
This is an updated version of the article that appeared in print.
Note: In May 2023, while this article was in production, the U.S. Food and Drug Administration approved fezolinetant (Veozah), a neurokinin 3 receptor antagonist, for treatment of moderate to severe vasomotor symptoms due to menopause. This article has been revised to incorporate information about this nonhormonal medication.
Am Fam Physician. 2023;108(1):28-39
Related Letter to the Editor: Clarification: Continuous vs Sequential Administration of Hormone Therapy for Menopausal Symptoms
Author disclosure: No relevant financial relationships.
Menopausal symptoms are widespread and significantly impact quality of life. Common symptoms of menopause are vasomotor (i.e., hot flashes and night sweats) and genitourinary (e.g., vulvovaginal irritation and dryness, dyspareunia, urinary problems), although women may also experience changes in sexual function, mood, and sleep. Estrogen-containing hormone therapy is effective treatment for vasomotor symptoms. Nonhormonal medications for vasomotor symptoms include selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, gabapentin, and fezolinetant, which is a neurokinin 3 receptor antagonist [updated]. Selective serotonin reuptake inhibitors should not be administered to women taking tamoxifen. Cognitive behavior therapy and clinical hypnosis are effective for short-term reduction of vasomotor symptoms and associated sleep disturbances, but data are lacking to support the effectiveness of other nonpharmacologic treatments such as herbal or botanical supplements, exercise, and acupuncture. Hormone-free vaginal moisturizers are noninferior to estrogen-based therapies for treating genitourinary syndrome of menopause. Other treatment options for vaginal dryness and dyspareunia associated with menopause include ospemifene and intravaginal dehydroepiandrosterone. Management of menopausal symptoms should involve shared decision-making that is informed by the best available evidence and individual risks and preferences.
The menopausal transition (perimenopause) is characterized by a persistent decline in ovarian function and hormonal fluctuations that may cause bothersome vasomotor (i.e., hot flashes and night sweats) and genitourinary symptoms (e.g., vulvovaginal irritation and dryness, dyspareunia, urinary problems), and affect mood, sleep, sexual function, bone health, and overall quality of life.1 Perimenopause usually begins during the fifth decade of life and lasts several years before completion of natural menopause, a clinical diagnosis made only after cessation of menses for 12 consecutive months. Vasomotor symptoms may affect as many as 80% of women worldwide and last, on average, a total of seven to eight years, including four to five years after the final menstrual period.2–4 Vasomotor symptoms account for 1.5 million excess outpatient visits per year and an additional $330 million in annual U.S. health care costs when left untreated.5 Genitourinary syndrome of menopause affects up to 50% of women worldwide; unlike vasomotor symptoms, it is progressive without treatment.6 Figure 1 suggests an approach for applying the best available evidence and an individualized risk-benefit assessment to guide shared decision-making about natural menopause symptoms.7–11,54 [updated] This article does not discuss surgical menopause or premature menopause (also known as primary ovarian insufficiency). The use of the term women is intended to include cisgender women and other people with ovaries.
| Long-term (18 years) follow-up of the Women's Health Initiative concluded that there was no significant increase in the risk of death from all causes or cardiovascular causes among women receiving hormone therapy with conjugated estrogen alone or conjugated estrogen and medroxyprogesterone during five to seven years of treatment. |
| Duavee, the combination drug containing conjugated estrogen plus bazedoxifene, a selective estrogen receptor modulator, is effective for vasomotor symptoms and osteoporosis prevention, precludes the need for endometrial protection with progesterone, and is well tolerated. |
| Although bioidentical synthetic estradiol is effective compared with placebo, there is no evidence to suggest greater effectiveness over other standard estrogens. |
| A 2018 meta-analysis of women in natural or treatment-induced menopause found that behavioral interventions significantly decreased short-term (i.e., less than 20 weeks) and medium-term (i.e., 20 weeks or longer) perceived severity, but not the frequency, of vasomotor symptoms. |
| Fezolinetant (Veozah), a neurokinin 3 receptor antagonist, received U.S. Food and Drug Adminstration approval in May 2023 for treatment of moderate to severe vasomotor symptoms due to menopause. Fezolinetant significantly reduces hot flashes when compared with placebo and may be considered in women who have contraindications to or prefer nonhormonal medications for vasomotor symptoms. [updated] |

What Are the Known Benefits and Harms of Hormone Therapy in the Treatment of Menopausal Vasomotor Symptoms?
