Am Fam Physician. 2021;103(7):407-416
Related letter to the Editor: Management of Syphilis in People with HIV Infection
Author disclosure: No relevant financial affiliations.
The HIV epidemic is an important public health priority. Transmissions continue to occur despite effective therapies that make HIV preventable and treatable. Approximately one-half of people with HIV are not receiving suppressive antiretroviral therapy (ART). Starting ART early, followed by continuous lifetime treatment, most effectively achieves durable virologic suppression and restoration of immune function that can improve clinical outcomes and prevent transmission to partners who are seronegative. National treatment guidelines include ART options that can be offered immediately after diagnosis, even before the results of baseline HIV drug-resistance testing are available. Initial ART selection should be guided by co-occurring conditions, including viral hepatitis, medications, and other factors such as pregnancy. Identifying and addressing psychosocial barriers to care is a key element of ensuring long-term adherence to treatment. The initial physical examination typically reveals no clinical manifestations of HIV in the absence of advanced disease. A comprehensive laboratory evaluation, including HIV viral load and CD4 lymphocyte monitoring, is necessary to guide decision-making for treatment, opportunistic infection prophylaxis, and vaccinations. The initial management of people with HIV presents a unique opportunity for family physicians to improve patients' long-term health care and reduce HIV transmissions.
One-half of the estimated 1.1 million people in the United States with HIV infection are not receiving antiretroviral therapy (ART) or are receiving ART that is not sufficiently effective to achieve key clinical outcomes. Key outcomes include preventing clinical progression to advanced HIV disease, allowing near-normal life expectancy, and reducing transmission risk (i.e., treatment as prevention).1–8 HIV disproportionately affects people of color and people with limited access to continuous, comprehensive health care.9,10 Family physicians are uniquely positioned to diagnose HIV early and ensure long-term quality care for patients.
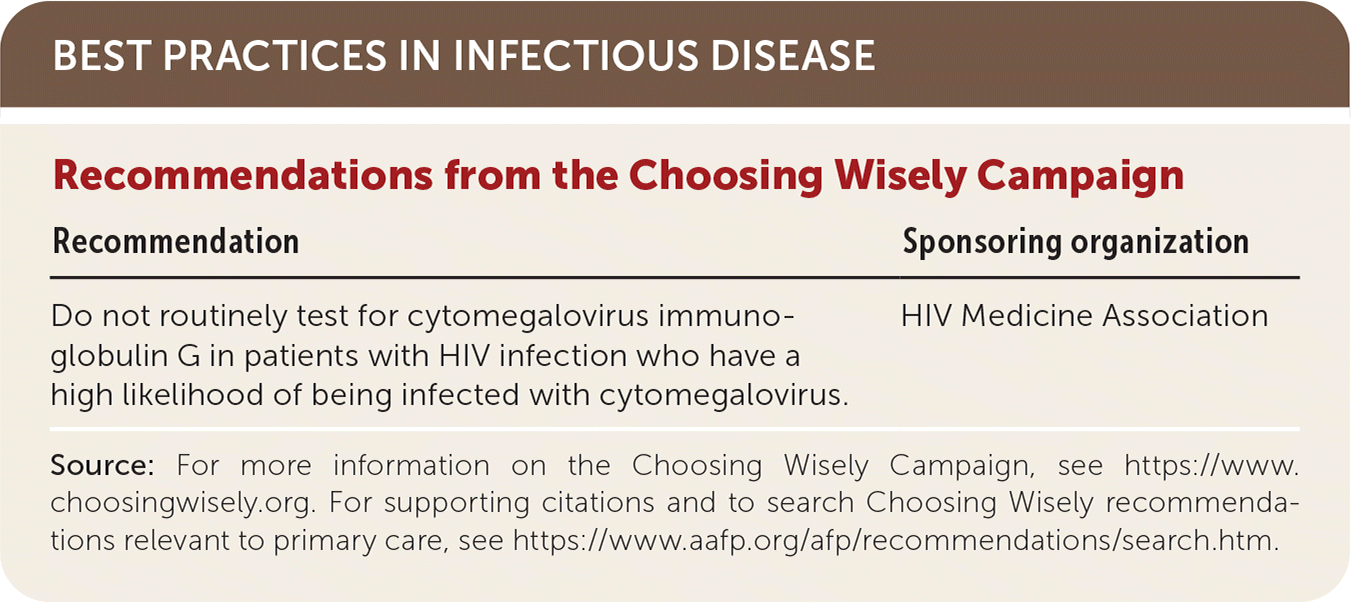
| Recommendation | Sponsoring organization |
|---|---|
| Do not routinely test for cytomegalovirus immunoglobulin G in patients with HIV infection who have a high likelihood of being infected with cytomegalovirus. | HIV Medicine Association |
Diagnosis
HIV screening and diagnostic testing are essential for timely ART initiation and transmission prevention because approximately 38% of new transmissions are from people with HIV who are unaware of their HIV status.2 Testing with the fourth-generation combination HIV antigen-antibody immunoassay is widely available and is recommended for screening people 15 to 65 years of age and for testing people with risk factors11–14 (Table 1). In addition to HIV-specific immunoglobulin M and immunoglobulin G antibodies, which typically develop three or more weeks following infection, the fourth-generation HIV test detects the p24 antigen that appears as early as two weeks after infection. Inclusion of the p24 antigen shortens the time frame for detecting HIV, increasing the likelihood of identifying people with HIV who recently acquired the infection (i.e., within the previous one to two months).

