
A more recent article on managing menopausal symptoms is available.
Am Fam Physician. 2016;94(11):884-889
Patient information: See related handout on treating menopausal symptoms, written by the authors of this article.
Author disclosure: No relevant financial affiliations.
The results of large clinical trials have led physicians and patients to question the safety of hormone therapy for menopause. In the past, physicians prescribed hormone therapy to improve overall health and prevent cardiac disease, as well as for symptoms of menopause. Combined estrogen/progestogen therapy, but not estrogen alone, increases the risk of breast cancer when used for more than three to five years. Therefore, in women with a uterus, it is recommended that physicians prescribe combination therapy only to treat menopausal symptoms such as vasomotor symptoms (hot flashes) and vaginal atrophy, using the smallest effective dosage for the shortest possible duration. Although estrogen is the most effective treatment for hot flashes, nonhormonal alternatives such as low-dose paroxetine, venlafaxine, and gabapentin are effective alternatives. Women with a uterus who are using estrogen should also take a progestogen to reduce the risk of endometrial cancer. Women who cannot tolerate adverse effects of progestogens may benefit from a combined formulation of estrogen and the selective estrogen receptor modulator bazedoxifene. There is no high-quality, consistent evidence that yoga, paced respiration, acupuncture, exercise, stress reduction, relaxation therapy, and alternative therapies such as black cohosh, botanical products, omega-3 fatty acid supplements, and dietary Chinese herbs benefit patients more than placebo. One systematic review suggests modest improvement in hot flashes and vaginal dryness with soy products, and small studies suggest that clinical hypnosis significantly reduces hot flashes. Patients with genitourinary syndrome of menopause may benefit from vaginal estrogen, nonhormonal vaginal moisturizers, or ospemifene (the only nonhormonal treatment approved by the U.S. Food and Drug Administration for dyspareunia due to menopausal atrophy). The decision to use hormone therapy depends on clinical presentation, a thorough evaluation of the risks and benefits, and an informed discussion with the patient.
Menopause is the physiologic transition when the ovaries stop releasing eggs, ovarian function decreases, and menstrual periods stop. Although some women go through the menopausal transition without symptoms, many women have hot flashes or genital tract symptoms, such as vulvar or vaginal dryness, painful intercourse, and urinary problems. When counseling patients who are going through menopause, clinicians should understand the benefits and risks of hormone therapy, nonhormonal prescription medications, and alternative treatments, and be familiar with the various delivery methods.
WHAT IS NEW ON THIS TOPIC: TREATMENTS FOR MENOPAUSAL SYMPTOMS
After a median of 13 years of follow-up, women taking combined estrogen/progestogen therapy in the Women's Health Initiative trial had a significantly increased risk of breast cancer and venous thromboembolism, and a reduction in hip fractures.
In 2014, a consensus conference endorsed new terminology: the term genitourinary syndrome of menopause replaces the terms vulvovaginal atrophy and atrophic vaginitis. This is partly because older terminology does not encompass the extent of genital tract symptoms that many women experience.
There is no evidence that using low-dose vaginal estrogen increases the risk of breast cancer recurrence.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Combined estrogen/progestogen therapy, but not estrogen alone, increases the risk of breast cancer after three to five years of use. | B | 3 |
| Systemic estrogen, alone or in combination with a progestogen, is the most effective therapy for menopausal hot flashes, and is approved by the U.S. Food and Drug Administration for this indication. | A | 9 |
| Because of the potential risks with long-term use of hormone therapy, clinicians should prescribe the lowest effective dosage for the shortest duration necessary to improve symptoms. | C | 8, 12 |
| There is no high-quality, consistent evidence that black cohosh, botanical products, omega-3 fatty acid supplements, or lifestyle modification alleviates hot flashes. | B | 19–21 |
| The decision to continue combined hormone therapy for more than three to five years should be made after reviewing the risks, benefits, and symptoms with the patient. | C | 12 |
| Effective nonhormonal therapies for genitourinary syndrome of menopause include vaginal moisturizers and oral ospemifene (Osphena). | B | 31, 32 |
Risks and Benefits of Hormone Therapy
In 2002, the first Women's Health Initiative (WHI) clinical trial was published, causing patients and clinicians to question the safety of menopausal hormone therapy.1 Before this study, many patients took hormones to improve overall health, prevent cardiac disease, and treat menopausal symptoms. The large study of approximately 16,000 women compared a combined oral regimen consisting of conjugated equine estrogen and medroxyprogesterone with placebo, and found that the combined regimen increased the risk of coronary artery disease, breast cancer, stroke, and venous thromboembolism (VTE). The study also found a decreased risk of colorectal cancer, hip fractures, and total fractures with combined hormone therapy.1 Critics of the study believe it is not appropriate to generalize results to all women going through menopause, partly because the average age of participants was 63 years.