Menopausal hormone therapy containing estrogen is effective for treating vasomotor symptoms of menopause.7,8,12 Unopposed estrogen is known to increase the risk of endometrial cancer and should not be administered without progesterone in women with a uterus. Estrogen-only hormone therapy may reduce breast cancer risk in those with a hysterectomy, whereas estrogen-progesterone hormone therapy may increase the risk in those with a uterus.13 Consensus guidelines support offering hormone therapy to women who are younger than 60 years and within 10 years of symptom onset, do not have contraindications to hormone therapy, and desire it for the treatment of moderate to severe vasomotor symptoms.7,8 Hormone therapy may be initiated cautiously in patients older than 60 years with risk-benefit analysis.7,10
EVIDENCE SUMMARY
The decision to initiate hormone therapy remains challenging. Several large systematic reviews, which included data from the Women's Health Initiative (WHI) and other randomized controlled trials (RCTs), have shown potential benefits and harms, although most participants were older than 60 years and postmenopausal.8,13–15 Table 1 presents cumulative data on the potential benefits and harms of hormone therapy for the prevention of chronic conditions; it is based on a large systematic review from 2022 that includes 20 RCTs and three large cohort studies.13
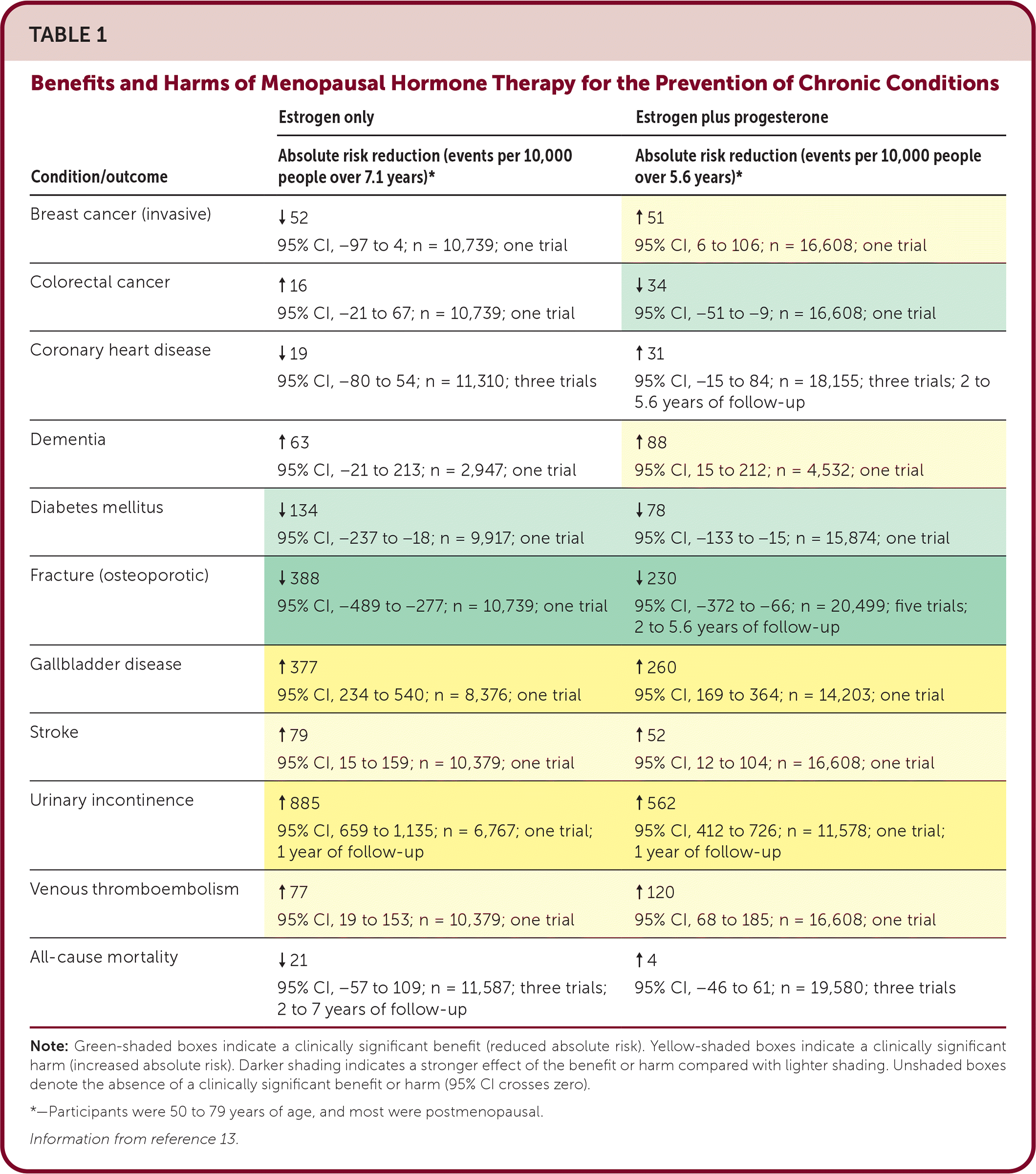
| Condition/outcome | Estrogen only | Estrogen plus progesterone |
|---|---|---|
| Absolute risk reduction (events per 10,000 people over 7.1 years)* | Absolute risk reduction (events per 10,000 people over 5.6 years)* | |
| Breast cancer (invasive) | ↓ 52 95% CI, −97 to 4; n = 10,739; one trial | ↑ 51 95% CI, 6 to 106; n = 16,608; one trial |
| Colorectal cancer | ↑ 16 95% CI, −21 to 67; n = 10,739; one trial | ↓ 34 95% CI, −51 to −9; n = 16,608; one trial |
| Coronary heart disease | ↓ 19 95% CI, −80 to 54; n = 11,310; three trials | ↑ 31 95% CI, −15 to 84; n = 18,155; three trials; 2 to 5.6 years of follow-up |
| Dementia | ↑ 63 95% CI, −21 to 213; n = 2,947; one trial | ↑ 88 95% CI, 15 to 212; n = 4,532; one trial |
| Diabetes mellitus | ↓ 134 95% CI, −237 to −18; n = 9,917; one trial | ↓ 78 95% CI, −133 to −15; n = 15,874; one trial |
| Fracture (osteoporotic) | ↓ 388 95% CI, −489 to −277; n = 10,739; one trial | ↓ 230 95% CI, −372 to −66; n = 20,499; five trials; 2 to 5.6 years of follow-up |
| Gallbladder disease | ↑ 377 95% CI, 234 to 540; n = 8,376; one trial | ↑ 260 95% CI, 169 to 364; n = 14,203; one trial |
| Stroke | ↑ 79 95% CI, 15 to 159; n = 10,379; one trial | ↑ 52 95% CI, 12 to 104; n = 16,608; one trial |
| Urinary incontinence | ↑ 885 95% CI, 659 to 1,135; n = 6,767; one trial; 1 year of follow-up | ↑ 562 95% CI, 412 to 726; n = 11,578; one trial; 1 year of follow-up |
