
Am Fam Physician. 2016;93(8):659-667
A more recent article on Cancer Screening in Older Adults is available.
Related letter: Physicians Making Eye Contact with Patients Is Important
Patient information: See related handout on cancer screening, written by the authors of this article.
Author disclosure: No relevant financial affiliations.
Although cancer is the second leading cause of death among persons 65 years and older, there is a paucity of clinical trial data about the effectiveness and harms of cancer screening in this population. Given the heterogeneous nature of the older population, cancer screening in these patients should not be based on age alone. Studies suggest that a life expectancy of at least 10 years is necessary to derive a survival benefit from screening for breast and colorectal cancers; therefore, screening for these cancers is not recommended in those with a life expectancy of less than 10 years. Prostate cancer screening, if performed at all, should not be performed after 69 years of age. Cervical cancer screening may be stopped after 65 years of age if the patient has an adequate history of negative screening results. An individualized approach to cancer screening decisions involves estimating life expectancy, determining the potential benefits and harms of screenings, and weighing those benefits and harms in relation to the patient's values and preferences.
Cancer is the second leading cause of death among those 65 years and older, and the incidence of cancer increases with age.1 Because of a lack of clinical trials that include older patients, there is a paucity of data about the effectiveness and harms of cancer screening in this population. Recommendations for cancer screening in older adults vary, particularly regarding when to stop screening. Such guidelines are based on evidence derived at population levels; are based on younger patients; and generally do not address individual variations in life expectancy, comorbid conditions, functional status, or personal preference.
Basing guidelines for cancer screening in older adults on age alone is problematic given the heterogeneous nature of this population. As Americans live longer, there is a need for more evidence-based guidance. This article reviews current guidelines and data, and offers suggestions on how physicians can incorporate these guidelines into their daily practice.
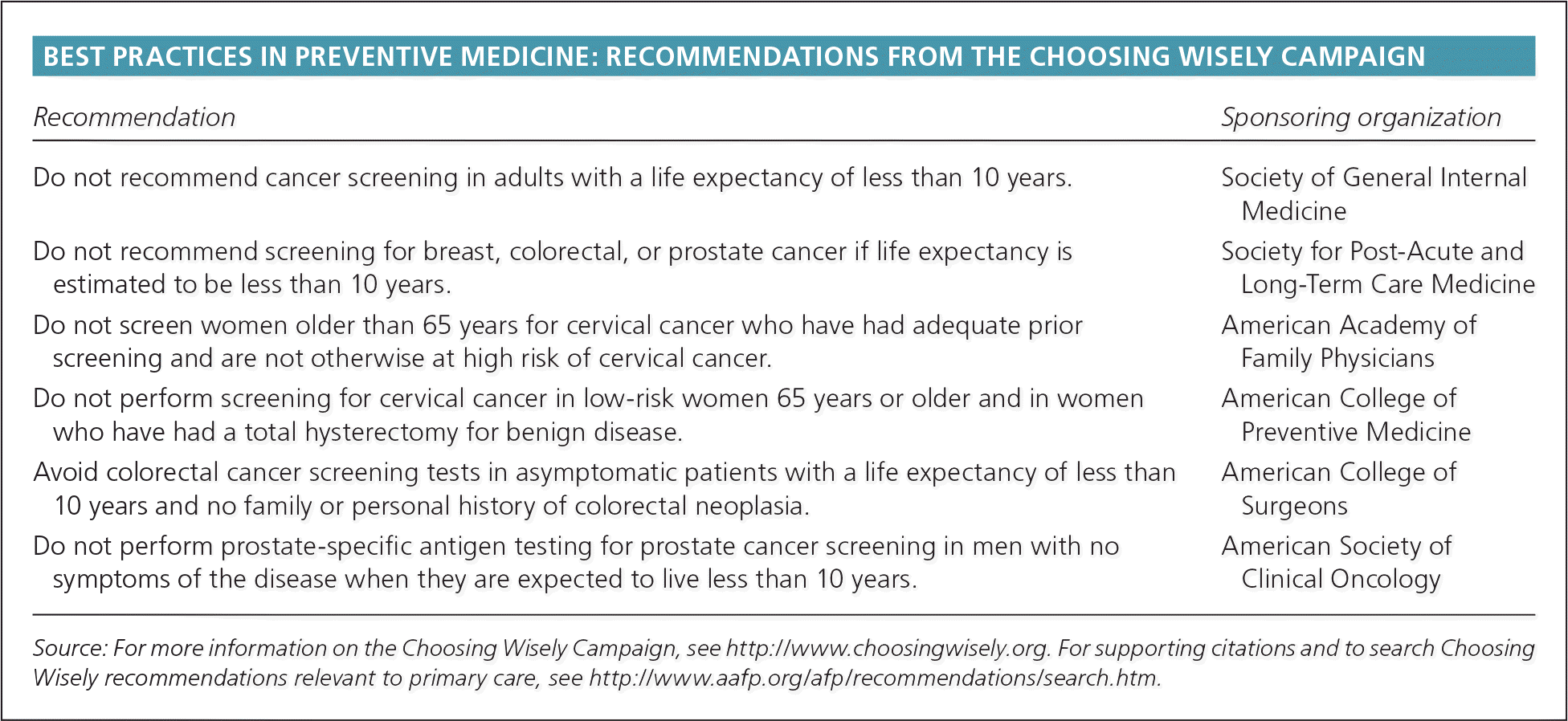
| Recommendation | Sponsoring organization |
|---|---|
| Do not recommend cancer screening in adults with a life expectancy of less than 10 years. | Society of General Internal Medicine |
| Do not recommend screening for breast, colorectal, or prostate cancer if life expectancy is estimated to be less than 10 years. | Society for Post-Acute and Long-Term Care Medicine |
| Do not screen women older than 65 years for cervical cancer who have had adequate prior screening and are not otherwise at high risk of cervical cancer. | American Academy of Family Physicians |
| Do not perform screening for cervical cancer in low-risk women 65 years or older and in women who have had a total hysterectomy for benign disease. | American College of Preventive Medicine |
| Avoid colorectal cancer screening tests in asymptomatic patients with a life expectancy of less than 10 years and no family or personal history of colorectal neoplasia. | American College of Surgeons |
