
Am Fam Physician. 2017;95(12):771-778
Related editorial: Dementia Care: More Than Just Prescription Drugs
Related letter: Prescribing Cholinesterase Inhibitors for Alzheimer Disease: Timing Matters
Author disclosure: No relevant financial affiliations.
Alzheimer disease comprises a syndrome of progressive cognitive and functional decline. Treatments should target cognitive and functional symptoms. Cholinesterase inhibitors, memantine, and a combination of a cholinesterase inhibitor and memantine have produced statistically significant but clinically small delays in various domains of cognitive and functional decline in select patients with Alzheimer disease. Vitamin E has been shown to delay functional decline in patients with mild to moderate Alzheimer disease, especially when taken in combination with a cholinesterase inhibitor. Structured programs of physical exercise improve physical function and reduce rates of neuropsychiatric symptoms in patients with mild to severe Alzheimer disease. Cognitive stimulation programs show benefit in maintenance of cognitive function and improved self-reported quality of life in patients with mild to moderate Alzheimer disease.
Dementia is a heterogeneous syndrome resulting in impairment in at least one cognitive domain severe enough to limit daily functioning.1 The most common etiologies of dementia in developed nations are Alzheimer disease, vascular dementia, mixed dementia (in which symptoms arise from a combination of multiple etiologies, most often Alzheimer disease and vascular dementia), Lewy body dementia/Parkinson disease dementia, and frontotemporal dementia.2–5
WHAT IS NEW ON THIS TOPIC: ALZHEIMER DISEASE
A 2014 randomized controlled trial in veterans with mild to moderate Alzheimer disease who were already receiving a cholinesterase inhibitor found that vitamin E slowed functional status decline (3.15 points less than placebo on a 78-point assessment scale over 4 years), with a delay in progression of about 6 months.
A 2012 Cochrane meta-analysis of 15 randomized controlled trials concluded that cognitive stimulation programs are beneficial for maintenance of cognitive function and self-reported quality of life in patients with mild to moderate dementia from Alzheimer disease. However, cognitive stimulation techniques are highly variable and lack standardization, and no effects were noted on functional status, behavior, or mood.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Cholinesterase inhibitors, including donepezil (Aricept, 5 to 10 mg per day), galantamine (Razadyne, at least 16 mg per day), or rivastigmine (Exelon, 6 to 12 mg per day orally or 9.5 mg per day transdermally), should be considered for treatment of cognitive and functional decline in patients with mild to moderate Alzheimer disease. | A | 11–15 |
| Memantine (Namenda, 20 mg per day) should be considered for treatment of cognitive and functional decline in patients with moderate to severe Alzheimer disease. | A | 16–21 |