| Venous thromboembolism | ↑ 77 95% CI, 19 to 153; n = 10,379; one trial | ↑ 120 95% CI, 68 to 185; n = 16,608; one trial |
| All-cause mortality | ↓ 21 95% CI, −57 to 109; n = 11,587; three trials; 2 to 7 years of follow-up | ↑ 4 95% CI, −46 to 61; n = 19,580; three trials |
In long-term (18 years) follow-up of the WHI, the use of conjugated estrogen alone significantly decreased the overall risk of breast cancer in women with a hysterectomy; however, in women with a uterus, the use of combination conjugated estrogen/medroxyprogesterone was associated with an increased risk of breast cancer but not breast cancer mortality.16 It also concluded that there were no significant increases in the risk of death from all causes or cardiovascular death among women receiving conjugated estrogen alone or conjugated estrogen/medroxyprogesterone during five to seven years of treatment.17
How Does the Difference Between Formulations of Hormone Therapy Affect Shared Decision-Making About Therapy Choice and Duration?
There are limited comparative data to guide decisions on dosing and formulations of hormone therapy that optimize effectiveness and reduce risk.7,8 Choice of regimen can generally be individualized based on patient preference, predominant symptoms (vasomotor vs. genitourinary or other), and known risks. Because of the potential risks with long-term use of hormone therapy, physicians should prescribe the lowest effective dose for the shortest duration necessary to treat symptoms based on risk assessment and shared decision-making.7,8 Women with a uterus who are using systemic estrogen should also receive progesterone.19
EVIDENCE SUMMARY
Estrogen is available in oral, transdermal, and vaginal forms in a variety of dosing regimens (Table 2), including some combined with progesterone (Table 3). Progesterone-based hormone therapy includes oral tablets, transdermal patches (combined with estrogen), levonorgestrel-releasing intrauterine system (used off-label for endometrial protection), and vaginal inserts (dehydroepiandrosterone [DHEA]). Oral and transdermal estrogen formulations designed for systemic delivery are generally preferred and similarly effective for vasomotor symptoms compared with placebo.20 Most vaginal formulations are minimally absorbed, with the exception of the high-dose estradiol vaginal ring (Femring) that is FDA-approved for vasomotor and vaginal symptoms.7,10 Guidelines state that hormone therapy does not have to be discontinued routinely in patients older than 60 years, but comorbidities and risks of long-term therapy should be carefully evaluated on an ongoing basis.7
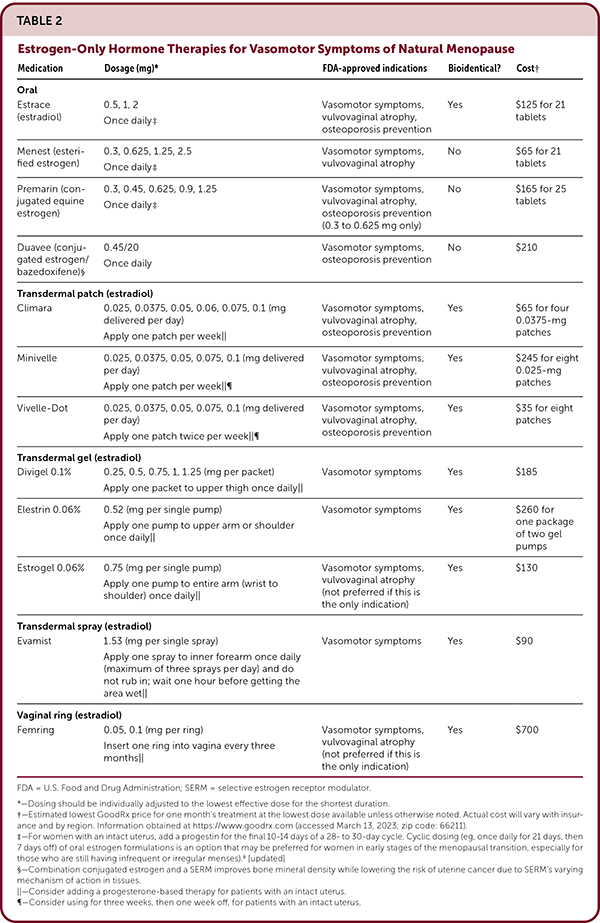
| Medication | Dosage (mg)* | FDA-approved indications | Bioidentical? | Cost† |
|---|---|---|---|---|
| Oral | ||||
| Estrace (estradiol) | 0.5, 1, 2 Once daily‡ |
Vasomotor symptoms, vulvovaginal atrophy, osteoporosis prevention | Yes | $125 for 21 tablets |