| Screen all people 15 to 65 years of age at least once; younger adolescents and older adults at increased risk should also be screened.* Retest people who are at increased risk of infection. |
| Screen all pregnant people, including those who present in labor or at delivery whose HIV status is unknown. |
| Screen all patients being considered for pre- and postexposure prophylaxis according to established guidelines. |
| Test when acute HIV infection is suspected in people with recent exposure history (i.e., within the previous two months) and symptoms of recent viral infection, including fever, chills, night sweats, fatigue, myalgia, lymphadenopathy, headache, sore throat, and diarrhea. |
| Test when chronic HIV infection is suspected, based on clinical presentation and risk or exposure history. Symptoms of chronic untreated HIV include fever, lymphadenopathy, malaise or fatigue, weight loss, and symptoms from undiagnosed opportunistic infections. |
The risk of HIV transmission from untreated people with acute or early HIV infection is much higher than from people with established chronic infection who are receiving suppressive ART. Therefore, improved identification of acute infection allows for earlier intervention, including ART initiation and education about transmission prevention (i.e., pre- and postexposure prophylaxis for partners who are seronegative).15–18 Acute symptomatic HIV is often unrecognized, and fewer than 50% of people will have an identifiable acute illness.19 Many respiratory illnesses, including those caused by SARS-CoV-2 or influenza, are more common; therefore, a careful assessment of recent risk behaviors and exposures may be a factor that prompts HIV testing.
Evaluation
HISTORY AND PHYSICAL EXAMINATION
After a diagnosis of HIV, the initial history should document details about other chronic health conditions, hospitalizations, medications and allergies, mental and behavioral health, substance use, sexual health history, and experience with HIV pre- or postexposure prophylaxis. For people with advanced HIV, which is generally indicated by a CD4 lymphocyte count of less than 200 cells per μL (0.20 × 109 per L), a full review of systems to assess for opportunistic infections or conditions associated with significant immunosuppression such as Pneumocystis jiroveci pneumonia, oropharyngeal candidiasis, tuberculosis, HIV-associated central nervous system and gastrointestinal disorders, and disseminated fungal or viral infections is recommended. When patients with chronic HIV infection reengage in care, clinicians should obtain a comprehensive history of previous ART regimens and treatment responses, including previous laboratory results. The initial examination generally shows no specific HIV-related manifestations unless symptomatic acute infection or advanced HIV disease is present.