| Do not perform prostate-specific antigen testing for prostate cancer screening in men with no symptoms of the disease when they are expected to live less than 10 years. | American Society of Clinical Oncology |
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Breast cancer screening should not be performed in those with a life expectancy of less than 10 years. | B | 12, 16 |
| Prostate cancer screening, if performed at all, should stop at 69 years of age. | B | 17, 20, 48–50 |
| Cervical cancer screening may be stopped after 65 years of age in patients who have had adequate prior negative screening results. | A | 21–23, 53–56 |
| Colorectal cancer screening should not be performed in patients who are older than 85 years or have a life expectancy of less than 10 years. | B | 8, 16, 61–64 |
| Routine colorectal cancer screening is not recommended in patients 76 to 85 years of age; screening in this age group should be performed on an individual basis after consideration of the patient's overall health and screening history. | B | 61–64 |
| Lung cancer screening with low-dose computed tomography is recommended for patients 55 to 80 years of age who have a 30 pack-year smoking history and currently smoke or have quit within the past 15 years. | B | 29, 30, 67–69 |
A Framework for Decision Making
The paradox of screening in the United States is that healthy older patients are often underscreened, whereas those in poor health are too often overscreened.2–5 For example, 18% of women with advanced dementia had a screening mammogram in 2002, despite a median survival of 3.3 years.5 A framework for guiding an individualized approach to cancer screening involves estimating life expectancy, determining the potential benefits and harms of screenings, and weighing those benefits and harms in relation to the patient's values and preferences.6 Estimating life expectancy is particularly important because there is lag time between cancer screening and its benefits, whereas the harms are often immediate and more prevalent in older adults.7 Studies suggest that a life expectancy of at least 10 years is necessary to derive a survival benefit from screening for breast or colorectal cancers.8
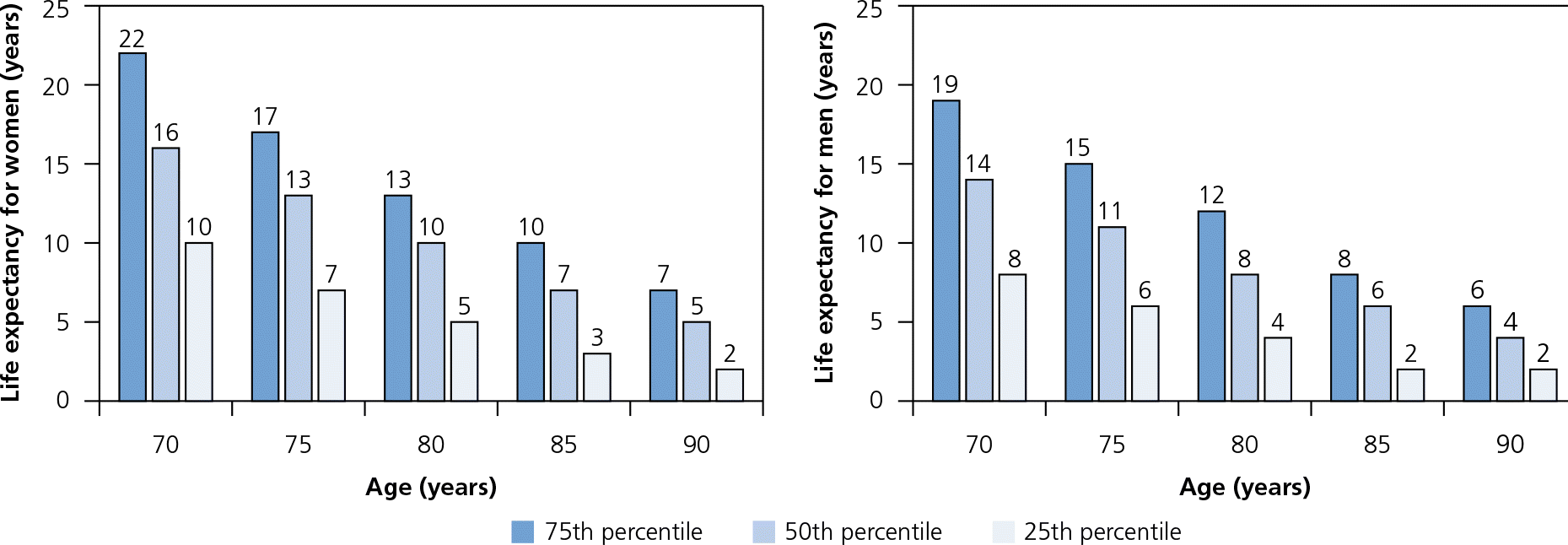
Table 1 lists the average life expectancy for different ages in the United States.9 In 2011, the average life expectancy was 6.5 years for 85-year-olds.9 However, there is tremendous variation in life expectancy within age groups (Figure 1).10 The healthiest quartile of 85-year-olds will live about 10 years, whereas the sickest quartile will live less than three years.6 Strong predictors of life expectancy other than age include the number and severity of comorbid conditions, and the presence of functional impairments.11 Prognostic tools (Table 2) can guide clinical judgment in estimating life expectancy.11
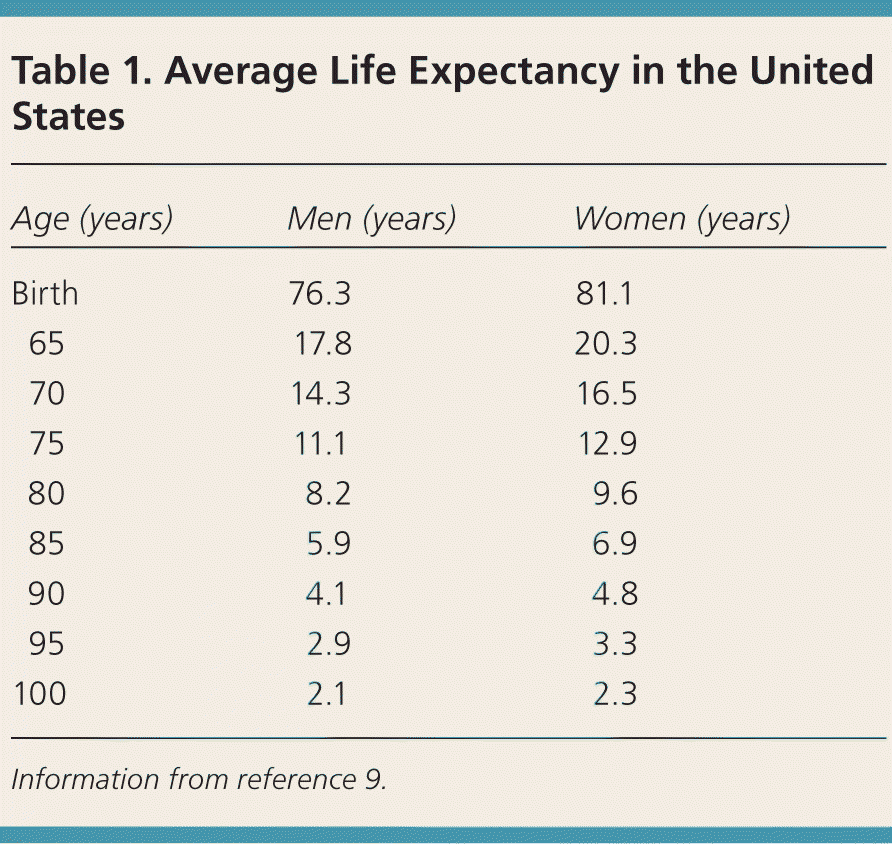
| Age (years) | Men (years) | Women (years) |
|---|---|---|
| Birth | 76.3 | 81.1 |
| 65 | 17.8 | 20.3 |
| 70 | 14.3 | 16.5 |
| 75 | 11.1 | 12.9 |
| 80 | 8.2 | 9.6 |
| 85 | 5.9 | 6.9 |
| 90 | 4.1 | 4.8 |
| 95 | 2.9 | 3.3 |
| 100 | 2.1 | 2.3 |

Screening Guidelines
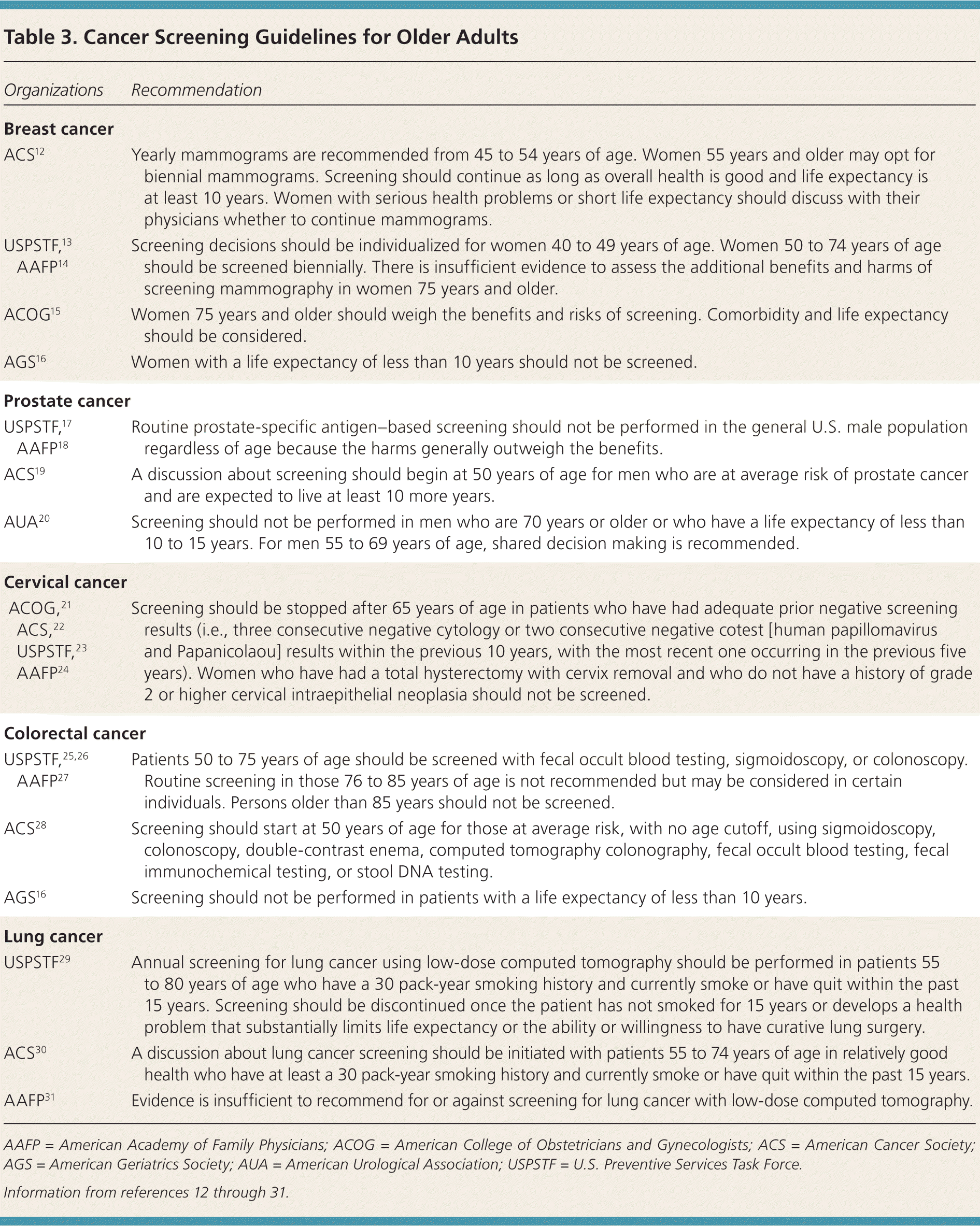
| Organizations | Recommendation |
|---|---|
| Breast cancer | |