| Menest (esterified estrogen) | 0.3, 0.625, 1.25, 2.5 Once daily for 21 days, then seven days off‡ |
Vasomotor symptoms, vulvovaginal atrophy | No | $65 for 21 tablets |
| Premarin (conjugated equine estrogen) | 0.3, 0.45, 0.625, 0.9, 1.25 Once daily for 25 days, then five days off‡ |
Vasomotor symptoms, vulvovaginal atrophy, osteoporosis prevention (0.3 to 0.625 mg only) | No | $165 for 25 tablets |
| Duavee (conjugated estrogen/bazedoxifene)§ | 0.45/20 Once daily |
Vasomotor symptoms, osteoporosis prevention | No | $210 |
| Transdermal patch (estradiol) | ||||
| Climara | 0.025, 0.0375, 0.05, 0.06, 0.075, 0.1 (mg delivered per day) Apply one patch per week|| |
Vasomotor symptoms, vulvovaginal atrophy, osteoporosis prevention | Yes | $65 for four 0.0375-mg patches |
| Minivelle | 0.025, 0.0375, 0.05, 0.075, 0.1 (mg delivered per day) Apply one patch per week||¶ |
Vasomotor symptoms, vulvovaginal atrophy, osteoporosis prevention | Yes | $245 for eight 0.025-mg patches |
| Vivelle-Dot | 0.025, 0.0375, 0.05, 0.075, 0.1 (mg delivered per day) Apply one patch twice per week||¶ |
Vasomotor symptoms, vulvovaginal atrophy, osteoporosis prevention | Yes | $35 for eight patches |
| Transdermal gel (estradiol) | ||||
| Divigel 0.1% | 0.25, 0.5, 0.75, 1, 1.25 (mg per packet) Apply one packet to upper thigh once daily|| |
Vasomotor symptoms | Yes | $185 |
| Elestrin 0.06% | 0.52 (mg per single pump) Apply one pump to upper arm or shoulder once daily|| |
Vasomotor symptoms | Yes | $260 for one package of two gel pumps |
| Estrogel 0.06% | 0.75 (mg per single pump) Apply one pump to entire arm (wrist to shoulder) once daily|| |
Vasomotor symptoms, vulvovaginal atrophy (not preferred if this is the only indication) | Yes | $130 |
| Transdermal spray (estradiol) | ||||
| Evamist | 1.53 (mg per single spray) Apply one spray to inner forearm once daily (maximum of three sprays per day) and do not rub in; wait one hour before getting the area wet|| |
Vasomotor symptoms | Yes | $90 |
| Vaginal ring (estradiol) | ||||
| Femring | 0.05, 0.1 (mg per ring) Insert one ring into vagina every three months|| |
Vasomotor symptoms, vulvovaginal atrophy (not preferred if this is the only indication) | Yes | $700 |
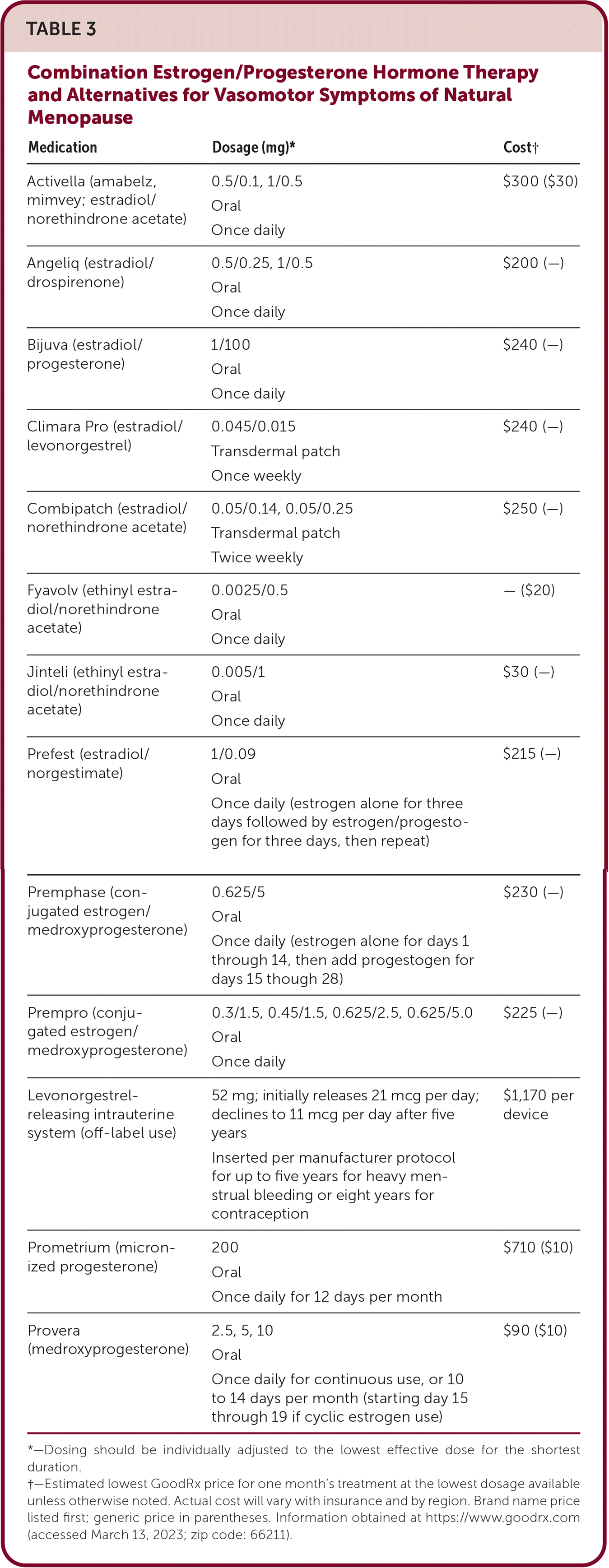
| Medication | Dosage (mg)* | Cost† |
|---|---|---|
| Activella (amabelz, mimvey; estradiol/norethindrone acetate) | 0.5/0.1, 1/0.5 Oral Once daily | $300 ($30) |
| Angeliq (estradiol/drospirenone) | 0.5/0.25, 1/0.5 Oral Once daily | $200 (—) |
| Bijuva (estradiol/progesterone) | 1/100 Oral Once daily | $240 (—) |
| Climara Pro (estradiol/levonorgestrel) | 0.045/0.015 Transdermal patch Once weekly | $240 (—) |
| Combipatch (estradiol/norethindrone acetate) | 0.05/0.14, 0.05/0.25 Transdermal patch Twice weekly | $250 (—) |
| Fyavolv (ethinyl estradiol/norethindrone acetate) | 0.0025/0.5 Oral Once daily | — ($20) |
| Jinteli (ethinyl estradiol/norethindrone acetate) | 0.005/1 Oral Once daily | $30 (—) |
