
A more recent article on medications for alcohol use disorder is available.
Am Fam Physician. 2016;93(6):457-465
Patient information: See related handout on medicine for alcohol use disorder, written by the authors of this article.
Author disclosure: No relevant financial affiliations.
The U.S. Preventive Services Task Force recommends that clinicians screen adults for alcohol misuse and provide persons engaged in risky or hazardous drinking behaviors with brief behavioral counseling to reduce alcohol misuse. However, only a minority of American adults with high-risk alcohol use receive treatment. Three medications are approved by the U.S. Food and Drug Administration to treat alcohol use disorder: acamprosate, disulfiram, and naltrexone. Acamprosate and naltrexone reduce alcohol consumption and increase abstinence rates, although the effects appear to be modest. Disulfiram has been used for years, but evidence supporting its effectiveness is inconsistent. Other medications may be beneficial to reduce heavy alcohol use. The anticonvulsants topiramate and gabapentin may reduce alcohol ingestion, although long-term studies are lacking. Antidepressants do not decrease alcohol use in patients without mood disorders, but sertraline and fluoxetine may help depressed patients decrease alcohol ingestion. Ondansetron may reduce alcohol use, particularly in selected subpopulations. Further study is needed for genetically targeted or as-needed medications to reduce alcohol use.
Excessive alcohol use is the third leading cause of preventable death in the United States.1 The Diagnostic and Statistical Manual of Mental Disorders, 5th ed., integrates the previous categories of alcohol abuse and alcohol dependence into the diagnosis of alcohol use disorder (AUD); Table 1 shows the complete criteria.2 The National Institutes of Health estimates that AUD affected 9% of adult men and 5% of adult women in the United States in 2013, and many more adults and adolescents engaged in high-risk alcohol use.3
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Acamprosate (Campral) increases abstinence rates in patients with alcohol use disorder. | A | 9, 12, 16 |
| There is inconsistent evidence supporting the use of disulfiram (Antabuse) to decrease alcohol intake in patients with alcohol use disorder. | B | 12, 18, 19 |
| Naltrexone (Revia) decreases alcohol consumption in patients with alcohol use disorder. | A | 9, 12, 21 |
| Topiramate (Topamax) may decrease alcohol intake in patients with alcohol use disorder. | B | 27–31 |
| Ondansetron (Zofran) may decrease alcohol intake in patients with alcohol use disorder. | B | 41, 42 |

| A. | A problematic pattern of alcohol use leading to clinically significant impairment or distress, as manifested by at least two of the following, occurring within a 12-month period: | ||
| 1. Alcohol is often taken in larger amounts or over a longer period than was intended. | |||