Other studies have helped refine information about hormone therapy. The 2004 publication of a conjugated estrogen–only arm of the WHI trial in women without a uterus showed that those taking estrogen alone had no significant change in risk of coronary heart disease or breast cancer and, similar to the trial of combined estrogen and progestogen, an increase in strokes and VTE.1,2 A 2013 poststoppage review of findings from both WHI trials after a median follow-up of 13 years revealed that women taking combined hormone therapy had a significantly increased risk of breast cancer and VTE, and a reduction in hip fractures. In contrast, women taking estrogen alone had a significantly reduced risk of breast cancer.3
The “timing hypothesis” suggests that starting hormone therapy early in menopause (compared with starting it 10 years or more after the onset of menopause) may be cardioprotective because of estrogen's apparent ability to slow the progression of atherosclerosis in younger women.3,4 Although the evidence suggests that beginning hormone therapy near the start of menopause decreases the risk of cardiac disease, further study is needed. Current guidelines recommend against using hormone therapy to prevent or treat cardiac disease.5 Further, the American Academy of Family Physicians recommends against using hormone therapy for the prevention of chronic conditions.6
Counseling patients about the options for treating the vasomotor symptoms of menopause is challenging. The North American Menopause Society provides a free mobile application called MenoPro to help clinicians assess the benefit-risk profile of hormone therapy when counseling patients. MenoPro is available at http://www.menopause.org/for-women/-i-menopro-i-mobile-app.
Vasomotor Symptoms
Vasomotor symptoms (hot flashes) are the most common indication for hormone therapy. During menopause, approximately 70% of women report hot flashes, which are more common in women with a higher body mass index, with lower income and education, who smoke cigarettes, or who are black.7 Treatment options include hormone therapy, nonhormonal prescription medications, and clinical hypnosis.