| Prefest (estradiol/norgestimate) | 1/0.09 Oral Once daily (estrogen alone for three days followed by estrogen/progestogen for three days, then repeat) | $215 (—) |
| Premphase (conjugated estrogen/medroxyprogesterone) | 0.625/5 Oral Once daily (estrogen alone for days 1 through 14, then add progestogen for days 15 though 28) | $230 (—) |
| Prempro (conjugated estrogen/medroxyprogesterone) | 0.3/1.5, 0.45/1.5, 0.625/2.5, 0.625/5.0 Oral Once daily | $225 (—) |
| Levonorgestrel-releasing intrauterine system (off-label use) | 52 mg; initially releases 21 mcg per day; declines to 11 mcg per day after five years Inserted per manufacturer protocol for up to five years for heavy menstrual bleeding or eight years for contraception | $1,170 per device |
| Prometrium (micronized progesterone) | 200 Oral Once daily for 12 days per month | $710 ($10) |
| Provera (medroxyprogesterone) | 2.5, 5, 10 Oral Once daily for continuous use, or 10 to 14 days per month (starting day 15 through 19 if cyclic estrogen use) | $90 ($10) |
Observational studies suggest that transdermal estrogen-containing hormone therapy does not increase the risk of venous thromboembolism compared with oral estrogen, but further data are needed to assess the risk of venous thromboembolism posed by various doses and formulations of estrogen and progestin.21 Although studies have shown that adding a progestin to estrogen therapy in those with a uterus is important to reduce endometrial cancer risk, the safest form of progesterone and optimal dosing schedule (e.g., daily vs. cyclic dosing 10 to 14 days per month) is less clear. Importantly, the WHI study included only combination hormone therapy with medroxyprogesterone used daily. Micronized progesterone may be less thrombogenic than other progestins but may be associated with a higher risk of endometrial cancer.7,19
Duavee is a combination drug containing conjugated estrogen and bazedoxifene, a selective estrogen receptor modulator; it is a reasonable alternative to a progesterone-containing regimen in women with a uterus.7 It is effective for vasomotor symptom relief and osteoporosis prevention, precludes the need for endometrial protection with progesterone, and is well tolerated.23
Patients who request bioidentical formulations can be offered FDA-approved formulations of synthetic estradiol (the dominant form of estrogen produced by ovaries during reproductive years) and, if a progestin is needed, micronized progesterone.7 Although effective compared with placebo, there is no evidence to suggest that bioidentical synthetic estradiol has greater effectiveness over other standard estrogens.24 Patients should be discouraged from seeking compounded bioidentical hormones, which are not regulated for safety or effectiveness.7
What Nonhormonal Pharmacologic Treatments Are Effective for Vasomotor Symptoms?