| ACS12 | Yearly mammograms are recommended from 45 to 54 years of age. Women 55 years and older may opt for biennial mammograms. Screening should continue as long as overall health is good and life expectancy is at least 10 years. Women with serious health problems or short life expectancy should discuss with their physicians whether to continue mammograms. |
| USPSTF,13 AAFP14 | Screening decisions should be individualized for women 40 to 49 years of age. Women 50 to 74 years of age should be screened biennially. There is insufficient evidence to assess the additional benefits and harms of screening mammography in women 75 years and older. |
| ACOG15 | Women 75 years and older should weigh the benefits and risks of screening. Comorbidity and life expectancy should be considered. |
| AGS16 | Women with a life expectancy of less than 10 years should not be screened. |
| Prostate cancer | |
| USPSTF,17 AAFP18 | Routine prostate-specific antigen–based screening should not be performed in the general U.S. male population regardless of age because the harms generally outweigh the benefits. |
| ACS19 | A discussion about screening should begin at 50 years of age for men who are at average risk of prostate cancer and are expected to live at least 10 more years. |
| AUA20 | Screening should not be performed in men who are 70 years or older or who have a life expectancy of less than 10 to 15 years. For men 55 to 69 years of age, shared decision making is recommended. |
| Cervical cancer | |
| ACOG,21 ACS,22 USPSTF,23 AAFP24 | Screening should be stopped after 65 years of age in patients who have had adequate prior negative screening results (i.e., three consecutive negative cytology or two consecutive negative cotest [human papillomavirus and Papanicolaou] results within the previous 10 years, with the most recent one occurring in the previous five years). Women who have had a total hysterectomy with cervix removal and who do not have a history of grade 2 or higher cervical intraepithelial neoplasia should not be screened. |
| Colorectal cancer | |
| USPSTF,25,26 AAFP27 | Patients 50 to 75 years of age should be screened with fecal occult blood testing, sigmoidoscopy, or colonoscopy. Routine screening in those 76 to 85 years of age is not recommended but may be considered in certain individuals. Persons older than 85 years should not be screened. |
| ACS28 | Screening should start at 50 years of age for those at average risk, with no age cutoff, using sigmoidoscopy, colonoscopy, double-contrast enema, computed tomography colonography, fecal occult blood testing, fecal immunochemical testing, or stool DNA testing. |
| AGS16 | Screening should not be performed in patients with a life expectancy of less than 10 years. |
| Lung cancer | |
| USPSTF29 | Annual screening for lung cancer using low-dose computed tomography should be performed in patients 55 to 80 years of age who have a 30 pack-year smoking history and currently smoke or have quit within the past 15 years. Screening should be discontinued once the patient has not smoked for 15 years or develops a health problem that substantially limits life expectancy or the ability or willingness to have curative lung surgery. |
| ACS30 | A discussion about lung cancer screening should be initiated with patients 55 to 74 years of age in relatively good health who have at least a 30 pack-year smoking history and currently smoke or have quit within the past 15 years. |
