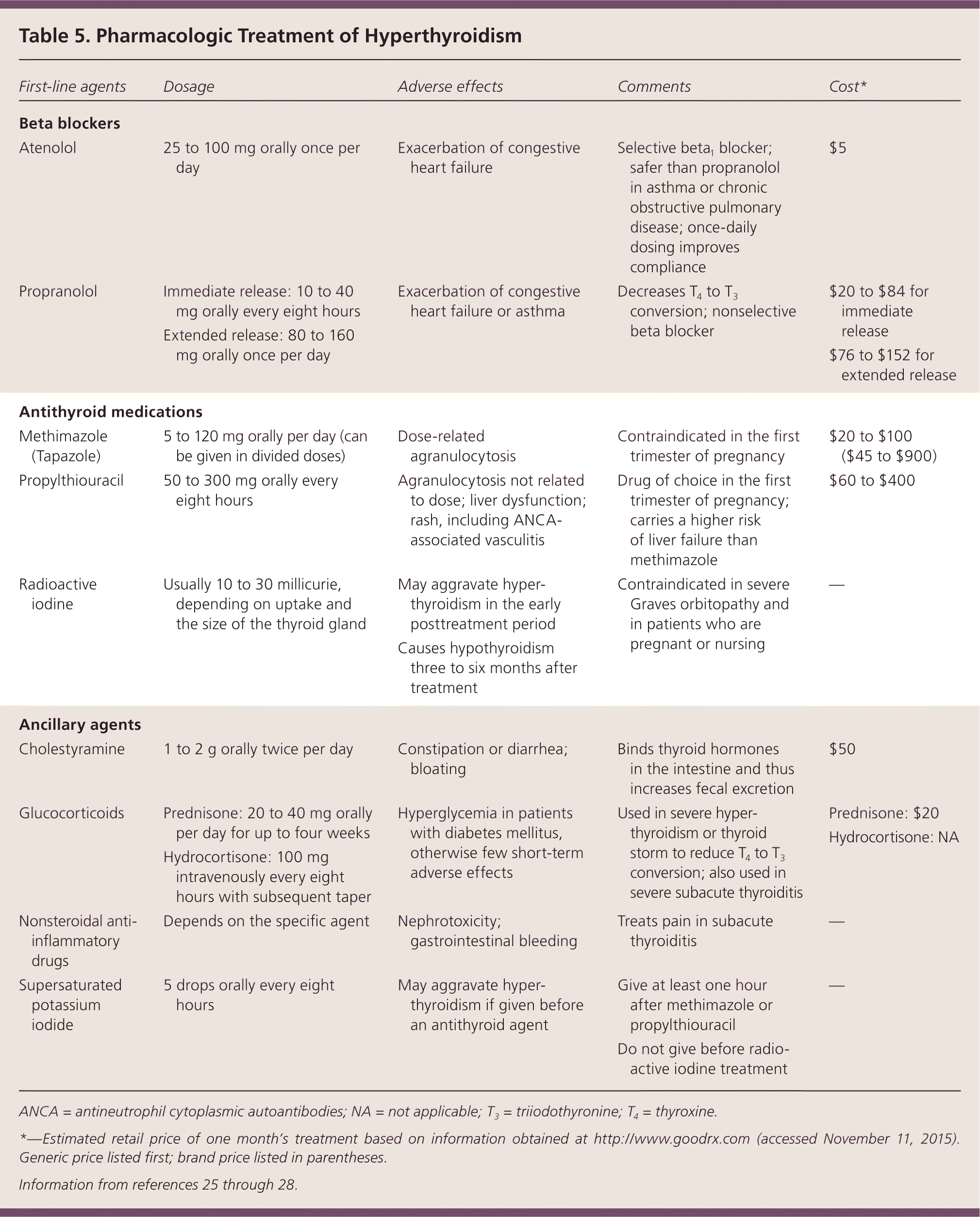
| First-line agents | Dosage | Adverse effects | Comments | Cost* |
|---|---|---|---|---|
| Beta blockers | ||||
| Atenolol | 25 to 100 mg orally once per day | Exacerbation of congestive heart failure | Selective beta1 blocker; safer than propranolol in asthma or chronic obstructive pulmonary disease; once-daily dosing improves compliance | $5 |
| Propranolol |
| Exacerbation of congestive heart failure or asthma | Decreases T4 to T3 conversion; nonselective beta blocker |
|
| Antithyroid medications | ||||
| Methimazole (Tapazole) | 5 to 120 mg orally per day (can be given in divided doses) | Dose-related agranulocytosis | Contraindicated in the first trimester of pregnancy | $20 to $100 ($45 to $900) |
| Propylthiouracil | 50 to 300 mg orally every eight hours | Agranulocytosis not related to dose; liver dysfunction; rash, including ANCA-associated vasculitis | Drug of choice in the first trimester of pregnancy; carries a higher risk of liver failure than methimazole | $60 to $400 |
| Radioactive iodine | Usually 10 to 30 millicurie, depending on uptake and the size of the thyroid gland |
| Contraindicated in severe Graves orbitopathy and in patients who are pregnant or nursing | — |
| Ancillary agents | ||||
| Cholestyramine | 1 to 2 g orally twice per day | Constipation or diarrhea; bloating | Binds thyroid hormones in the intestine and thus increases fecal excretion | $50 |
| Glucocorticoids |
| Hyperglycemia in patients with diabetes mellitus, otherwise few short-term adverse effects | Used in severe hyperthyroidism or thyroid storm to reduce T4 to T3 conversion; also used in severe subacute thyroiditis |
|
| Nonsteroidal anti-inflammatory drugs | Depends on the specific agent | Nephrotoxicity; gastrointestinal bleeding | Treats pain in subacute thyroiditis | — |
| Supersaturated potassium iodide | 5 drops orally every eight hours | May aggravate hyperthyroidism if given before an antithyroid agent |
| — |
GRAVES DISEASE