| 2. There is a persistent desire or unsuccessful efforts to cut down or control alcohol use. | |||
| 3. A great deal of time is spent in activities necessary to obtain alcohol, use alcohol, or recover from its effects. | |||
| 4. Craving, or a strong desire or urge to use alcohol. | |||
| 5. Recurrent alcohol use resulting in a failure to fulfill major role obligations at work, school, or home. | |||
| 6. Continued alcohol use despite having persistent or recurrent social or interpersonal problems caused or exacerbated by the effects of alcohol. | |||
| 7. Important social, occupational, or recreational activities are given up or reduced because of alcohol use. | |||
| 8. Recurrent alcohol use in situations in which it is physically hazardous. | |||
| 9. Alcohol use is continued despite knowledge of having a persistent or recurrent physical or psychological problem that is likely to have been caused or exacerbated by alcohol. | |||
| 10. Tolerance, as defined by either of the following: | |||
| a. A need for markedly increased amounts of alcohol to achieve intoxication or desired effect. | |||
| b. A markedly diminished effect with continued use of the same amount of alcohol. | |||
| 11. Withdrawal, as manifested by either of the following: | |||
| a. The characteristic withdrawal syndrome for alcohol. | |||
| b. Alcohol (or a closely related substance, such as a benzodiazepine) is taken to relieve or avoid withdrawal symptoms. | |||
| Specify if: | |||
| In early remission: After full criteria for alcohol use disorder were previously met, none of the criteria for alcohol use disorder have been met for at least 3 months but for less than 12 months (with the exception that Criterion A4, “Craving, or a strong desire or urge to use alcohol,” may be met). | |||
| In sustained remission: After full criteria for alcohol use disorder were previously met, none of the criteria for alcohol use disorder have been met at any time during a period of 12 months or longer (with the exception that Criterion A4, “Craving, or a strong desire or urge to use alcohol,” may be met). | |||
| Specify if: | |||
| In a controlled environment: This additional specifier is used if the individual is in an environment where access to alcohol is restricted. | |||
Guidelines
The U.S. Preventive Services Task Force (USPSTF) recommends screening adults for alcohol misuse and providing persons engaged in risky or hazardous drinking behaviors with brief behavioral counseling to reduce alcohol misuse.4
Table 2 lists USPSTF-recommended screening methods that have been validated in primary care settings.4,5 Although the CAGE questionnaire is familiar to clinicians, its accuracy varies in ambulatory settings, and it is not recommended by the USPSTF. Individuals who engage in high-risk drinking should be counseled to decrease their alcohol use, and patients diagnosed with AUD should be offered treatment, such as brief behavioral interventions, support