| The addition of memantine should be considered for treatment of cognitive and functional symptoms in patients with moderate to severe Alzheimer disease or mixed dementia who are already receiving a cholinesterase inhibitor. | B | 19, 20 |
| The addition of vitamin E (2,000 IU per day) should be considered for treatment of mild to moderate Alzheimer disease in patients who are already receiving a cholinesterase inhibitor. | B | 22 |
| A structured physical exercise program should be recommended for patients with Alzheimer disease of any severity. | A | 32–36 |
| Cognitive stimulation programs should be recommended for patients with mild to moderate cognitive impairment. | B | 37 |
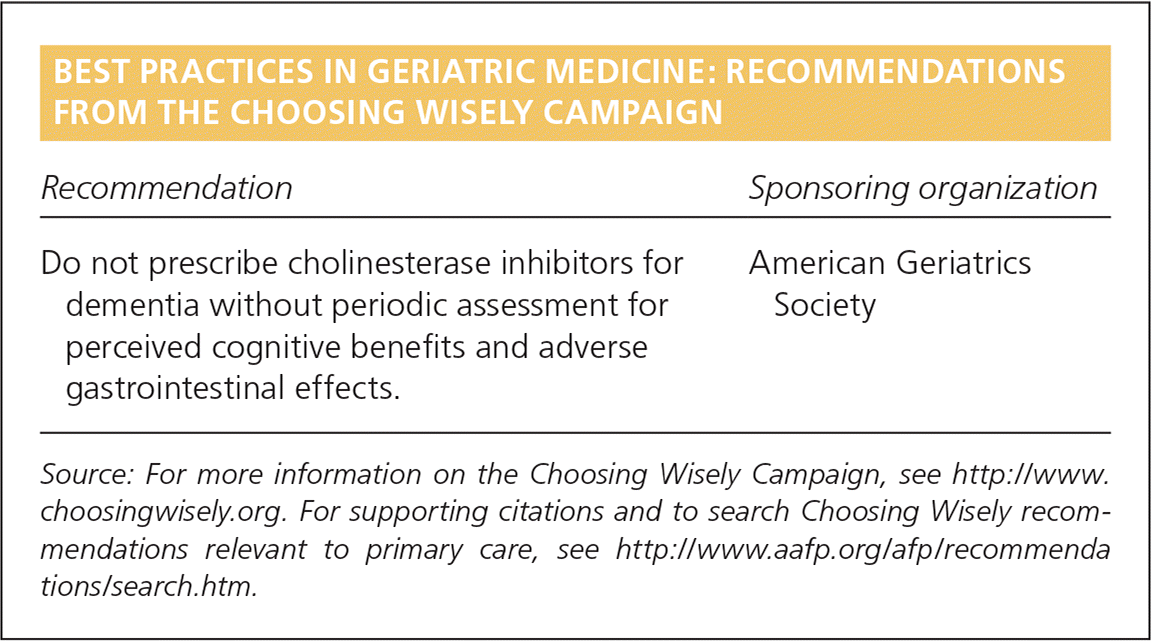
| Recommendation | Sponsoring organization |
|---|---|
| Do not prescribe cholinesterase inhibitors for dementia without periodic assessment for perceived cognitive benefits and adverse gastrointestinal effects. | American Geriatrics Society |
This review will focus on the management of cognitive and functional symptoms of dementia due to Alzheimer disease. A previous review discussed management of behavioral disorders in dementia (https://www.aafp.org/afp/2016/0815/p276.html).6
Assessment
Alzheimer disease exists on a spectrum of mild to severe, and evidence for the effectiveness of treatments varies depending on the severity.4,5,7,8 Progressive decline in physical, social, and executive function accompanies progressive cognitive decline, and the severity of the syndrome is based on measures of cognitive function and daily functional status.
Cognitive function can be evaluated using brief cognitive screening instruments, such as the Saint Louis University Mental Status Examination (http://aging.slu.edu/index.php?page=saint-louis-university-mental-status-slums-exam), the Mini-Mental State Examination (which now requires a fee), or the Montreal Cognitive Assessment.9 Functional status is best assessed with questions about activities of daily living (ADLs; e.g., bathing, dressing, feeding, toileting) and instrumental ADLs (e.g., using a telephone; shopping for and preparing food; housekeeping; managing medications, finances, and transportation). Standardized tools can also be used to evaluate functional status, such as the Bristol Activities of Daily Living Scale, the Barthel Index, or the Functional Independence Measure. However, these tools are less practical in clinical encounters than a functional status history based on ADLs and instrumental ADLs.9
In addition to their use at the time of diagnosis, standardized measures of cognition and function are useful in the periodic assessment of patients undergoing treatment for dementia.
Pharmacotherapy for Cognitive and Functional Symptoms
Table 1 summarizes pharmacologic treatments for Alzheimer disease, including cholinesterase inhibitors, memantine (Namenda), and vitamin E, and describes titration schedules and adverse effects for each medication. There are no curative therapies for Alzheimer disease and other common etiologies of dementia. The goal of current pharmacologic therapies is to delay the progression of symptoms of neurocognitive and physical decline.