Graves disease requires one of the three treatment options: an antithyroid medication (methimazole [Tapazole] or propylthiouracil), radioactive iodine (I-131) ablation of the thyroid gland, or surgical thyroidectomy. The choice of treatment depends on the benefits vs. risks in a specific clinical situation and on the patient's preference25 (eTable A).
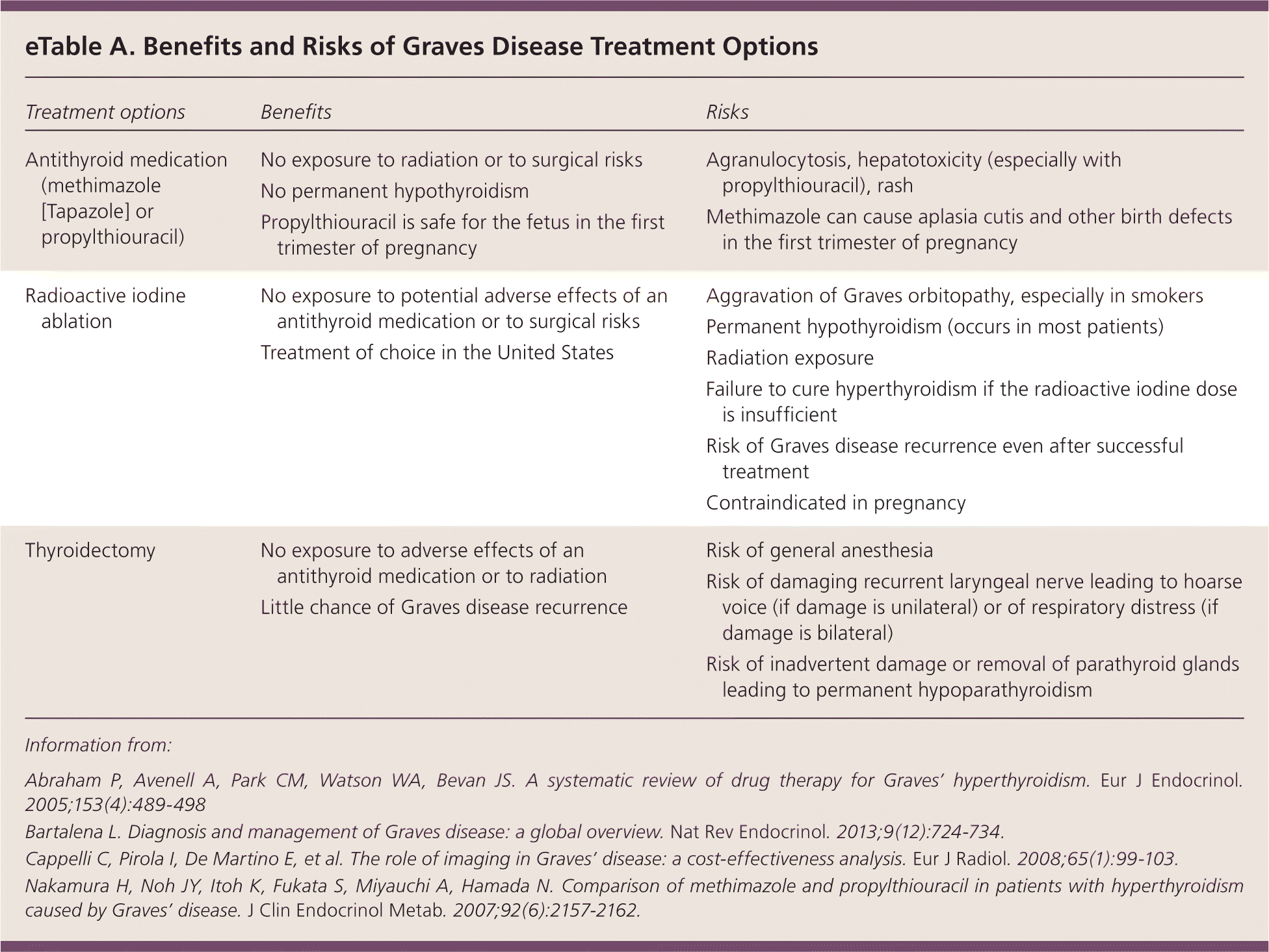
| Treatment options | Benefits | Risks |
|---|---|---|
|
|
|
|
|
|
|
|
|
Antithyroid Medications. Antithyroid medications are thionamides; they inhibit thyroid peroxidase, blocking the synthesis of T3 and T4. Thionamides can serve as a long-term therapy or as a bridge to I-131 ablation or thyroidectomy, with the goal of normalizing thyroid function and preventing exacerbation of hyperthyroidism after I-131 ablation or avoiding surgical risks associated with uncontrolled hyperthyroidism. Because Graves disease remits in up to 30% of patients treated with thionamides, these medications can be used as the initial treatment, with ablation or thyroidectomy performed if remission does not occur.25,26 Once medical therapy is discontinued, relapse occurs in 30% to 70% of patients, mostly within the first year.27 After discontinuation, thyroid function should be monitored every one to three months for six to 12 months, and the patient should be instructed to contact the physician if symptoms recur.
Because use of propylthiouracil has a higher risk of causing severe liver injury, as highlighted in the U.S. Food and Drug Administration's boxed warning, methimazole is preferred except during the first trimester of pregnancy (can cause birth defects) and in patients with an adverse reaction to methimazole.28,29 For patients using methimazole, the prevalence of agranulocytosis is 0.17%, the incidence of hepatitis is 3.17 per 1,000 person-years, and the incidence of acute hepatic failure is 0.32 per 1,000 person-years.30,31 Patients should be instructed to discontinue medication use and contact their physician if they develop jaundice, acholic stools, dark urine, arthralgias, abdominal pain, nausea, vomiting, fever, or sore throat. A baseline complete blood count (CBC) with differential and a hepatic panel should be obtained before initiating an antithyroid medication. Subsequent routine monitoring of CBC is unnecessary, but CBC with differential should be obtained if fever and/or pharyngitis develop.