programs such as Alcoholics Anonymous, individual and group therapy, and medications. A study of more than 43,000 American adults found that only 24% of those with AUD received treatment.6 Possible reasons for low treatment rates include the social stigma of AUD, a lack of understanding of AUD as a treatable condition, and a lack of clinician familiarity with pharmacotherapy and other treatment options for the disorder. Patients with AUD are at risk of alcohol withdrawal and may require medical management for withdrawal before initiating treatment.7
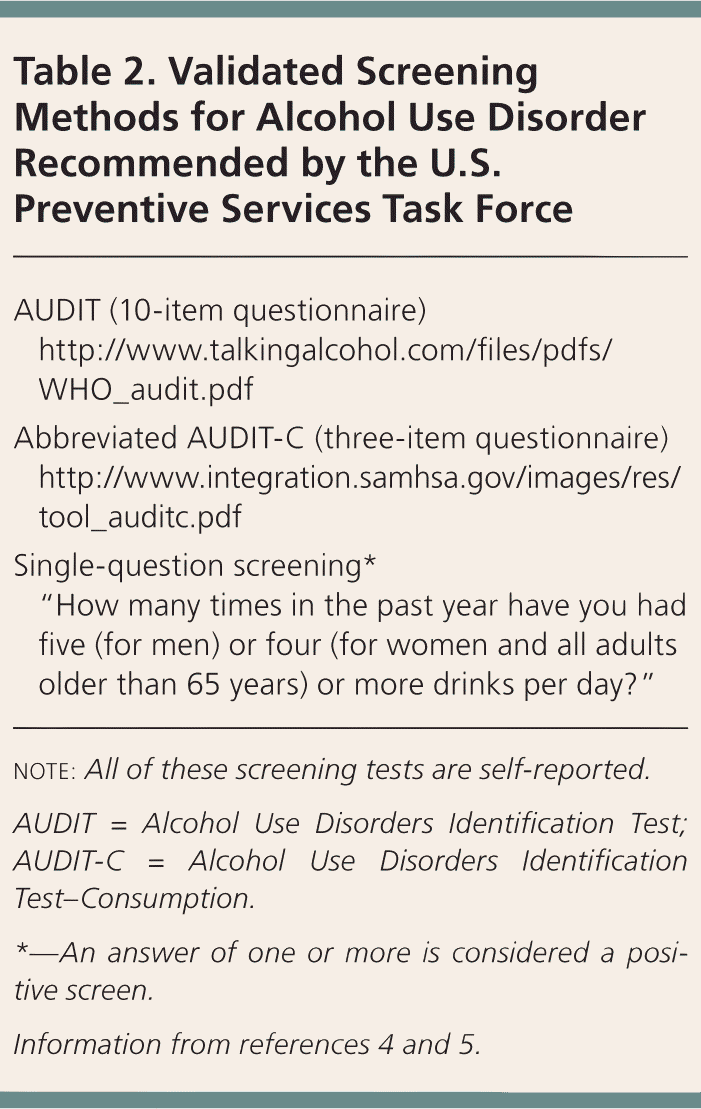
| AUDIT (10-item questionnaire) | |
| http://www.talkingalcohol.com/files/pdfs/WHO_audit.pdf | |
| Abbreviated AUDIT-C (three-item questionnaire) | |
| http://www.integration.samhsa.gov/images/res/tool_auditc.pdf | |
| Single-question screening* | |
| “How many times in the past year have you had five (for men) or four (for women and all adults older than 65 years) or more drinks per day?” | |
A Substance Abuse and Mental Health Services Administration/National Institute on Alcohol Abuse and Alcoholism Consensus Panel recommends pharmacotherapy along with behavioral interventions for AUD.8 However, less than 10% of patients with AUD are treated with medications.9 It is difficult to assess the benefit of medications because most studies assess outcomes such as alcoholic drinks per day and drinking days over a period of time, rather than abstinence and complications of alcohol abuse (e.g., mortality, cirrhosis, alcohol-related arrests, job loss). Most studies of medications for AUD also include counseling, so it is difficult to assess medication effects without counseling.
The Department of Veterans Affairs recommends the consideration of naltrexone (Revia, Vivitrol) and/or acamprosate (Campral) for AUD treatment, along with counseling.10 The United Kingdom's National Institute for Health and Care Excellence recommends the consideration of acamprosate or naltrexone to treat AUD, with disulfiram (Antabuse) as a second-line medication.11 The Substance Abuse and Mental Health Services Administration/National Institute on Alcohol Abuse and Alcoholism Consensus Panel provides a guide for the use of acamprosate, disulfiram, and naltrexone.8
No medications are approved for the treatment of AUD in adolescents younger than 18 years; therefore, these patients should be referred for subspecialist treatment. None of the medications used to treat AUD have been proven completely safe during pregnancy or lactation, so they should be used cautiously in women of childbearing age.
Medications for the Treatment of AUD
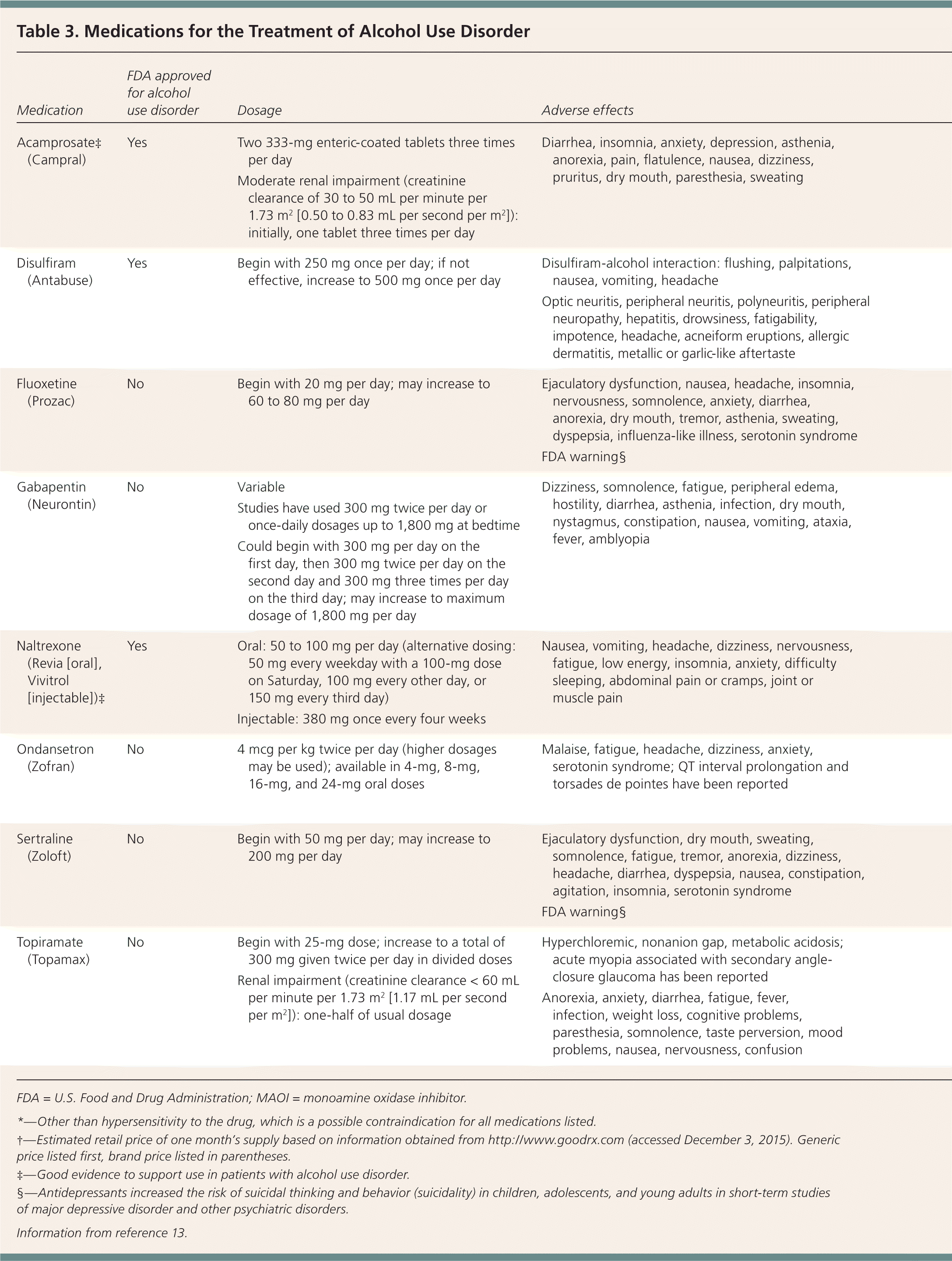
An Agency for Healthcare Research and Quality (AHRQ) review that included 135 studies of pharmacologic treatment of AUD in outpatient settings found moderate evidence to support the use of naltrexone and acamprosate, and insufficient evidence to support the use of disulfiram. The review also concluded that the evidence was lacking for most other medications, including those for off-label use and those undergoing trials. However, there is some evidence for topiramate (Topamax) and valproic acid (Depakene).12