For patients with contraindications to hormone therapy and those who desire alternatives for vasomotor symptoms, selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) are shown to be effective for managing hot flashes, but there are limited data from head-to-head comparison trials for drugs in both classes.25,26 Fezolinetant (Veozah), a neurokinin 3 receptor antagonist, significantly reduces vasomotor symptoms when compared with placebo and received U.S. Food and Drug Administration approval for this indication in May 2023.52–54 [updated] Other nonhormonal medication options include gabapentin, clonidine, and oxybutynin, but data supporting effectiveness are limited.27–30 Table 4 lists nonhormonal treatments for vasomotor symptoms. [updated]
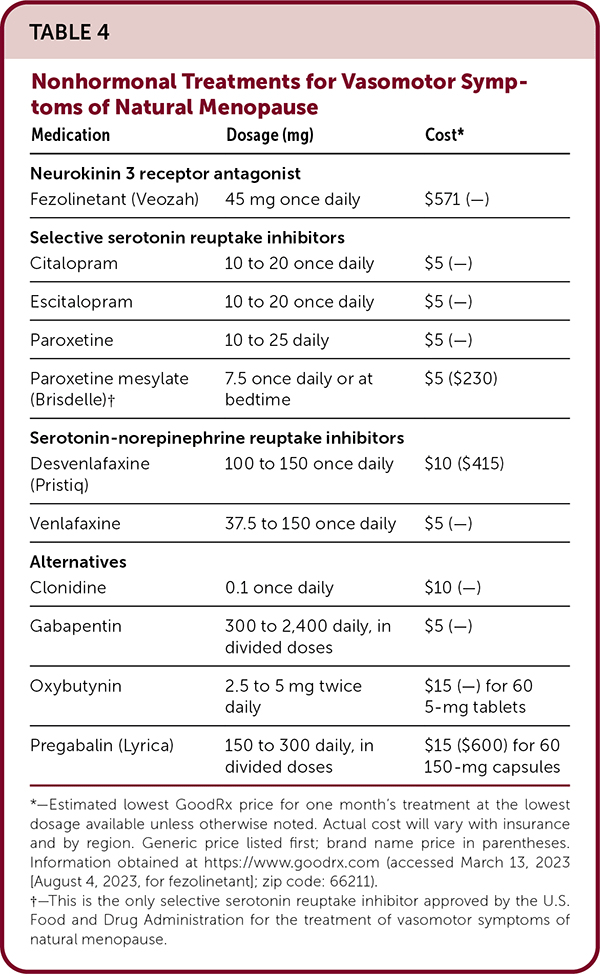
| Medication | Dosage (mg) | Cost* |
|---|---|---|
| Neurokinin 3 receptor antagonist | ||
| Fezolinetant (Veozah) | 45 mg once daily | $571 (—) |
| Selective serotonin reuptake inhibitors | ||
| Citalopram | 10 to 20 once daily | $5 (—) |
| Escitalopram | 10 to 20 once daily | $5 (—) |
| Paroxetine | 10 to 25 daily | $5 (—) |
| Paroxetine mesylate (Brisdelle)† | 7.5 once daily or at bedtime | $5 ($230) |
| Serotonin-norepinephrine reuptake inhibitors | ||
| Desvenlafaxine (Pristiq) | 100 to 150 once daily | $10 ($415) |
| Venlafaxine | 37.5 to 150 once daily | $5 (—) |
| Alternatives | ||
| Clonidine | 0.1 once daily | $10 (—) |
| Gabapentin | 300 to 2,400 daily, in divided doses | $5 (—) |
| Oxybutynin | 2.5 to 5 mg twice daily | $15 (—) for 60 5-mg tablets |
| Pregabalin (Lyrica) | 150 to 300 daily, in divided doses | $15 ($600) for 60 150-mg capsules |
EVIDENCE SUMMARY
Low-dose paroxetine mesylate (Brisdelle; 7.5 mg) used once daily or at bedtime is the only medication in the SSRI and SNRI classes that is FDA-approved for the treatment of vasomotor symptoms, although higher doses may be needed for effectiveness.26 All SSRIs inhibit CYP2D6 activity to some degree, and they may reduce the effectiveness of tamoxifen. An association between SSRIs and breast cancer recurrence and mortality has yet to be clarified and causality is unclear.31 Patients who prefer SSRIs or SNRIs for control of vasomotor symptoms may need to try several medications to find one that works well and has tolerable or minimal adverse effects. Nausea, dizziness, dry mouth, and other adverse effects are worse with SNRIs.25
The neurokinin 3 receptor antagonist, fezolinetant, is the newest nonhormonal treatment option for vasomotor symptoms; it works by disrupting the thermoregulatory response to estrogen deficiency that is believed to cause hot flashes.52,53 In a large phase 3 randomized controlled trial (n = 500), fezolinetant, 45 mg, significantly reduced vasomotor symptom frequency and severity when compared with placebo at four and 12 weeks; these effects persisted at 52 weeks of treatment.52 The most commonly reported adverse effect was headache; serious adverse effects were infrequent.52 Head-to-head trials are needed to compare the effectiveness of neurokinin 3 receptor antagonists with menopausal hormone therapy and nonhormonal medications for control of vasomotor symptoms. [updated]
A large meta-analysis found that gabapentin reduced the frequency of hot flashes compared with placebo. However, it appears to be less effective than estrogen and is associated with significant adverse effects, including dizziness, headaches, and somnolence.27
Clonidine is another treatment option, but most studies are of limited size and quality and several decades old. Dry mouth, insomnia, and drowsiness were common adverse effects.28 Oxybutynin may be effective for moderate to severe hot flashes and may be an option in women who have concomitant overactive bladder.29,30
What Nonpharmacologic Treatments Are Effective for Vasomotor Symptoms?