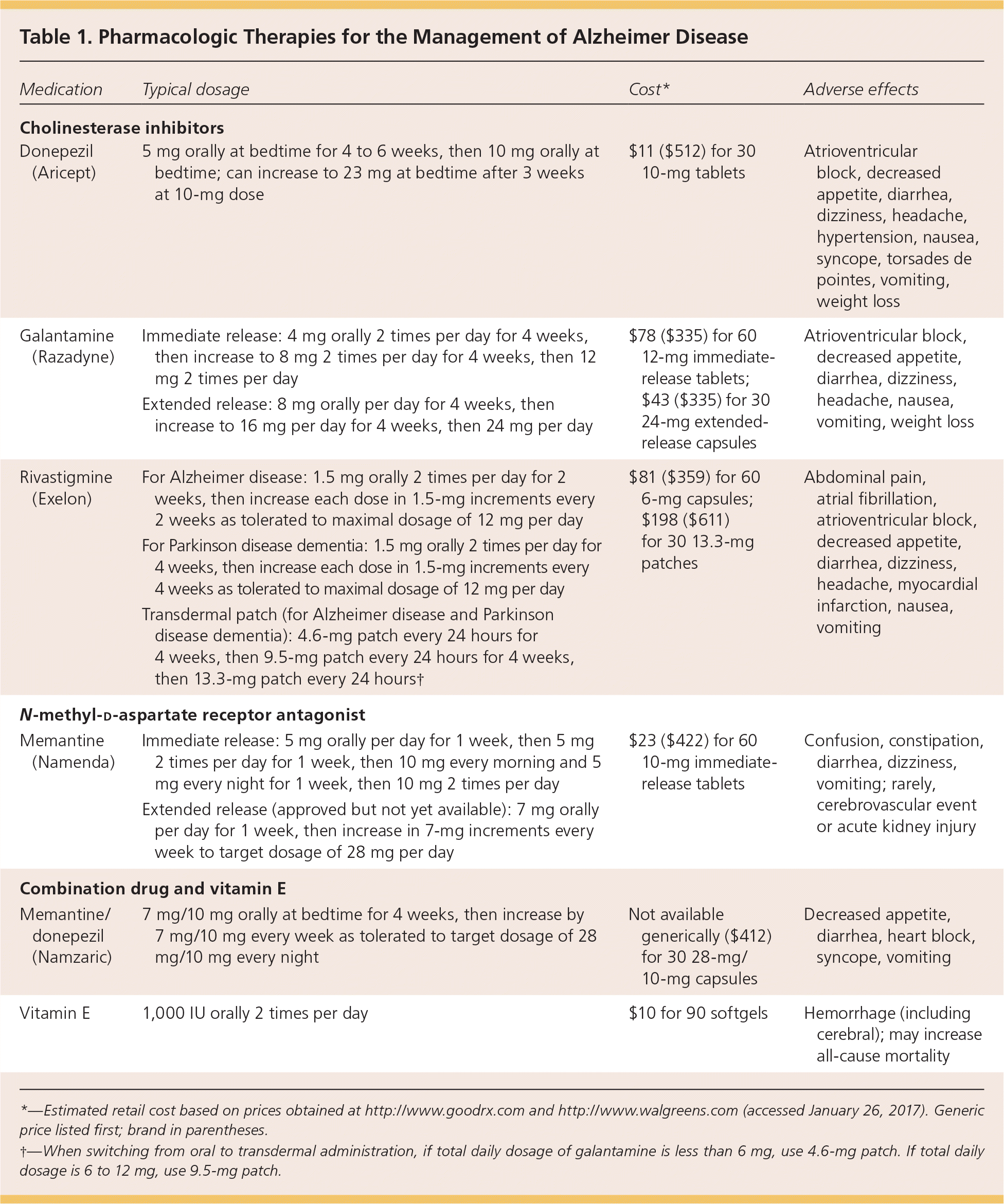
| Medication | Typical dosage | Cost* | Adverse effects |
|---|---|---|---|
| Cholinesterase inhibitors | |||
| Donepezil (Aricept) | 5 mg orally at bedtime for 4 to 6 weeks, then 10 mg orally at bedtime; can increase to 23 mg at bedtime after 3 weeks at 10-mg dose | $11 ($512) for 30 10-mg tablets | Atrioventricular block, decreased appetite, diarrhea, dizziness, headache, hypertension, nausea, syncope, torsades de pointes, vomiting, weight loss |
| Galantamine (Razadyne) | Immediate release: 4 mg orally 2 times per day for 4 weeks, then increase to 8 mg 2 times per day for 4 weeks, then 12 mg 2 times per day | $78 ($335) for 60 12-mg immediate-release tablets; $43 ($335) for 30 24-mg extended-release capsules | Atrioventricular block, decreased appetite, diarrhea, dizziness, headache, nausea, vomiting, weight loss |
| Extended release: 8 mg orally per day for 4 weeks, then increase to 16 mg per day for 4 weeks, then 24 mg per day | |||
| Rivastigmine (Exelon) | For Alzheimer disease: 1.5 mg orally 2 times per day for 2 weeks, then increase each dose in 1.5-mg increments every 2 weeks as tolerated to maximal dosage of 12 mg per day | $81 ($359) for 60 6-mg capsules; $198 ($611) for 30 13.3-mg patches | Abdominal pain, atrial fibrillation, atrioventricular block, decreased appetite, diarrhea, dizziness, headache, myocardial infarction, nausea, vomiting |
| For Parkinson disease dementia: 1.5 mg orally 2 times per day for 4 weeks, then increase each dose in 1.5-mg increments every 4 weeks as tolerated to maximal dosage of 12 mg per day | |||