Estrogen is the most effective therapy for hot flashes and is approved by the U.S. Food and Drug Administration (FDA) for this indication.8,9 Although some women may prefer lifestyle modification, there is no evidence that lowering the ambient temperature; using fans; exercising; or avoiding triggers, such as alcohol and spicy foods, improves hot flashes.10 Because all systemic estrogen formulations are effective, patient preference should guide the choice of therapy, unless there are contraindications such as undiagnosed vaginal bleeding or a history of breast cancer, VTE, or severe liver disease. Table 1 summarizes the estrogen formulations available for the treatment of hot flashes.
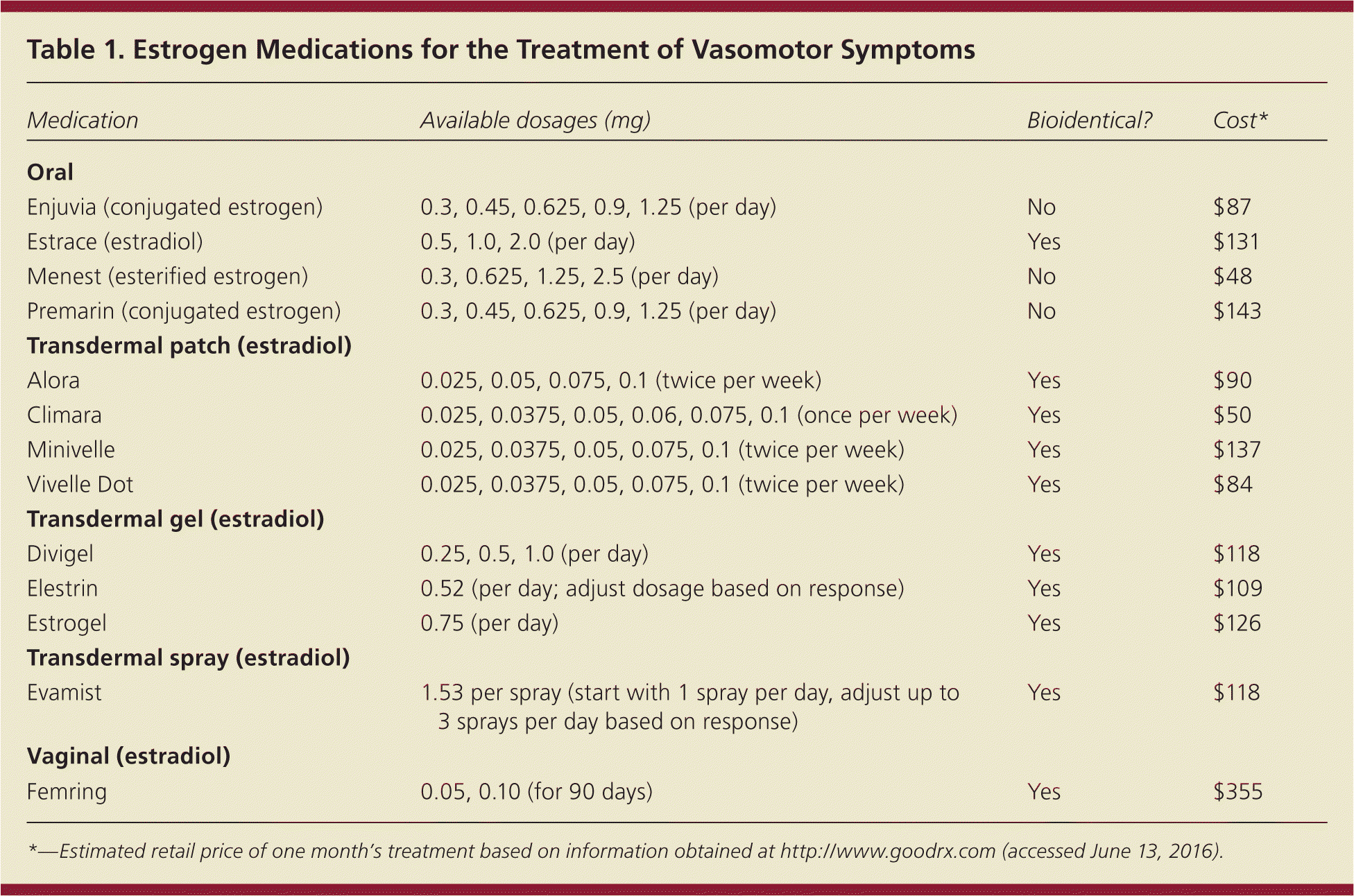
| Medication | Available dosages (mg) | Bioidentical? | Cost* |
|---|---|---|---|
| Oral | |||
| Enjuvia (conjugated estrogen) | 0.3, 0.45, 0.625, 0.9, 1.25 (per day) | No | $87 |
| Estrace (estradiol) | 0.5, 1.0, 2.0 (per day) | Yes | $131 |
| Menest (esterified estrogen) | 0.3, 0.625, 1.25, 2.5 (per day) | No | $48 |
| Premarin (conjugated estrogen) | 0.3, 0.45, 0.625, 0.9, 1.25 (per day) | No | $143 |
| Transdermal patch (estradiol) | |||
| Alora | 0.025, 0.05, 0.075, 0.1 (twice per week) | Yes | $90 |
| Climara | 0.025, 0.0375, 0.05, 0.06, 0.075, 0.1 (once per week) | Yes | $50 |
| Minivelle | 0.025, 0.0375, 0.05, 0.075, 0.1 (twice per week) | Yes | $137 |
| Vivelle Dot | 0.025, 0.0375, 0.05, 0.075, 0.1 (twice per week) | Yes | $84 |
| Transdermal gel (estradiol) | |||
| Divigel | 0.25, 0.5, 1.0 (per day) | Yes | $118 |
| Elestrin | 0.52 (per day; adjust dosage based on response) | Yes | $109 |
| Estrogel | 0.75 (per day) | Yes | $126 |
| Transdermal spray (estradiol) | |||
| Evamist | 1.53 per spray (start with 1 spray per day, adjust up to 3 sprays per day based on response) | Yes | $118 |
| Vaginal (estradiol) | |||
| Femring | 0.05, 0.10 (for 90 days) | Yes | $355 |
Although there are no randomized trials, observational studies have shown that transdermal estrogen, which avoids the first-pass liver effect, may have a lower risk of VTE compared with oral estrogen.11 All estrogen medications should be given at the lowest effective dosage for the shortest duration necessary to improve symptoms.8,12 If necessary, the dosage may be increased after evaluating for effectiveness during the first eight weeks of therapy, with reassessment annually or as needed.