Cognitive behavior therapy (CBT) and clinical hypnosis are effective for short-term reduction of vasomotor symptoms and associated sleep disturbances.9,32–34 Black cohosh and isoflavones are shown to be effective compared with placebo but not significantly more than hormonal preparations, and they lack standard formulations.35–37 Additional nonpharmacologic therapies include other dietary supplements, exercise, and acupuncture, but there are low-quality data available to support effectiveness.9,34,38–41
EVIDENCE SUMMARY
A 2018 meta-analysis of women in natural or treatment-induced menopause found that behavioral interventions significantly decreased short-term (less than 20 weeks) and medium-term (20 weeks or longer) perceived severity, but not frequency, of vasomotor symptoms.32 The interventions included mostly group-based, one- to two-hour CBT, relaxation therapy, or mindfulness-based stress reduction sessions occurring weekly for two to 10 weeks.32 Telephone-based CBT has been shown to improve sleep in perimenopausal and postmenopausal women with insomnia due to hot flashes.33 Two small RCTs of weekly clinical hypnosis sessions over a five-week period appear to reduce hot flash severity and frequency in breast cancer survivors and postmenopausal women with frequent hot flashes.9 Regular exercise, yoga, biofeedback, and other mindfulness-based therapies are likely safe and may reduce stress associated with menopausal symptoms but are generally not effective for vasomotor symptoms.34
The effectiveness and safety of the herbal supplement black cohosh (Cimicifuga racemosa) have been debated. A meta-analysis of six RCTs found that isopropanolic black cohosh extract at dosages of 40 to 120 mg per day was more effective for reducing vasomotor symptoms than placebo (standardized mean difference = −0.694; 95% CI, −0.831 to −0.557); it also showed similar effectiveness to low-dose transdermal estradiol in a single three-month RCT not included in the meta-analysis.36 Black cohosh is not currently recommended over standard treatments for menopause symptoms because of the lack of regulatory oversight of herbal supplements.9 Isoflavones are found in dietary sources of soy proteins and have been studied for several decades for their potential benefit in menopause, but conflicting or heterogeneous data prevent their inclusion in current guidelines as standard therapies.34,37 Use of other plant-based therapies, including ginseng and probiotics, is unproven.34,38 Despite numerous studies of acupuncture for control of hot flashes, meta-analyses of RCTs remain inconclusive as to any significant benefit over sham acupuncture, hormone therapy, or nonhormonal medications for menopausal vasomotor symptoms.39–41
How Should Genitourinary Syndrome of Menopause Be Managed?
Genitourinary syndrome of menopause includes symptoms of vulvovaginal dryness, itching, irritation, dyspareunia, dysuria, urinary frequency and urgency, nocturia, and recurrent urinary tract infections. Effective treatment options include hormone-free vaginal moisturizers and lubricants, low-dose vaginal estrogen (i.e., cream, tablet, or ring insert), oral ospemifene (Osphena), and intravaginal DHEA7,11,42–46 (Table 5). Choice of therapy is guided by symptom severity and patient preference. Loss of sexual function and decreased libido are potential sequelae of changes due to genitourinary syndrome of menopause, and treatment is similar to that of hypoactive sexual desire disorder.47
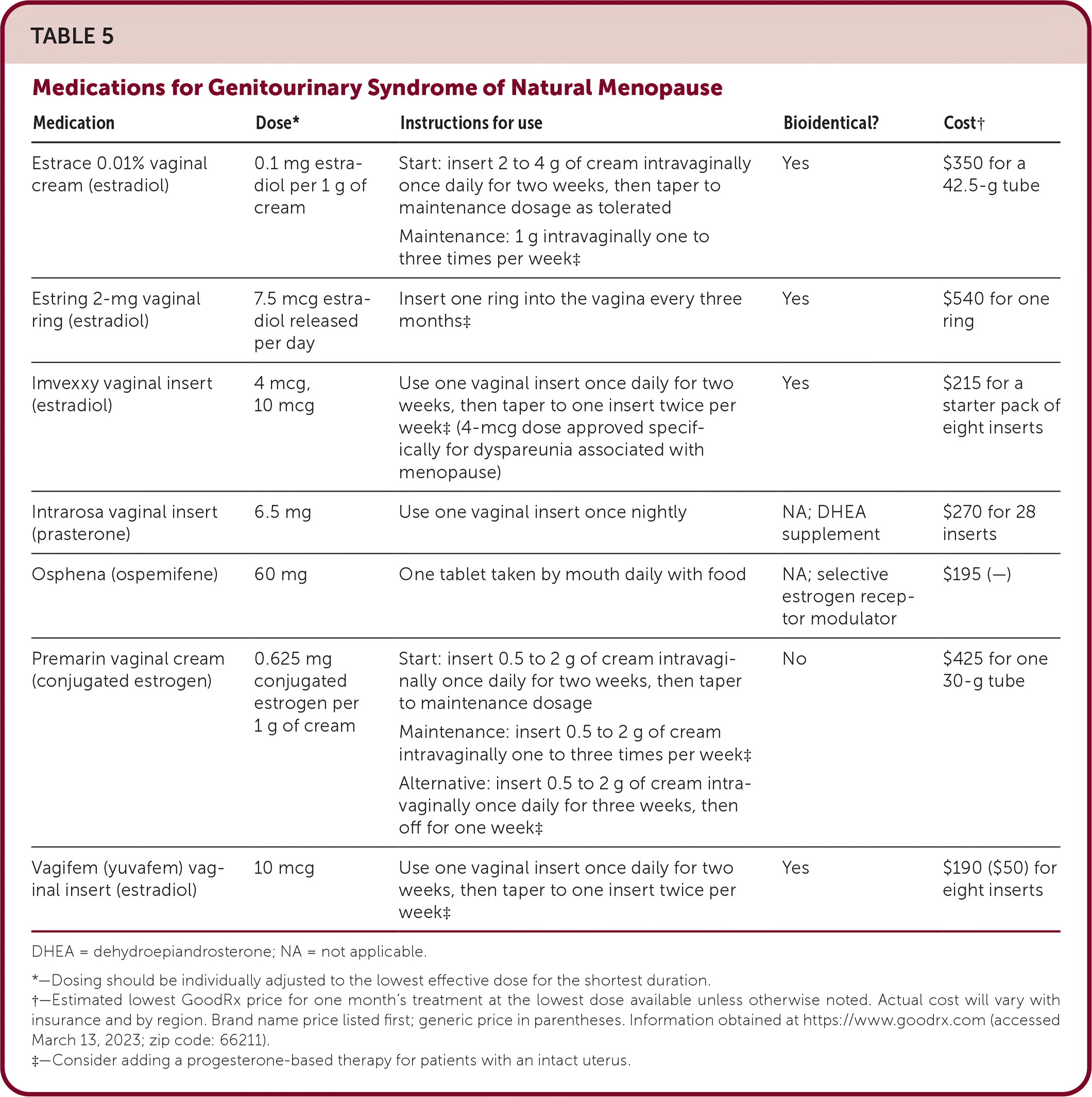
| Medication | Dose* | Instructions for use | Bioidentical? | Cost† |
|---|---|---|---|---|
| Estrace 0.01% vaginal cream (estradiol) | 0.1 mg estradiol per 1 g of cream | Start: insert 2 to 4 g of cream intravaginally once daily for two weeks, then taper to maintenance dosage as tolerated Maintenance: 1 g intravaginally one to three times per week‡ | Yes | $350 for a 42.5-g tube |
| Estring 2-mg vaginal ring (estradiol) | 7.5 mcg estradiol released per day | Insert one ring into the vagina every three months‡ | Yes | $540 for one ring |
| Imvexxy vaginal insert (estradiol) | 4 mcg, 10 mcg | Use one vaginal insert once daily for two weeks, then taper to one insert twice per week‡ (4-mcg dose approved specifically for dyspareunia associated with menopause) | Yes | $215 for a starter pack of eight inserts |