Free T4 and total T3 should be obtained four weeks after starting a thionamide and every four to eight weeks thereafter with the dosage adjusted based on results. Once free T4 and total T3 levels normalize, they should be monitored every three months. Serum TSH is of limited value early in the treatment course because levels may remain suppressed for several months after treatment is started. An antithyroid medication should be continued for 12 to 18 months, then tapered or discontinued if the TSH level is normal at the time. Elevated or above-normal TSH levels (greater than 4.0 mIU per mL) at antithyroid drug discontinuation is associated with an increased likelihood of permanent remission.27
Radioactive Iodine Ablation. Radioactive iodine ablation of the thyroid gland is the most common treatment of Graves disease in the United States. It is contraindicated in pregnancy. Moderate to severe Graves orbitopathy is a relative contraindication, especially in patients who smoke, because radioactive iodine may exacerbate the eye disease.32,33 In mild cases of Graves orbitopathy, radioactive iodine ablation can be performed with concomitant glucocorticoid therapy. Nonradioactive iodine impedes radioactive iodine uptake by iodide transporter; therefore, exposure to large amounts of nonradioactive iodine (e.g., iodinated contrast, amiodarone) should be avoided within three months before radioactive iodine ablation. Pregnancy should be ruled out within 48 hours before radioactive iodine ablation and avoided for six months thereafter.1 A thionamide should be discontinued at least five days before the treatment but can be restarted three to five days after to maintain control of thyroid function, because it may take up to 12 weeks to achieve the full effect of radioactive iodine.