Because all formulations of unopposed estrogen increase the risk of endometrial hyperplasia and cancer in women with a uterus, these patients should use estrogen plus progestogen for endometrial protection; a continuous regimen appears to be more effective than sequential therapy at reducing the risk of endometrial neoplasia.13 Patients can take an estrogen/progestogen combination product (Table 2) or an estrogen product used in conjunction with a separate progestogen.
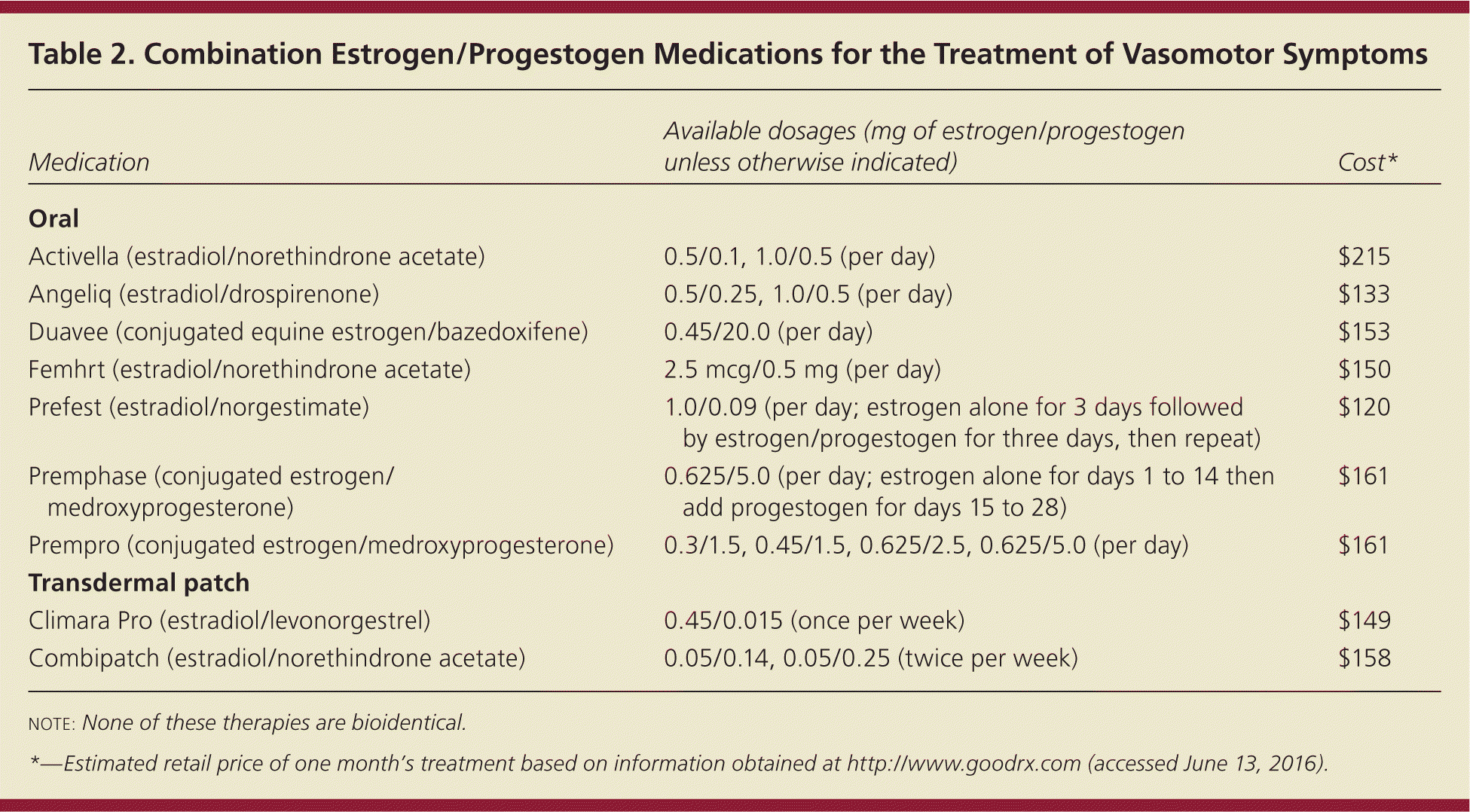
| Medication | Available dosages (mg of estrogen/progestogen unless otherwise indicated) | Cost* |
|---|---|---|
| Oral | ||
| Activella (estradiol/norethindrone acetate) | 0.5/0.1, 1.0/0.5 (per day) | $215 |
| Angeliq (estradiol/drospirenone) | 0.5/0.25, 1.0/0.5 (per day) | $133 |
| Duavee (conjugated equine estrogen/bazedoxifene) | 0.45/20.0 (per day) | $153 |
| Femhrt (estradiol/norethindrone acetate) | 2.5 mcg/0.5 mg (per day) | $150 |
| Prefest (estradiol/norgestimate) | 1.0/0.09 (per day; estrogen alone for 3 days followed by estrogen/progestogen for three days, then repeat) | $120 |
| Premphase (conjugated estrogen/medroxyprogesterone) | 0.625/5.0 (per day; estrogen alone for days 1 to 14 then add progestogen for days 15 to 28) | $161 |
| Prempro (conjugated estrogen/medroxyprogesterone) | 0.3/1.5, 0.45/1.5, 0.625/2.5, 0.625/5.0 (per day) | $161 |
| Transdermal patch | ||
| Climara Pro (estradiol/levonorgestrel) | 0.45/0.015 (once per week) | $149 |
| Combipatch (estradiol/norethindrone acetate) | 0.05/0.14, 0.05/0.25 (twice per week) | $158 |
Progestogens can cause fatigue, dysphoria, and fluid retention.14 Patients with a uterus who cannot tolerate these adverse effects may benefit from Duavee, a combination of 0.45-mg conjugated equine estrogen and the selective estrogen receptor modulator bazedoxifene, which is approved by the FDA for treating hot flashes and preventing osteoporosis. Bazedoxifene does not