LABORATORY TESTING
The diagnosis of HIV should be established with documented laboratory test results. Abnormal results from home-based, point-of-care, or rapid tests should be followed with standard, instrument-based laboratory assays. After the diagnosis is confirmed, comprehensive testing should be ordered (Table 2).4,20 Testing should include primary care screening and HIV-specific baseline laboratory testing with a CD4 count, quantitative plasma HIV RNA (viral load), and HIV drug-resistance assessment. The CD4 count reflects the degree of immune system impairment and helps determine if opportunistic infection prophylaxis is indicated. The HIV viral load indicates how much active viral replication is occurring. Regular viral load monitoring during treatment is the most important indicator of a response to ART. HIV drug-resistance testing guides the initial selection of the ART regimen by identifying clinically significant mutations in the genes (reverse transcriptase, protease, integrase) targeted by commonly used antiretroviral drugs. Commercially available resistance tests are generally highly sensitive and accurate. Studies have established the clinical and cost-effectiveness of baseline resistance testing.21
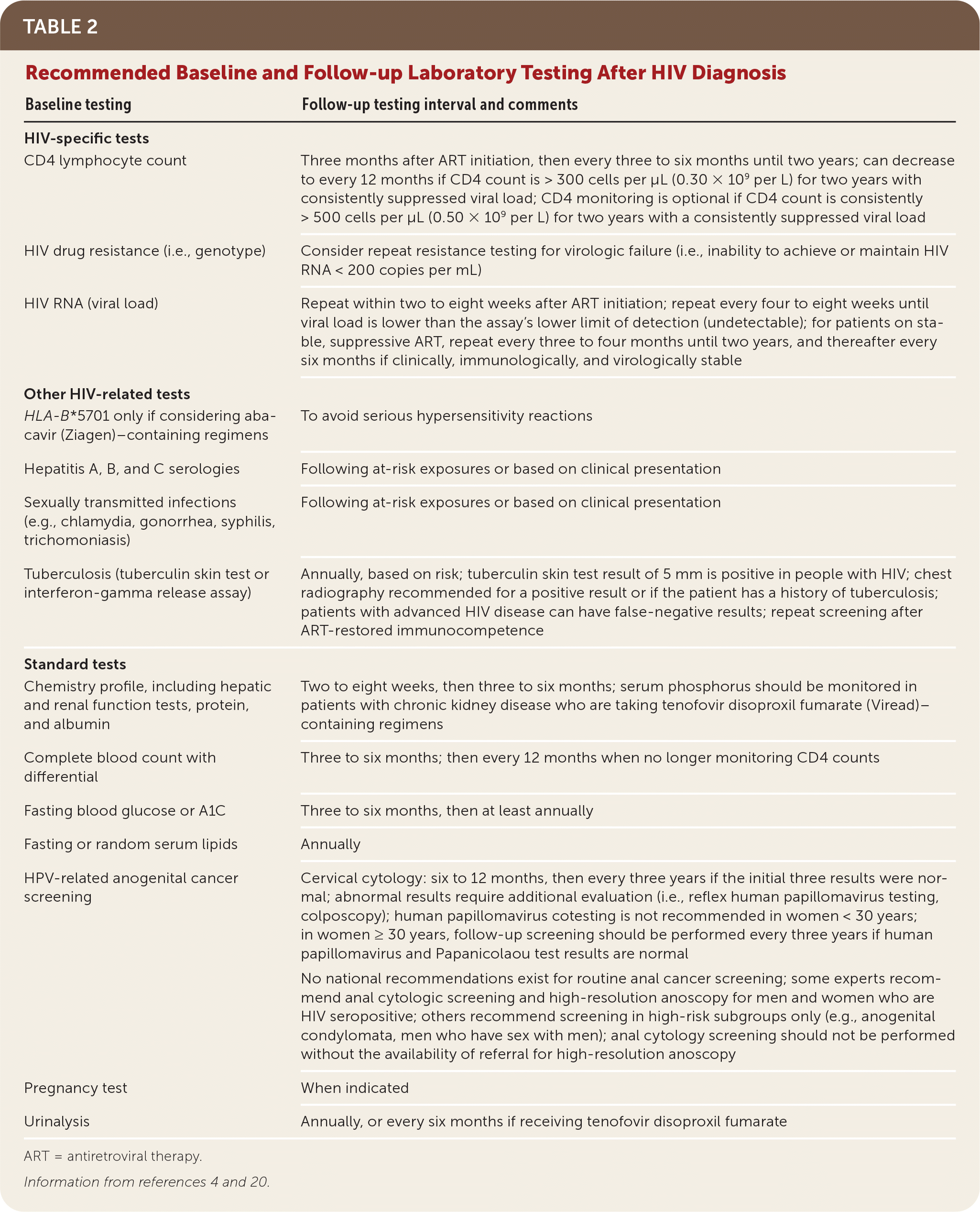
| Baseline testing | Follow-up testing interval and comments |
|---|---|
| HIV-specific tests | |
| CD4 lymphocyte count | Three months after ART initiation, then every three to six months until two years; can decrease to every 12 months if CD4 count is > 300 cells per μL (0.30 × 109 per L) for two years with consistently suppressed viral load; CD4 monitoring is optional if CD4 count is consistently > 500 cells per μL (0.50 × 109 per L) for two years with a consistently suppressed viral load |
| HIV drug resistance (i.e., genotype) | Consider repeat resistance testing for virologic failure (i.e., inability to achieve or maintain HIV RNA < 200 copies per mL) |
| HIV RNA (viral load) | Repeat within two to eight weeks after ART initiation; repeat every four to eight weeks until viral load is lower than the assay's lower limit of detection (undetectable); for patients on stable, suppressive ART, repeat every three to four months until two years, and thereafter every six months if clinically, immunologically, and virologically stable |
| Other HIV-related tests | |
| HLA-B*5701 only if considering abacavir (Ziagen)–containing regimens | To avoid serious hypersensitivity reactions |
| Hepatitis A, B, and C serologies | Following at-risk exposures or based on clinical presentation |
| Sexually transmitted infections (e.g., chlamydia, gonorrhea, syphilis, trichomoniasis) | Following at-risk exposures or based on clinical presentation |
| Tuberculosis (tuberculin skin test or interferon-gamma release assay) | Annually, based on risk; tuberculin skin test result of 5 mm is positive in people with HIV; chest radiography recommended for a positive result or if the patient has a history of tuberculosis; patients with advanced HIV disease can have false-negative results; repeat screening after ART-restored immunocompetence |
| Standard tests | |
| Chemistry profile, including hepatic and renal function tests, protein, and albumin | Two to eight weeks, then three to six months; serum phosphorus should be monitored in patients with chronic kidney disease who are taking tenofovir disoproxil fumarate (Viread)–containing regimens |
| Complete blood count with differential | Three to six months; then every 12 months when no longer monitoring CD4 counts |
| Fasting blood glucose or A1C | Three to six months, then at least annually |
| Fasting or random serum lipids | Annually |
| HPV-related anogenital cancer screening | Cervical cytology: six to 12 months, then every three years if the initial three results were normal; abnormal results require additional evaluation (i.e., reflex human papillomavirus testing, colposcopy); human papillomavirus cotesting is not recommended in women < 30 years; in women ≥ 30 years, follow-up screening should be performed every three years if human papillomavirus and Papanicolaou test results are normal |