| Transdermal patch (for Alzheimer disease and Parkinson disease dementia): 4.6-mg patch every 24 hours for 4 weeks, then 9.5-mg patch every 24 hours for 4 weeks, then 13.3-mg patch every 24 hours† | |||
| N-methyl-d-aspartate receptor antagonist | |||
| Memantine (Namenda) | Immediate release: 5 mg orally per day for 1 week, then 5 mg 2 times per day for 1 week, then 10 mg every morning and 5 mg every night for 1 week, then 10 mg 2 times per day | $23 ($422) for 60 10-mg immediate-release tablets | Confusion, constipation, diarrhea, dizziness, vomiting; rarely, cerebrovascular event or acute kidney injury |
| Extended release (approved but not yet available): 7 mg orally per day for 1 week, then increase in 7-mg increments every week to target dosage of 28 mg per day | |||
| Combination drug and vitamin E | |||
| Memantine/donepezil (Namzaric) | 7 mg/10 mg orally at bedtime for 4 weeks, then increase by 7 mg/10 mg every week as tolerated to target dosage of 28 mg/10 mg every night | Not available generically ($412) for 30 28-mg/10-mg capsules | Decreased appetite, diarrhea, heart block, syncope, vomiting |
| Vitamin E | 1,000 IU orally 2 times per day | $10 for 90 softgels | Hemorrhage (including cerebral); may increase all-cause mortality |
CHOLINESTERASE INHIBITORS
Patients with Alzheimer disease, as well as those with vascular and Lewy body dementia, have reduced cerebral cholinergic function, which is implicated in cognitive losses.10 Cholinesterase inhibitors reversibly bind to cholinesterase, the enzyme responsible for degradation of acetylcholine within the synaptic cleft, thereby increasing cholinergic transmission between neurons.