stimulate the endometrium.15,16 Like all products containing estrogen, Duavee is contraindicated in women who have a history of breast cancer or VTE. The levonorgestrel-releasing intrauterine system (Mirena) is an off-label option for providing local progestogen to the endometrium for patients who cannot tolerate systemic therapy.17
Despite the effectiveness of estrogen, some patients may prefer nonhormonal medications or may have contraindications to using estrogen. Several antidepressants reduce hot flashes8,18 (Table 3). Low-dose paroxetine (Brisdelle) is the only nonhormonal medication approved by the FDA to treat hot flashes, although other dosages can be used. Patients taking tamoxifen should not use paroxetine because of inhibition of the hepatic enzyme cytochrome P450 2D6, which decreases the effectiveness of tamoxifen.18 Desvenlafaxine (Khedezla) and venlafaxine do not inhibit CYP2D6 and are appropriate alternatives. Starting at a low dose and titrating as necessary should be considered for antidepressants other than low-dose paroxetine. Other nonhormonal options include gabapentin (Neurontin) and pregabalin (Lyrica). These medications can cause dizziness and other adverse effects, and should be titrated from a lower dosage.18 Although clonidine is somewhat more effective than placebo, adverse events, such as hypotension, dizziness, and rebound hypertension, limit its usefulness for menopausal symptoms.10 Stellate ganglion block of the C6-T2 anterior cervical spine has shown promise in uncontrolled studies and one small randomized trial, but larger studies are needed.10,18
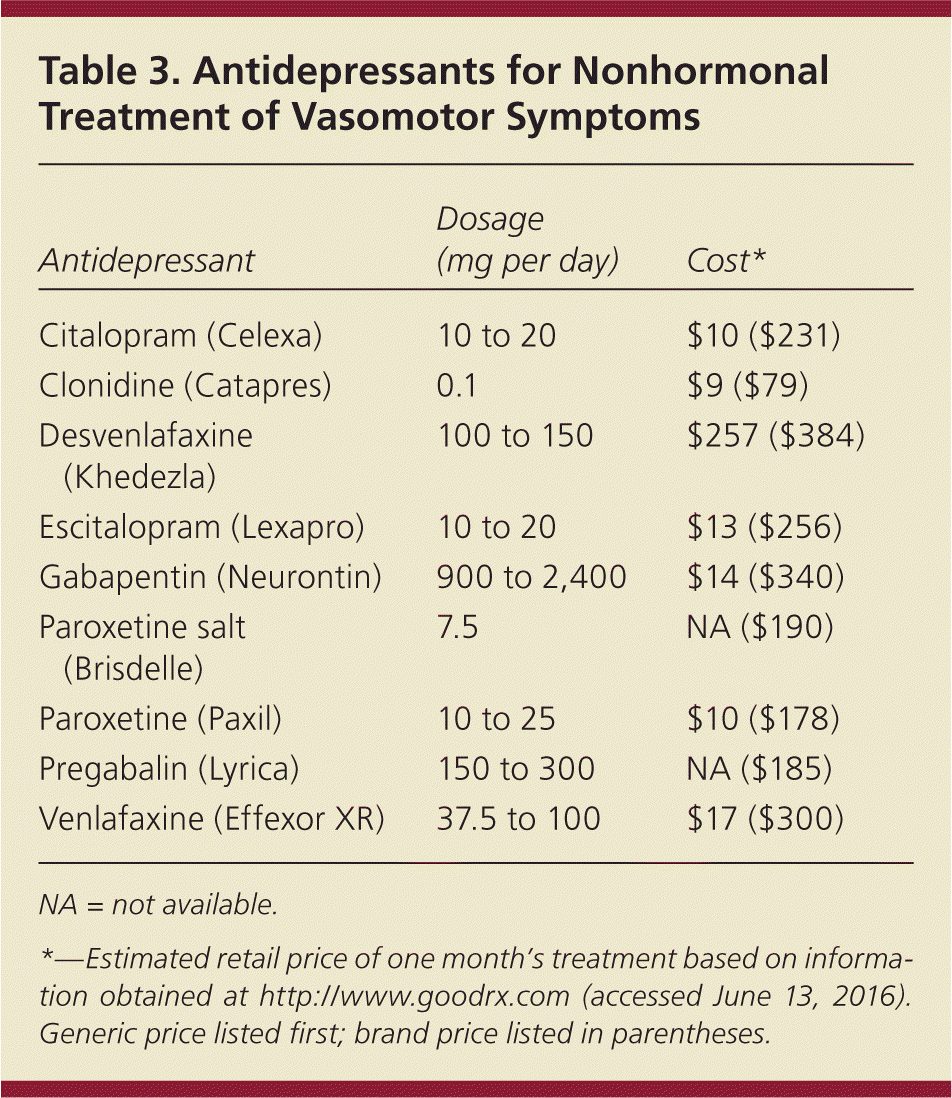
| Antidepressant | Dosage (mg per day) | Cost* |
|---|---|---|
| Citalopram (Celexa) | 10 to 20 | $10 ($231) |
| Clonidine (Catapres) | 0.1 | $9 ($79) |
| Desvenlafaxine (Khedezla) | 100 to 150 | $257 ($384) |
| Escitalopram (Lexapro) | 10 to 20 | $13 ($256) |
| Gabapentin (Neurontin) | 900 to 2,400 | $14 ($340) |