| Intrarosa vaginal insert (prasterone) | 6.5 mg | Use one vaginal insert once nightly | NA; DHEA supplement | $270 for 28 inserts |
| Osphena (ospemifene) | 60 mg | One tablet taken by mouth daily with food | NA; selective estrogen receptor modulator | $195 (—) |
| Premarin vaginal cream (conjugated estrogen) | 0.625 mg conjugated estrogen per 1 g of cream | Start: insert 0.5 to 2 g of cream intravaginally once daily for two weeks, then taper to maintenance dosage Maintenance: insert 0.5 to 2 g of cream intravaginally one to three times per week‡ Alternative: insert 0.5 to 2 g of cream intravaginally once daily for three weeks, then off for one week‡ | No | $425 for one 30-g tube |
| Vagifem (yuvafem) vaginal insert (estradiol) | 10 mcg | Use one vaginal insert once daily for two weeks, then taper to one insert twice per week‡ | Yes | $190 ($50) for eight inserts |
EVIDENCE SUMMARY
In a 2008 double-blind RCT, a 10-mcg vaginal estradiol tablet used once daily for two weeks, then twice weekly, was superior to placebo for reducing the severity of urogenital symptoms in eight weeks, and treatment effects remained significant at 52 weeks.48 There appears to be no difference between hormonal preparations and their nonhormonal equivalents. A 2018 RCT directly compared three combinations: vaginal estradiol, 10 mcg with plain lubricant; a placebo tablet with a vaginal moisturizer; and a placebo tablet with plain lubricant.42 At 12 weeks, all three formulations significantly reduced atrophic symptom severity and increased sexual function regardless of preparation.42
Over-the-counter, hormone-free vaginal products are reasonable first-line therapies for genitourinary syndrome of menopause.44 Vaginal moisturizers are used several times per week to mitigate symptoms related to vaginal dryness, and vaginal lubricants are recommended on an as-needed basis for dyspareunia.8,11,44
Women who prefer vaginal estrogen may also benefit from improved urge incontinence and prevention of recurrent urinary tract infection.7 Endometrial protection with progesterone is generally not necessary with low-dose vaginal estrogen, but there are only short-term data to support this.7,11 Patients with a uterus who experience vaginal bleeding while using vaginal estrogen should be evaluated and endometrial biopsy considered.7,8 Patients with a history of breast cancer should discuss the use of vaginal estrogen with their oncologists before initiation.7,8,11
FDA-approved non-estrogen therapies for vaginal atrophy and dyspareunia include oral ospemifene and intravaginal DHEA. A 12-week multicenter RCT of postmenopausal women with moderate to severe vaginal dryness found that ospemifene, a selective estrogen receptor modulator, was superior to placebo for reducing vaginal dryness (primary outcome) and improving dyspareunia and sexual function scores (secondary outcomes).45 Ospemifene was well tolerated and had no serious treatment-related adverse effects, including endometrial hyperplasia or carcinoma.45 A 12-week RCT comparing daily intravaginal 0.50% DHEA (6.5 mg) application with placebo found significant improvement in dyspareunia and vaginal dryness for menopausal women with moderate to severe symptoms at baseline.46
This article updates previous articles on this topic by Hill, et al.,49 Hill and Hill,50 and Cutson and Meuleman.51
Data Sources: A PubMed search was completed using the key terms menopause, hot flashes, menopausal symptoms, vaginal moisturizer, vaginal estrogen, endometrial hyperplasia. The search included meta-analyses, randomized controlled trials, clinical trials, and review articles. A search was also performed using Essential Evidence Plus. Search dates: May, August, September, and December 2022; and January and April 2023.
Literature search for the updated version was completed using the terms neurokinin and menopause: Search date: August 4, 2023.
The opinions and assertions contained herein are the private views of the authors and not to be construed as official or reflecting the views of the U.S. Air Force, the U.S. Department of Defense, or the U.S. government.