There are three commonly prescribed cholinesterase inhibitors: donepezil (Aricept), galantamine (Razadyne), and rivastigmine (Exelon). Randomized controlled trials (RCTs) of these agents have demonstrated some evidence of benefit on cognitive and functional status in persons with Alzheimer disease, although these benefits tend to have only minor clinical significance.11–15 For example, a 24-week pharmaceutical company–sponsored RCT showed a small but statistically significant improvement in cognitive scores and functional status in patients with Alzheimer disease who were treated with donepezil.11 However, the mean cognitive benefit with 5-mg and 10-mg doses was only 2.5 and 2.9 points, respectively, on a 70-point scale. These effects did not persist after a washout period following treatment cessation, which confirmed that donepezil is not a disease-modifying treatment.
Similarly, in a subsequent, non–industry-sponsored RCT of donepezil, a small but statistically significant cognitive improvement in Mini-Mental State Examination scores (0.8-point improvement on the 30-point scale) was demonstrated after two years of treatment with 5 or 10 mg of donepezil compared with placebo.12 However, there were no benefits in institutionalization rates or progression of disability after at least three years of therapy.
Disparities in patient inclusion and exclusion criteria may account for the minor differences between the results of these two trials. In both cases, however, the statistically significant changes in rating scale scores are likely not clinically significant.
A 2004 Cochrane review demonstrated improvement in global function and cognitive symptoms (four points on a 70-point scale) in patients with mild to moderate Alzheimer disease who received at least 16 mg of galantamine per day for at least six months.15 A 2006 Cochrane review demonstrated benefits in cognitive function (two or three points on a 70-point scale), ADLs, and behavior in patients with mild to moderate Alzheimer disease who were treated with donepezil for 12, 24, or 52 weeks.13 A 2015 Cochrane review also demonstrated statistically significant but small improvements in cognitive function and ADLs after six months of treatment with rivastigmine.14
Overall, the small beneficial effects in functional and/or cognitive scores have not translated into consistent benefits in patient-oriented outcomes, such as patient-rated quality of life or institutionalization.11–15 Many experts in dementia care use cholinesterase inhibitors to temporarily improve cognitive and functional performance in patients with mild to moderate Alzheimer disease, but evidence of clinically meaningful benefit is lacking. Furthermore, there are no published head-to-head trials comparing the various cholinesterase inhibitors.
MEMANTINE
Memantine is a partial N-methyl-d-aspartate (NMDA) receptor antagonist thought to exert a protective effect on cortical and hippocampal neurons by limiting damaging glutamatergic excitation at the NMDA receptor. Memantine was approved by the U.S. Food and Drug Administration in 2003 and is the only drug in its class.
Memantine has been studied in patients with Alzheimer disease, vascular dementia, and mixed dementia. RCTs comparing memantine with placebo generally demonstrate benefits in cognition, global status, and functional status in persons with moderate to severe dementia, although benefits are small and not consistently seen in those with less-severe disease.16–21 For example, a 28-week RCT showed lower clinician-assessed dementia severity scores and higher functional status scores in persons with moderate to severe Alzheimer disease who received memantine (10 mg twice daily) compared with those who received placebo.16 This trial is significant because more patients in the control group discontinued the study because of adverse effects. Patients receiving memantine generally report few adverse effects.16–21
A 2006 Cochrane review of studies evaluating memantine in persons with dementia concluded that there was a small but statistically significant benefit at six months in those with moderate to severe dementia, including dementia due to Alzheimer disease, but not in those with milder disease.21 However, as with the studies on cholinesterase inhibitors, the magnitude of benefit was small: 2.97 points on a 100-point cognitive assessment scale, 1.27 points on a 54-point ADL scale, and 2.76 points on a 144-point behavior scale.
Given the clinical benefit, albeit small, and tolerability of memantine, it is a reasonable choice for use in the treatment of cognitive and functional symptoms in patients with moderate to severe Alzheimer disease.