| Paroxetine salt (Brisdelle) | 7.5 | NA ($190) |
| Paroxetine (Paxil) | 10 to 25 | $10 ($178) |
| Pregabalin (Lyrica) | 150 to 300 | NA ($185) |
| Venlafaxine (Effexor XR) | 37.5 to 100 | $17 ($300) |
Some patients may want to use alternative therapies or compounded formulations. Clinicians should counsel patients that there is no high-quality, consistent evidence that black cohosh, many botanical products, and omega-3 fatty acid supplements are effective for treating hot flashes.19–21 One recent systematic review found a modest benefit for soy-based foods and supplements in reducing hot flashes and vaginal dryness compared with placebo. Red clover and some non-Chinese herbal medicines also variably improved symptoms.22 Overall, the low quality and heterogeneity of the studies included in this review do not allow definitive conclusions about the benefits of plant-based complementary therapies. Yoga, paced respiration, acupuncture, exercise, stress reduction, and relaxation therapy also have not been proven to alleviate hot flashes.23 However, clinical hypnosis (five 45-minute sessions weekly) has been shown to reduce hot flashes by 74%, compared with a 17% reduction in patients who received only education and encouragement.24
Celebrity endorsements and online marketing have given increased attention to compounded bioidentical hormone formulations. Bioidentical hormones, such as estrone, 17-beta estradiol, and estriol, are identical to human hormones. Data are limited about the safety and effectiveness of compounded bioidentical formulations. There is concern that these unregulated formulations may have similar adverse effects as estrogen medications, such as endometrial hyperplasia. If a patient prefers a bioidentical hormone, FDA-approved medications containing the bioidentical hormone estradiol can be considered; for women with a uterus, micronized progestogen (100 mg or 200 mg per day) should be added.