| AAFP31 | Evidence is insufficient to recommend for or against screening for lung cancer with low-dose computed tomography. |

| Overdiagnosis: When screening results in the detection (and often treatment) of asymptomatic disease that would not have become clinically important during a patient's lifetime and would not have impacted morbidity or mortality. |
| Lead-time bias: When screening results in the earlier diagnosis of disease, leading to an apparent increase in survival time when measured from the time of diagnosis (“five-year survival”), even though overall mortality and the overall length of a person's life may be the same in screened and unscreened groups. |
| Length-time bias: Screening tests are more likely to detect indolent, slow-growing tumors than aggressive, rapidly growing tumors, leading to an apparent improvement in survival. |
| Healthy participant bias: Those who choose to be screened may be in better health and have more favorable health habits in general, causing improvements in survival and other health outcomes that are unrelated to screening. |
BREAST CANCER
Mammography is the only screening test shown to reduce breast cancer deaths in randomized controlled trials (RCTs).33 Even though advancing age is the greatest risk factor for breast cancer,13 RCTs evaluating mammography have not included women older than 74 years.13,33,34 As a result, the U.S. Preventive Services Task Force (USPSTF) states there is insufficient evidence to recommend for or against screening women 75 years and older.35 In contrast, the American Cancer Society (ACS) recommends continuing screening for as long as the patient is in good health and has a life expectancy of at least 10 years.12 The American Geriatrics Society also does not recommend screening for patients with a life expectancy of less than 10 years.16
In the only RCT of mammography that includes women 70 to 74 years of age, a subgroup analysis of this age group did not demonstrate a significant reduction in breast cancer mortality (relative risk = 1.12; 95% confidence interval, 0.73 to 1.72).36
Observational, retrospective cohort studies have shown that regular screening mammography in women 75 years and older is associated with detection of earlier-stage disease and lower breast cancer mortality.37–39 However, these findings may be affected by lead-time, length-time, and selection bias.37–39 In addition, a mortality benefit was not demonstrated in older women with severe or multiple comorbidities.38
In the absence of clinical trial data, statistical modeling studies have assessed the impact of screening mammography for women 70 to 84 years of age. Studies suggest a mortality benefit from mammography screening beyond 69 years of age, with one to four fewer breast cancer deaths per 1,000 women.40–42 However, the rate of false positives and overdiagnosis increased with age, particularly in the oldest age groups.42
Mortality benefit must be weighed against the potential harms of screening. Harms can include pain, anxiety, and complications from subsequent testing and treatment. In patients 66 to 74 years of age, false-positive rates from mammography approach 50% for those screened annually and 30% for those screened biennially over 10 years.43 Although the sensitivity and specificity of mammography increase with age,42 overdiagnosis also increases because of reduced life expectancy and an increased proportion of slower-growing cancers.44 In other