Most patients develop permanent hypothyroidism between two and six months after radioactive iodine ablation and require thyroid hormone supplementation.1,33 Free T4 and total T3 should be measured four to eight weeks after ablation; if hyperthyroidism persists, these indices should be monitored every four to six weeks and thyroid hormone replacement started in the early stages of hypothyroidism.1
Thyroidectomy. This treatment option is preferred in patients with goiter-induced compressive symptoms and in patients with contraindications to radioactive iodine ablation or thionamides. Besides general anesthesia risk, thyroidectomy carries a risk of inadvertently injuring parathyroid glands and recurrent laryngeal nerves.34
TOXIC ADENOMA OR TOXIC MULTINODULAR GOITER
Antithyroid medications can control hyperthyroidism, but do not induce remission of hyperthyroidism associated with toxic adenoma or toxic multinodular goiter. Therefore, radioactive iodine ablation and thyroidectomy are the main treatment options for these conditions. Thyroidectomy is favored if a nodule or goiter causes compressive symptoms. Antithyroid medications may be used for long-term treatment in select patients who refuse ablation or who have a contraindication to thyroidectomy.35,36
THYROIDITIS
Painless thyroiditis and subacute thyroiditis are self-limiting conditions that usually resolve spontaneously within six months. There is no role for antithyroid medications or radioactive iodine ablation in the treatment of thyroiditis. Beta blockers may be used if needed to control adrenergic symptoms. Pain associated with subacute thyroiditis may be relieved with a nonsteroidal anti-inflammatory drug.5
THYROID STORM
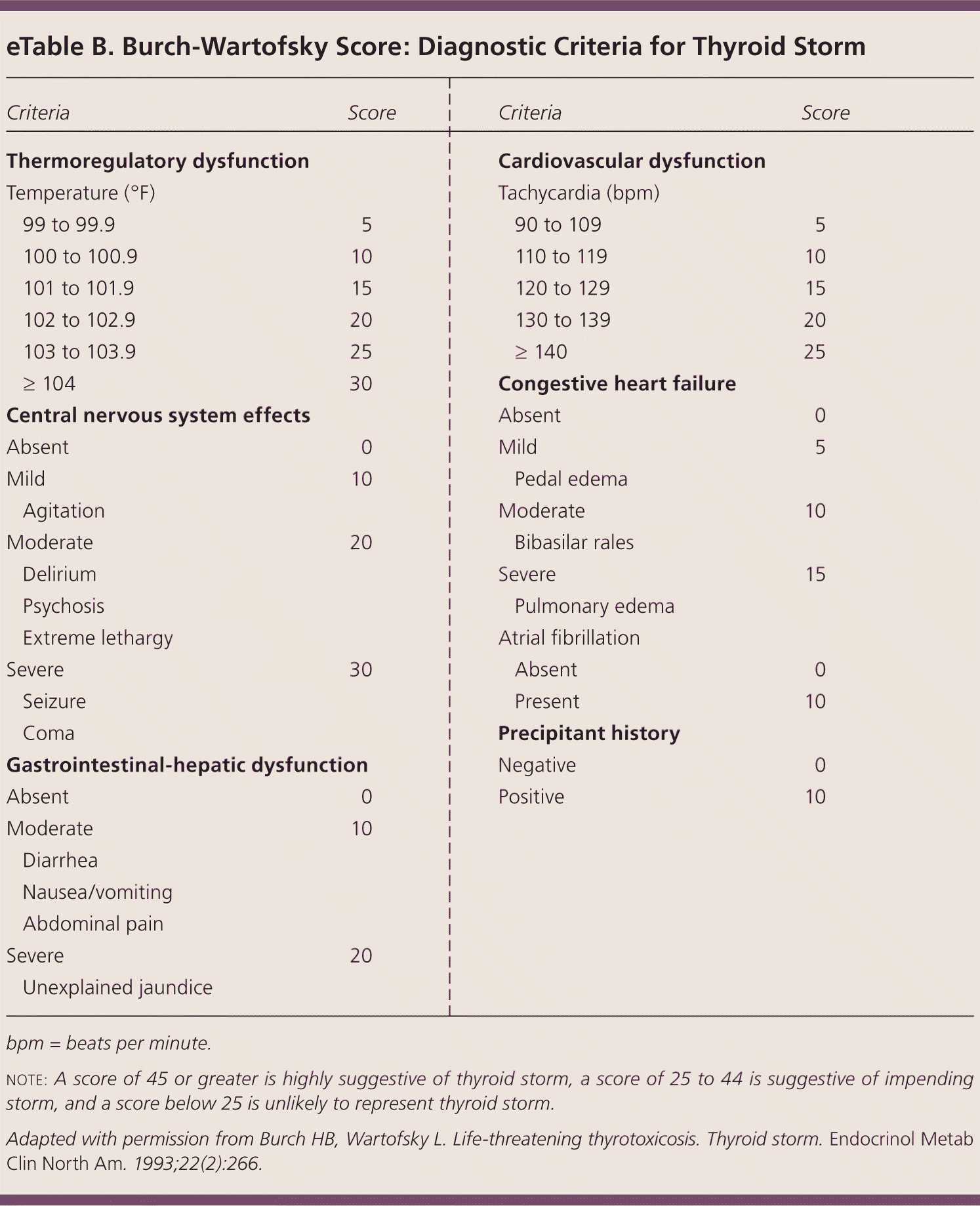
| Criteria | Score | |
|---|---|---|
| Thermoregulatory dysfunction | ||
| Temperature (°F) | ||
| 99 to 99.9 | 5 | |
| 100 to 100.9 | 10 | |
| 101 to 101.9 | 15 | |
| 102 to 102.9 | 20 | |
| 103 to 103.9 | 25 | |
| ≥ 104 | 30 | |
| Central nervous system effects | ||
| Absent | 0 | |
| Mild | 10 | |
| Agitation | ||
| Moderate | 20 | |
| Delirium | ||
| Psychosis | ||
| Extreme lethargy | ||
| Severe | 30 | |
| Seizure | ||
| Coma | ||
| Gastrointestinal-hepatic dysfunction | ||
| Absent | 0 | |
| Moderate | 10 | |
| Diarrhea | ||
| Nausea/vomiting | ||
| Abdominal pain | ||
| Severe | 20 | |
| Unexplained jaundice | ||
| Cardiovascular dysfunction | ||
| Tachycardia (bpm) | ||
| 90 to 109 | 5 | |
| 110 to 119 | 10 | |
| 120 to 129 | 15 | |
| 130 to 139 | 20 | |
| ≥ 140 | 25 | |
| Congestive heart failure | ||
| Absent | 0 | |