COMBINATION THERAPY
An increasing body of evidence describes concomitant use of cholinesterase inhibitors and memantine in patients with moderate to severe dementia, including Alzheimer disease and mixed dementia. For example, a 2014 RCT demonstrated small increases in cognitive scores, ADL scores, global outcomes scores, and behavioral scores among persons with moderate to severe Alzheimer disease who received donepezil/memantine (Namzaric) compared with those who received donepezil plus placebo.22 Although the changes in scores met thresholds for clinical significance, they were very small (3.4-point improvement on a 100-point scale). A subsequent RCT comparing memantine with placebo in patients with mild to moderate Alzheimer disease who were already receiving a cholinesterase inhibitor showed no difference in outcomes between the groups.23
Many experts in dementia care treat patients with moderate to severe dementia due to Alzheimer disease with memantine in addition to a cholinesterase inhibitor. This approach is reasonable, given the tolerability of memantine and lack of other beneficial treatments for cognitive and functional decline. However, clinicians must be wary of drawing conclusions about clinically significant benefits from small but statistically significant improvements in an ideal study population.
VITAMIN E
Vitamin E (alpha tocopherol) has been hypothesized to exert a protective effect on cortical neurons in patients with dementia through its antioxidant properties. Data from multiple RCTs suggest benefit in patients with mild to moderate dementia due to Alzheimer disease who received 2,000 IU per day.22,23
A recent RCT compared vitamin E (2,000 IU per day) with memantine (10 mg twice per day), a combination of vitamin E and memantine, or placebo in veterans with mild to moderate Alzheimer disease who were already receiving a cholinesterase inhibitor.22 Those who received vitamin E had a slower decline in functional status (3.15 points less than placebo on a 78-point scale over four years), with an estimated delay in functional decline progression of 6.2 months; there was also a smaller increase in time required from a caregiver than in all other groups over 2.27 years. There were no significant differences among treatment groups in mortality or serious adverse events, including heart failure, falls, syncope, or bleeding.
Although the clinical significance of these effects was marginal, it is reasonable to prescribe 2,000 IU of vitamin E per day for patients with mild to moderate dementia due to Alzheimer disease, especially in those already receiving cholinesterase inhibitors.
INEFFECTIVE MEDICATIONS
Statins have been investigated for treatment and prevention of dementia due to Alzheimer disease. Simvastatin (Zocor), 40 mg per day, and atorvastatin (Lipitor), 80 mg per day, were evaluated in studies of patients with mild to moderate dementia and were found to have no beneficial effect on cognitive or functional outcomes.24,25
Nonsteroidal anti-inflammatory drugs have also been advanced as a potential treatment to limit amyloid-induced neuronal inflammation in patients with Alzheimer disease. However, results of RCTs evaluating naproxen, aspirin, and diclofenac have not demonstrated arrest or slowing of cognitive decline.26–28
Ginkgo has been studied in the prevention and treatment of dementia due to Alzheimer disease. A 2007 Cochrane review concluded that there was inconsistent evidence of benefit.29
Omega-3 fatty acids have been evaluated in the prevention and treatment of dementia. Although observational studies have suggested an inverse association between intake of dietary omega-3 fatty acids and incident dementia, RCTs of omega-3 fatty acid supplementation in patients with mild to moderate Alzheimer disease have not demonstrated benefits in cognitive or functional decline.30,31
CONTROVERSIES IN THE PHARMACOLOGIC MANAGEMENT OF DEMENTIA
There is no definitive evidence on the appropriate duration of therapy for cognitive and functional decline in patients with dementia. Similarly, there is no clear evidence to support discontinuing therapy at any particular point in the progression of disease severity.
Nonpharmacologic Therapy for Cognitive and Functional Symptoms
Table 2 summarizes the evidence for nonpharmacologic therapies in the management of Alzheimer disease.