words, women with breast cancer diagnosed at an older age are more likely to die of something else, compared with younger women. In addition, treatment of breast cancer in advanced age is associated with greater morbidity, including an increased risk of postoperative complications and toxicity from chemotherapy.45,46 The psychological impact of a false-positive result can be substantial for some women. Although few studies include women 75 years and older, one meta-analysis showed that psychological distress can persist for three years following a false-positive mammogram result.47
PROSTATE CANCER
Most prostate cancers have a good prognosis even without treatment, although some cancers are aggressive. Prostate cancer increases with age, and 75% of diagnoses are in those 65 years and older.48
Screening recommendations are largely based on two large RCTs conducted in the United States and Europe that evaluated the effect of prostate-specific antigen (PSA) testing on mortality. The U.S. study, including men 55 to 74 years of age, showed no reduction in prostate cancer mortality after 13 years.49 The European trial, including men 50 to 74 years of age, showed a small reduction in prostate cancer mortality after 11 years (number needed to screen = 1,055) in men 55 to 69 years of age, but no mortality benefit in those 70 years and older.50 Based on these trials, and considering the known harms of overtreatment for prostate cancer, the USPSTF recommends against PSA-based prostate cancer screening in average-risk men regardless of age.17 The American Urological Association recommends a discussion of the harms and benefits of PSA screening in men 55 to 69 years of age, and they do not recommend screening in men who are 70 years and older or who have a life expectancy of less than 10 to 15 years.20 Additionally, the ACS recommends that men expected to live at least 10 years receive information about the uncertainties, risks, and potential benefits of screening starting at 50 years of age.19
Overdiagnosis from PSA screening increases with age. A modeling study of men 55 to 69 years of age found that 43% of prostate cancers detected with screening were overdiagnosed. When screening was extended to 74 years of age, 48% of cancers detected were overdiagnosed.51 Many men with elevated PSA levels undergo prostate biopsy, and at least one-third of these men will have pain, fever, bleeding, infection, urinary incontinence, or erectile dysfunction; about 1% require hospitalization.52
CERVICAL CANCER
Data from the National Cancer Institute show that 20% of new cases of cervical cancer and 34% of deaths from cervical cancer between 2008 and 2012 occurred in women 65 years and older.53 Most new cases in older women (72%) occur in those who have never been adequately screened.54 Coordinated guidelines for cervical cancer screening were issued in 2012 by the American College of Obstetricians and Gynecologists,21 ACS,22 and USPSTF.23 All advise stopping cervical cancer screening after 65 years of age in patients who have had adequate prior negative screening results (i.e., three consecutive negative cytology results or two consecutive negative cotest [human papillomavirus and Papanicolaou (Pap)] results within the previous 10 years, with the most recent one occurring in the previous five years). Additionally, screening should not be performed in women who have had a total hysterectomy with cervix removal and do not have a history of grade 2 or higher cervical intraepithelial neoplasia.21–23