| Medication | FDA approved for alcohol use disorder | Dosage | Adverse effects | Contraindications* | Comments | Cost† |
|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
APPROVED BY THE U.S. FOOD AND DRUG ADMINISTRATION
Acamprosate. This drug appears to be most effective at maintaining abstinence in patients who are not currently drinking alcohol.14 Acamprosate seems to interact with glutamate at the N-methyl-d-aspartate receptor, although its exact mechanism is unclear.15 It is safe in patients with impaired hepatic function but should be avoided in patients with severe renal dysfunction. A systematic review of 27 studies including 7,519 patients using acamprosate showed a number needed to treat (NNT) of 12 to prevent a return to any drinking.9 A Cochrane review of 24 trials including 6,915 patients concluded that acamprosate reduced drinking compared with placebo (NNT = 9).16 One randomized trial found no difference between acamprosate and placebo, although outcomes improved significantly in both groups. This may be because enrolled patients were highly motivated to decrease alcohol use, increasing the likelihood of success with any treatment.17
Disulfiram. There are limited trials to support the effectiveness of disulfiram. It does not reduce the craving for alcohol, but it causes unpleasant symptoms when alcohol is ingested because it inhibits aldehyde dehydrogenase and alcohol metabolism. Compliance is a major limitation, and disulfiram is more effective when taken under supervision. One trial randomized 243 patients to naltrexone, acamprosate, or disulfiram with supervision over 12 weeks and determined that patients taking disulfiram had fewer heavy drinking days, lower weekly consumption, and a longer period of abstinence compared with the other drugs.18 However, a 2014 meta-analysis of 22 randomized trials found that in open-label studies, disulfiram was more effective than naltrexone, acamprosate, and no disulfiram, but blinded studies did not demonstrate benefit for disulfiram.19 In a systematic review of two studies including 492 patients, disulfiram did not reduce drinking rates.9 As noted earlier, the AHRQ review found insufficient evidence to support disulfiram's effectiveness.12
Naltrexone. Naltrexone, an opioid antagonist, reduces alcohol consumption in patients with AUD, and is more successful in those who are abstinent before starting the medication.8 The opioid receptor system mediates the pleasurable effects of alcohol. Alcohol ingestion stimulates endogenous opioid release and increases dopamine transmission. Naltrexone blocks these effects, reducing euphoria and cravings.20 Naltrexone is available in oral and injectable long-acting formulations.