| Mild | 5 | |
| Pedal edema | ||
| Moderate | 10 | |
| Bibasilar rales | ||
| Severe | 15 | |
| Pulmonary edema | ||
| Atrial fibrillation | ||
| Absent | 0 | |
| Present | 10 | |
| Precipitant history | ||
| Negative | 0 | |
| Positive | 10 | |

| Supportive treatment | |
| Airway maintenance | |
| Oxygen | |
| IV fluids | |
| Cooling blanket (do not use salicylate to treat fever because salicylates increase free T4 and free T3 levels) | |
| Inhibit T4 and T3 synthesis | |
| Methimazole (Tapazole) orally, rectally, via nasogastric tube, or IV, 20 to 40 mg every eight hours | |
| Propylthiouracil orally, rectally, or via nasogastric tube, 200 to 400 mg every eight hours | |
| Inhibit T4 and T3 release | |
| Saturated solution of potassium iodide, five drops orally every six hours to be started at least one hour after administration of an antithyroid agent | |
| Heart rate control | |
| Esmolol (Brevibloc) IV, 50 to 100 mcg per kg per minute | |
| Propranolol, 60 to 80 mg orally every four hours | |
| Metoprolol IV, 5 to 10 mg every two to four hours | |
| If beta-blockade is contraindicated, use diltiazem IV, 0.25 mg per kg over two minutes, then 10 mg per hour IV infusion or 60 to 90 mg orally every six to eight hours | |
| Inhibit T4 to T3 conversion | |
| Hydrocortisone 100 mg IV every eight hours (also suppresses autoimmune process in Graves disease) | |
| Treat precipitating cause | |
DRUG-ASSOCIATED HYPERTHYROIDISM
Amiodarone-induced thyrotoxicosis can be classified as type 1 (thyroid hormone overproduction, treated with antithyroid medications) or type 2 (thyroid tissue destruction, treated with steroids). Amiodarone should not be discontinued unless it can be stopped safely, without triggering cardiac complications.38,39
Hyperthyroidism associated with use of other medications (e.g., lithium, interferon alfa, tyrosine kinase inhibitors, highly active antiretroviral therapy) is usually self-limited. The physician should determine whether the medication may be discontinued safely or replaced with a different medication.
Data Sources: A PubMed search was performed in Clinical Queries using the key terms hyperthyroidism, thyrotoxicosis, Graves disease, toxic multinodular goiter, toxic adenoma, and thyroiditis. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. Also searched were Essential Evidence Plus, the Cochrane database, the National Guideline Clearinghouse database, and UpToDate. Search dates: December 26, 2014, and August 24, 2015.
The author thanks Dr. Harold E. Carlson and Dr. Marie C. Gelato for reviewing this manuscript.