Data show that more than one-half of all new cases of cervical cancer occur in women who have never or rarely been screened.54 The percentage of abnormal cytology results decreases with age and with subsequent screenings. In the Centers for Disease Control and Prevention's National Breast and Cervical Cancer Early Detection Program, the detection rate for grade 3 or higher cervical intraepithelial neoplasia was two per 1,000 cytology tests in women 65 years and older, compared with 14.6 per 1,000 tests in those 18 to 29 years of age.55 Modeling studies indicate no substantial benefit to screening beyond 65 years of age, but there is increased risk of potential harms, including false-positive results, and unnecessary colposcopies and cervical biopsies.56
Furthermore, the risk of cytologic abnormalities after hysterectomy is low. Dysplasia was rarely identified after hysterectomy (0.19 per 1,000 women screened) in a cross-sectional study of more than 5,000 cytology tests among women older than 50 years.57 In another study of nearly 10,000 Pap tests in a similar population, the rate was 0.42 high-grade lesion per 1,000 screens.58
Underscreening of eligible women for cervical cancer is common. A survey of about 70 million U.S. women 21 to 65 years of age revealed that an estimated 8.2 million (11.4%) had not been screened for cervical cancer in the previous five years, with higher percentages of non-screening among women 60 to 65 years of age (12.6%).59
Overscreening is also common. The National Health Interview Survey, which included 27,404 women 65 years or older, showed that 56% of participants were screened for cervical cancer.60 Stratified by life expectancy, 31% of those with a life expectancy of less than 10 years received a Pap test. Among women who had a hysterectomy for benign reasons, 34% to 56% received a Pap test.
COLORECTAL CANCER
In its 2008 guideline and in a new draft recommendation statement, the USPSTF recommends screening for colorectal cancer using fecal occult blood testing, sigmoidoscopy, or colonoscopy beginning at 50 years of age and continuing until 75 years of age.25,26 Screening those older than 85 years is not recommended. Routine screening in those 76 to 85 years of age is also not recommended because of a small net benefit in this population; however, there may be considerations to support colorectal screening in an individual patient in this age group. For example, screening may be more likely to benefit those who have never been screened previously or those in the highest quartile of life expectancy.61,62
Rather than basing guidelines on age alone, the American Geriatrics Society recommends against colorectal cancer screening in patients with a life expectancy less than 10 years because the immediate risk of harms outweighs the minimal likelihood of benefit.16 The ACS recommends colorectal cancer screening starting at 50 years of age for those at average risk, without an explicit age cutoff.28
Meta-analysis data demonstrate a lag time of 10 years before patients receive any benefit from colorectal cancer screening. At least 1,000 persons would need to undergo fecal occult blood testing at least twice to prevent one death from colorectal cancer in 10.3 years.8 It also takes 9.4 years to prevent one colorectal cancer–related death among every 1,000 persons who are screened using flexible sigmoidoscopy.63