A Cochrane review that included 50 randomized trials and 7,793 patients found that oral naltrexone decreased heavy drinking (NNT = 10) and slightly decreased daily drinking (NNT = 25). The number of heavy drinking days and the amount of alcohol consumed also decreased. Injectable naltrexone did not decrease heavy drinking, but the sample size was small.21
A subsequent systematic review of 53 randomized trials including 9,140 patients found that oral naltrexone increased abstinence rates (NNT = 20) and decreased heavy drinking (NNT = 12). There was no difference between naltrexone and acamprosate. Injectable naltrexone did not demonstrate benefit.9 A randomized trial of 627 veterans with AUD who received injectable naltrexone or placebo found that 380 mg of naltrexone given intramuscularly decreased heavy drinking days over six months but did not increase abstinence rates.22
Another meta-analysis found no difference in heavy drinking between acamprosate and naltrexone; however, it favored acamprosate for abstinence and naltrexone for cravings.14 Studies of combination therapy with acamprosate and naltrexone have produced mixed results. The COMBINE study did not show that combined therapy was more effective than either agent alone.23 Another study showed that relapse rates were lower with combined therapy compared with placebo or acamprosate alone, but not compared with naltrexone alone.24 It is unclear if and when combination therapy should be used, although it may be reasonable to consider it if monotherapy fails. Opioid antagonists may also be helpful when used as needed during high-risk situations, such as social events or weekends.25
Naltrexone is well tolerated and is not habit-forming. Because it is metabolized by the liver, hepatotoxicity is possible, although uncommon. Patients with AUD may have liver dysfunction; therefore, caution is warranted. Naltrexone can precipitate severe opioid withdrawal in patients who are opioid-dependent, so these agents should not be used together, and opioids should not be used for at least seven days before starting naltrexone.8 Pain management is challenging for patients taking naltrexone; these patients should carry a medical alert card.
OFF-LABEL MEDICATIONS
Anticonvulsants. There are several anticonvulsants that may help patients with AUD decrease alcohol consumption, but data are limited. A Cochrane review of 25 trials including 2,641 patients showed that those taking an anticonvulsant (i.e., topiramate, gabapentin [Neurontin], valproate, levetiracetam [Keppra], oxcarbazepine [Trileptal], zonisamide [Zonegran], carbamazepine [Tegretol], pregabalin [Lyrica], or tiagabine [Gabitril]) consumed 1.5 fewer drinks per day than those taking placebo. There was no difference in abstinence rates compared with naltrexone, but anticonvulsants were associated with fewer heavy drinking days and a longer time to relapse; many of the studies were of low quality.26
Topiramate appears to decrease alcohol consumption. The AHRQ review concluded that there is moderate evidence that topiramate decreases number of drinking days, heavy drinking days, and drinks per day based on two randomized trials.12,27,28 An open-label study compared topiramate plus psychotherapy with psychotherapy alone in hospitalized patients after alcohol withdrawal treatment. The topiramate group had lower rates of depression and anxiety and a lower relapse rate after four months.29 However, a randomized trial of 106 patients did not show a difference in alcohol consumption between topiramate therapy and placebo.30 Another randomized trial found that topiramate increased abstinence rates in patients with a specific genetic polymorphism.31 Such targeted medication use for specific populations warrants further study.