Deciding When to Discontinue Hormone Therapy
Because of the potential increased risk of breast cancer in women taking combined estrogen/progestogen therapy, some clinicians advise that patients discontinue therapy after three to five years. An analysis of women in the WHI combined hormone trial who had hot flashes when starting therapy showed that after discontinuation, 56% had moderate to severe hot flashes compared with 21% of women who took placebo.25
Clinicians should inform patients that some women will have a difficult time stopping hormone therapy.25 There are limited data to inform patients and clinicians about the best way to discontinue hormone therapy. Some patients will be successful with abrupt discontinuation, whereas others may benefit from weaning. The American College of Obstetricians and Gynecologists recommends against routine discontinuation based on age or treatment duration, and advises that the decision to continue or stop hormone therapy should be individualized based on the patient's symptoms and medical history.12
Transitioning from Contraception to Hormone Therapy
Transitioning from contraception to hormone therapy may be challenging because oral contraceptives have higher dosages than typical hormone therapy regimens. Also, measuring follicle-stimulating hormone levels after stopping oral contraceptives can be inaccurate during perimenopause.26 One small study found that a rise in follicle-stimulating hormone level without a change in estradiol levels two weeks after stopping oral contraceptives is evidence that it is safe to transition to hormone therapy.26 Others suggest discontinuation of contraception when women are in their mid-50s because spontaneous conception is rare at this age.27
Genitourinary Syndrome of Menopause
Genitourinary syndrome of menopause refers to bothersome genital symptoms from changes in the vulva, vagina, and lower genital tract that are caused by diminished estrogen. This condition affects up to one-half of women during menopause.28 In 2014, a consensus conference endorsed the new term genitourinary syndrome of menopause to replace the terms vulvovaginal atrophy and atrophic vaginitis, partly because the older terminology does not encompass the extent of genital tract symptoms many women experience.29 Decreased estrogen can cause several changes to genital anatomy that lead to patient discomfort. Thinning of the vulvar mucosa may cause vulvar burning, irritation, or constriction of the introitus, resulting in entry dyspareunia. Narrowing of the vagina and decreased lubrication can cause painful intercourse or coital bleeding.30 Diminished estrogen may also lead to recurrent urinary tract infections and urinary urgency.29 Genitourinary syndrome of menopause is often progressive without treatment.28