The benefit-to-risk ratio worsens with advancing age and increased comorbidity. In a meta-analysis, the pooled incidence of adverse events (i.e., perforation, bleeding, or cardiopulmonary complications) was 26 per 1,000 colonoscopies in patients 65 years and older, and it increased to 35 per 1,000 in those 80 years and older.64
Overscreening for colon cancer is common. Of the 27,404 participants in the National Health Interview Survey, 31% of those 85 years and older and 46% of those 75 to 84 years of age were screened. Stratified by life expectancy, 41% of those with a life expectancy of less than 10 years received a screening colonoscopy.60 In another study, 46% of Medicare beneficiaries 75 to 79 years of age and 33% of those 80 years or older with a previous normal result on screening colonoscopy received another colonoscopy within seven years, most with no clear indication. In this study, older patients with three or more medical conditions were actually more likely to undergo early repeated screening colonoscopy.65
Conversely, 23% of all persons older than 75 years in the United States have never been screened for colorectal cancer. A recent modeling study found that colorectal cancer screening for older adults remains cost-effective and may be indicated beyond 75 years of age, but only in those who have not had previous screenings.66
LUNG CANCER
The USPSTF recommends annual lung cancer screening using low-dose computed tomography in adults 55 to 80 years of age who have at least a 30 pack-year smoking history and currently smoke or have quit within the past 15 years. Screening should be discontinued once the patient has not smoked for 15 years or develops a health problem that substantially limits life expectancy or the ability or willingness to have curative lung surgery.29 The ACS recommends initiating a discussion about screening in those 55 to 74 years of age who are in relatively good health.30 The USPSTF and ACS guidelines are primarily based on the results from the National Lung Screening Trial (NLST).67
The NLST is a large, good-quality trial conducted in the United States that compared three annual screenings with either low-dose computed tomography or single-view posteroanterior chest radiography in current or former smokers between 55 and 74 years of age. The computed tomography group had a significant reduction in lung cancer and all-cause mortality (20% and 7% reduction, respectively) compared with the radiography group. The number needed to screen over 6.5 years was 320 to prevent one lung cancer death and 210 to prevent one death from any cause.68 Of note, more than 95% of positive screening results were false positives. Additionally, patients enrolled in the NLST were relatively healthy, and less than 10% were older than 70 years. The NLST excluded persons who were unlikely to complete curative lung cancer surgery or who had medical conditions with a substantial risk of death during the eight-year trial, which may limit its applicability to many older adults.