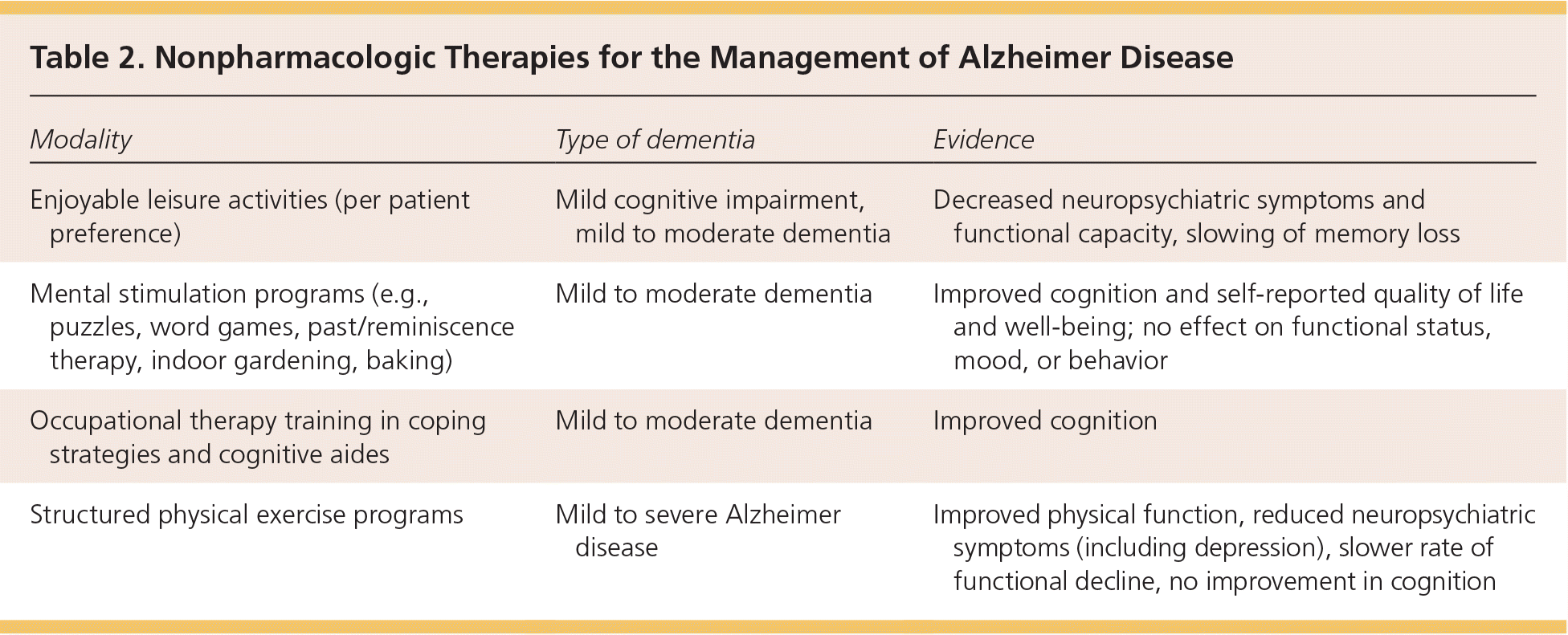
| Modality | Type of dementia | Evidence |
|---|---|---|
| Enjoyable leisure activities (per patient preference) | Mild cognitive impairment, mild to moderate dementia | Decreased neuropsychiatric symptoms and functional capacity, slowing of memory loss |
| Mental stimulation programs (e.g., puzzles, word games, past/reminiscence therapy, indoor gardening, baking) | Mild to moderate dementia | Improved cognition and self-reported quality of life and well-being; no effect on functional status, mood, or behavior |
| Occupational therapy training in coping strategies and cognitive aides | Mild to moderate dementia | Improved cognition |
| Structured physical exercise programs | Mild to severe Alzheimer disease | Improved physical function, reduced neuropsychiatric symptoms (including depression), slower rate of functional decline, no improvement in cognition |
EXERCISE
Multiple RCTs have evaluated structured exercise programs among community-dwelling and institutionalized patients with mild to severe Alzheimer disease.32–36 These trials have generally demonstrated no improvement in cognitive function. However, they have shown benefits in physical function, neuropsychiatric symptoms such as depression, and rates of functional decline. Although no RCTs have shown clear improvement in cognition with structured exercise programs, there is clearly a role for exercise in improving neuropsychiatric symptoms and slowing functional decline, and physicians should encourage safe physical exercise in patients with dementia due to Alzheimer disease at any stage of severity.8,32–36
MENTAL STIMULATION PROGRAMS AND ENJOYABLE LEISURE ACTIVITIES
RCTs have evaluated cognitive stimulation activities such as puzzles, word games, indoor gardening, discussions of the past/reminiscence therapy, and baking. A 2012 Cochrane review of 15 RCTs concluded that cognitive stimulation programs are beneficial for maintenance of cognitive function and self-reported quality of life in patients with mild to moderate dementia due to Alzheimer disease.37 However, this meta-analysis also noted that cognitive stimulation techniques are highly variable and lack standardization, and that studies evaluating these techniques were of poor quality and found no effects on functional status, behavior, or mood. When cognitive and quality-of-life benefits were observed, they were noted immediately after the programs ended and were sustained for up to three months.
Enjoyable leisure activities have been shown to slow memory loss in patients with mild cognitive impairment and mild to moderate dementia. Enjoyable leisure activities have also been associated with improved functional capacity and reduced neuropsychiatric symptoms in patients with dementia.38