Treatment options (Table 4) include vaginal estrogen, nonhormonal vaginal moisturizers, and the newer oral systemic estrogen agonist-antagonist ospemifene (Osphena). It is reasonable to try nonhormonal therapy as a first-line option to alleviate vulvovaginal symptoms caused by genitourinary syndrome of menopause. Although not as effective as estrogen, vaginal moisturizers, such as Replens, are an effective treatment for mild vaginal dryness and related dyspareunia.31 Ospemifene is the only FDA-approved nonhormonal therapy for dyspareunia caused by menopausal atrophy. Short-term studies of ospemifene showed improvement in vaginal dryness.32 This medication is taken with food once per day, and should not be used by women with a history of breast cancer or thromboembolic disease. Hot flashes are the most bothersome adverse effect.
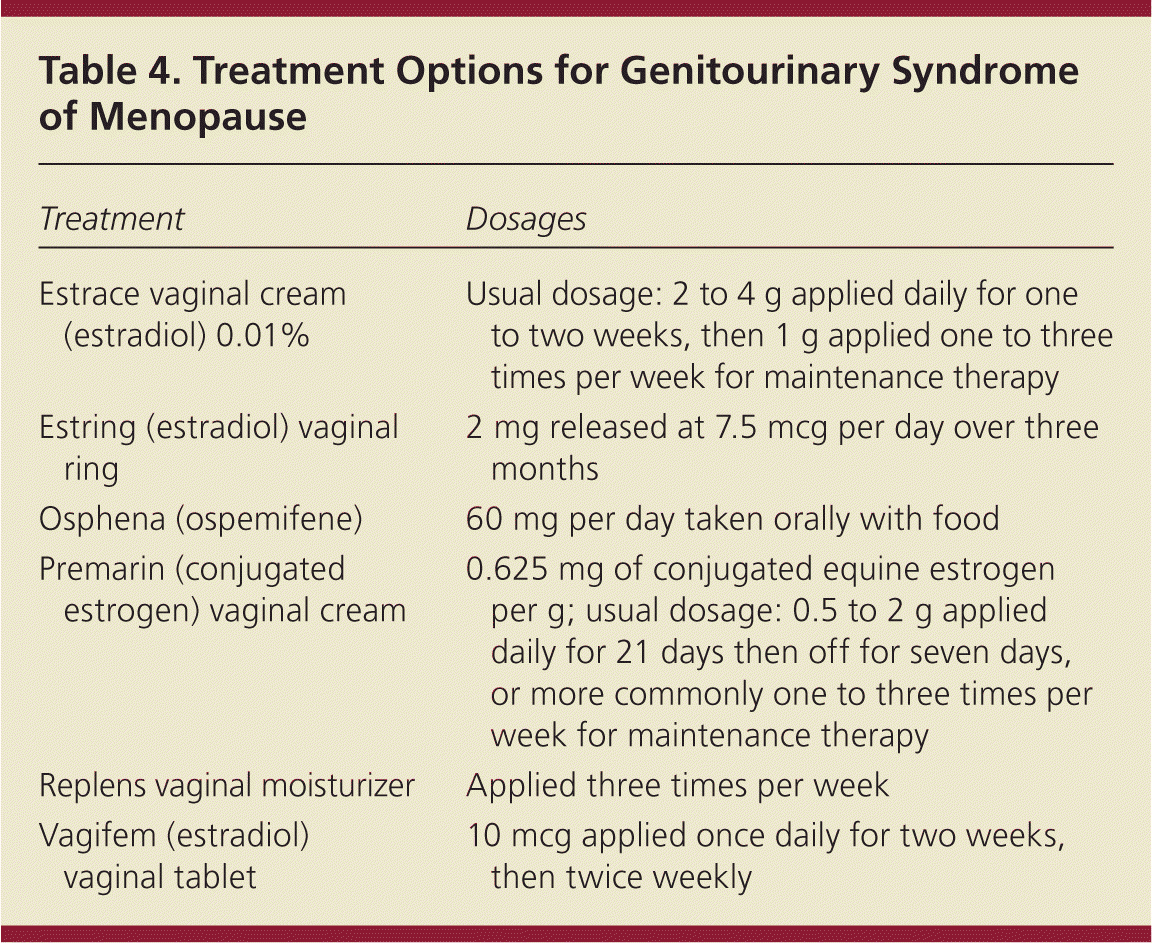
| Treatment | Dosages |
|---|---|
| Estrace vaginal cream (estradiol) 0.01% | Usual dosage: 2 to 4 g applied daily for one to two weeks, then 1 g applied one to three times per week for maintenance therapy |
| Estring (estradiol) vaginal ring | 2 mg released at 7.5 mcg per day over three months |
| Osphena (ospemifene) | 60 mg per day taken orally with food |
| Premarin (conjugated estrogen) vaginal cream | 0.625 mg of conjugated equine estrogen per g; usual dosage: 0.5 to 2 g applied daily for 21 days then off for seven days, or more commonly one to three times per week for maintenance therapy |
| Replens vaginal moisturizer | Applied three times per week |
| Vagifem (estradiol) vaginal tablet | 10 mcg applied once daily for two weeks, then twice weekly |
Patients who have genitourinary syndrome of menopause that does not respond to nonhormonal medications or who have a narrow introitus or vagina may benefit from low-dose vaginal estrogen. All estrogen formulations for genital atrophy appear to have similar effectiveness regardless of administration method.33 Patient preference should guide the choice of formulation. Creams have the advantage of both intravaginal and vulvar application, but they can be messy to apply. Some patients may prefer the convenience of vaginal estradiol tablets (Vagifem) or the vaginal low-dose estradiol ring (Estring), which provides three months of continuous therapy. Femring, another vaginal ring, provides a systemic dosage of estrogen for treating hot flashes.
Women using low-dose vaginal estrogen for less than one year should not require a progestogen to decrease the risk of endometrial cancer, but there are no long-term data.28 Clinicians should consider an endometrial biopsy and/or transvaginal ultrasonography if spotting or bleeding occurs while using low-dose vaginal estrogen.28 The decision to use vaginal estrogen formulations for women with a history of hormone-dependent cancers should be based on the woman's preference after consultation with a clinician experienced with hormone therapy. Clinicians can inform patients that there is no evidence that using low-dose local estrogen increases the risk of breast cancer recurrence.34
This review updates previous articles on this topic by Hill and Hill,35 by Carroll,36 by Morelli and Naquin,37 and by Cutson and Meuleman.38
Data Sources: An evidence-based search included PubMed, Essential Evidence Plus, Cochrane Database of Systematic Reviews, U.S. Food and Drug Administration, UpToDate, and a professional search by our institutional library team. Search dates: January and May 2016.