Three randomized trials suggest a possible benefit from gabapentin. A study of 150 patients found higher abstinence rates in those taking gabapentin compared with placebo (NNT = 8), as well as lower rates of heavy drinking, improved mood, fewer cravings, and improved sleep.32 A study of 60 males with an average alcohol consumption of 17 drinks per day in the previous 90 days who underwent alcohol withdrawal treatment and were treated with gabapentin or placebo found that those in the gabapentin group had fewer heavy drinking days and drank less during the 30-day trial.33 A small study of 21 patients had similar results and also found that gabapentin was more effective at improving sleep over the first six weeks of therapy. Dosages of gabapentin used in the study varied from 300 mg twice per day to 1,800 mg at bedtime.34 Longer studies are needed to evaluate gabapentin for AUD.
Pregabalin is classified as a controlled substance, and there are limited data regarding its use in AUD. A randomized trial comparing pregabalin and naltrexone in 71 patients found no difference in drinking outcomes or cravings, but the pregabalin group had less anxiety, hostility, and psychotic symptomatology.35
Antidepressants. Antidepressants are not effective in decreasing alcohol use in persons without coexisting mental health disorders.36 Antidepressants can be helpful in some instances, however, because patients with AUD often have coexisting mental health disorders. A trial randomized 170 patients with alcohol dependence and depression to 14 weeks of cognitive behavior therapy plus sertraline (Zoloft; 200 mg per day), naltrexone (100 mg per day), both medications, or double placebo. Those taking a combination of sertraline and naltrexone had higher abstinence rates and a longer delay before relapse to heavy drinking compared with those taking placebo or either agent alone. Neither agent alone was superior to placebo.37 A study of patients with AUD and major depression found that 20 to 40 mg per day of fluoxetine (Prozac) reduced drinking, drinking days, and heavy drinking days over 12 weeks.38 There is inconclusive evidence regarding the effectiveness of treating AUD with the atypical antipsychotics olanzapine (Zyprexa) and quetiapine (Seroquel).
Ondansetron. Ondansetron (Zofran) may decrease alcohol consumption in patients with AUD. In three studies, ondansetron (4 mcg per kg twice per day) combined with cognitive behavior therapy decreased alcohol consumption and cravings and increased abstinence in young adults with early AUD.39–41 In another trial, a higher dosage of ondansetron (16 mcg per kg twice per day) combined with cognitive behavior therapy decreased depression, anxiety, and hostility.42 This effect may be due to the serotonin-3 antagonist properties of ondansetron. In another randomized trial, men taking ondansetron (8 mg twice per day) had fewer heavy drinking days compared with those taking placebo, although they did not have increased abstinence rates.43 The combination of ondansetron (4 mcg per kg twice per day) and naltrexone (25 mg twice per day) may be effective in treating early AUD.43 The dosages commonly studied (4 to 16 mcg per kg twice per day) are much lower than the current available formulations of 4-mg and 8-mg tablets.
Other. There is inconclusive evidence to support baclofen (Lioresal) and various supplements for AUD. Gamma hydroxybutyrate is used in some countries to treat AUD; however, because of its central nervous system effects and its potential use as a date rape drug, it is not recommended.44
Data Sources: A PubMed search was conducted in Clinical Queries using the terms alcoholic intoxication, alcoholism, alcohol-related diseases, alcohol use disorder, treatment, and medications. The search included randomized controlled trials, meta-analyses, systematic reviews, clinical trials, and reviews. Also searched were Essential Evidence Plus, the Cochrane database, the Agency for Healthcare Research and Quality evidence reports, the National Guideline Clearinghouse database, and the U.S. Preventive Services Task Force. Search dates: September 9, 2014; January 11, 2015; February 21, 2015; February 28, 2015; August 13, 2015; and January 16, 2016.
note: This review updates a previous article on this topic by Williams.45