To inform its recommendations, the USPSTF initiated a modeling study to further assess the long-term benefits and harms of lung cancer screening and to evaluate different screening intervals and eligibility criteria. The modeling scenarios suggested that extending the screening eligibility to 80 years of age has a mortality benefit while maintaining a modest level of harm related to false positives, overdiagnosis, and radiation-related lung cancer deaths.69 Screening beyond 80 years of age was not recommended because of concerns about increased operative mortality, comorbidity, and reduced eligibility for surgery with curative intent. The modeling scenarios assumed that screened older adults were healthy and without comorbidities influencing life expectancy.
Ultimately, the applicability of lung cancer screening guidelines is uncertain with advancing age and increased comorbidity. The decision to screen for lung cancer in older adults should be individualized and take into account life expectancy, potential benefits and harms, and the patient's values and preferences. A recent analysis concluded that the greatest benefit of screening is in smokers, with relatively little, if any, benefit in former smokers.70
Implications for Practice
Although cancer screening decisions for older adults should be individualized, there is little information on how clinicians should approach such complex, shared decisions with patients. Many physicians recommend screening to older patients with advanced illness who would not benefit,71 and feel uncomfortable and unprepared to talk about stopping screening.72,73 Additionally, many older patients perceive cancer screening as a routine part of their health and may feel taken aback by the prospect of not being screened.74,75
Studies show that most older adults with serious illness want to know their prognosis so that they can make medical decisions76 and want to discuss the possibility of stopping cancer screening with their physicians.75 Many family caregivers of patients with dementia are open to discussions about screening cessation that focus on quality of life, burdens, and benefits.77 Given the uncertainty of the relative benefits and harms of cancer screening in older adults, the patient's preferences should be a key consideration. Decision aids (Table 2) can improve patient involvement, knowledge, and realistic perceptions of outcomes.78
Experts recommend that physicians introduce the idea of stopping cancer screening in advance to set up the expectation that a time will come when the burdens of screening will outweigh the potential benefits.79 Discussions should be revisited periodically, in the context of the patient's health status, to help him or her weigh the benefits and harms of screening, and to clarify patient preferences. It is important to convey that a decision to stop cancer screening does not translate into decreased health care. Rather, discussions can focus on health promotion strategies that are most likely to benefit patients in the more immediate future, such as exercise and immunizations.
Data Sources: A PubMed search was completed in Clinical Queries using the key terms cancer, screening, older adults, and elderly. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. We also performed a literature search using the following sources: Cochrane Database of Systematic Reviews, USPSTF, National Guideline Clearinghouse, Agency for Healthcare Research and Quality Evidence Reports, Essential Evidence Plus, and UpToDate. Search dates: January 1, 2015, to March 2, 2016.
note: This review updates a previous article on this topic by Albert, et al.80