Cognitive stimulation programs and engagement in enjoyable leisure activities are safe and effective interventions in patients with dementia due to Alzheimer disease at any stage of severity. Such programs are most easily implemented in institutional settings, but community-dwelling patients with Alzheimer disease should also be encouraged to engage in these activities.
OCCUPATIONAL THERAPY
A small RCT (n = 135) showed that 10 sessions of occupational therapy to train patients with mild to moderate dementia and their caregivers in techniques for coping with cognitive impairment improved functional capacity.39 This study included community-dwelling patients with mild to moderate dementia of multiple etiologies, including Alzheimer disease.39 Occupational therapists evaluated participants' home environments and helped them and their caregivers develop compensatory strategies to adapt ADLs to patients' disabilities, as well as environmental strategies to adapt the patients' environment to their cognitive limitations. It is difficult to draw generalizable conclusions from this small study, but beneficial effects from tailored occupational therapy seem intuitive and highly plausible.
Limitations of the Evidence
There are important limitations in the literature on pharmacologic management of dementia. First, most studies include patients with dementia due to Alzheimer disease and not other types of dementia, so results are applicable only to Alzheimer disease. Additionally, the definition of probable or likely Alzheimer disease (or other types of dementia) and of dementia severity varies among studies. Second, recruitment and retention of participants with dementia can be difficult, and participation—particularly in the later stages of disease—requires caregiver support and often consent from a surrogate decision-maker. Third, study populations are often not reflective of populations seen in clinical practice; most patients enrolled in the studies have undergone extensive workup to define the likely etiology of dementia and may have classic presentations of Alzheimer disease or other dementia syndromes. Additionally, patients with neuropsychiatric or behavioral symptoms are often excluded from study populations. Finally, outcome measures across studies are inconsistent. Multiple validated tools and scales are used to assess important aspects of dementia progression, including cognition, functional status, and caregiver burden, and there is no consensus on which tools to use to assess outcomes. This leads to difficulty in comparing results across studies, as well as difficulty in extrapolating clinical significance from small, statistically significant changes in scores across studies.
Data Sources: A PubMed search was completed using the search terms dementia, cognitive impairment, cholinesterase inhibitor, and memantine. We also searched the Cochrane Database of Systematic Reviews, UpToDate, and DynaMed using the same search terms. The Essential Evidence database was searched using the terms Alzheimer disease and dementia. Search dates: September, November, and December 2015, and January 2016.