| No national recommendations exist for routine anal cancer screening; some experts recommend anal cytologic screening and high-resolution anoscopy for men and women who are HIV seropositive; others recommend screening in high-risk subgroups only (e.g., anogenital condylomata, men who have sex with men); anal cytology screening should not be performed without the availability of referral for high-resolution anoscopy | |
| Pregnancy test | When indicated |
| Urinalysis | Annually, or every six months if receiving tenofovir disoproxil fumarate |
Initial Management
ANTIRETROVIRAL THERAPY
ART should be started as soon as possible after diagnosis to reduce HIV-related morbidity and mortality and reduce transmission risk.4,20 Durable suppression of viremia facilitates immunologic recovery and may mitigate HIV-associated inflammation and immune activation that contributes to end-organ damage. Physicians should regularly emphasize the importance of medication adherence because suboptimal ART adherence can promote HIV drug resistance and complicate or limit future treatment options.
Patients and clinicians should attempt to identify and address potential barriers to treatment and adherence, including concerns for unintended disclosure of HIV status, housing and food instability, difficulties in attending appointments because of transportation challenges or conflicting priorities, and lack of patient readiness and motivation. Psychiatric disorders, substance use, and psychosocial challenges should not be reasons to withhold ART. Patient-centered strategies to support adherence and engagement in care are pivotal to achieving desired health outcomes. It is essential to establish how medical services and treatment costs will be covered. In addition to insurance with or without copays, HIV-specific programs such as the Ryan White HIV/AIDS Treatment Extension Act of 2009 Part B and AIDS Drug Assistance Programs can lower the cost barrier for patients. Social services, case management, HIV education and counseling, and additional support services are often critical for a successful care plan.
ART can be initiated before baseline drug-resistance test results are obtained for patients who are pregnant, patients with acute or recent HIV infection, and when the patient and physician decide to start ART immediately after diagnosis. Benefits of ART initiation immediately or soon after diagnosis include increased patient engagement in care, faster time to virologic suppression, and decreased transmission risk.22 Once drug-resistance testing results are available, treatment can be adjusted if indicated.
Immediate ART initiation may not be advisable if a serious untreated opportunistic infection is present, specifically one involving the central nervous system.4,20 In such cases, opportunistic infection treatment should begin before ART to decrease the risk of immune reconstitution inflammatory syndrome, which is an exaggerated inflammatory reaction resulting from activation of latent infections, malignancies, or other conditions that occur with ART-induced immune system recovery. Expert guidance is recommended.4,23 Patients who are not ready to start ART immediately after diagnosis should be followed closely and supported, with frequent reevaluation of medication readiness and barriers to initiating treatment.
Initial ART selection for patients who have not received treatment previously is guided by patient preference, baseline laboratory results (including hepatic and renal function tests), adverse effect and drug interaction profiles, viral hepatitis coinfection and other comorbidities, and drug allergies (Table 34,20). Once-daily and single-tablet regimens are widely available and covered by many HIV/AIDS drug assistance programs.
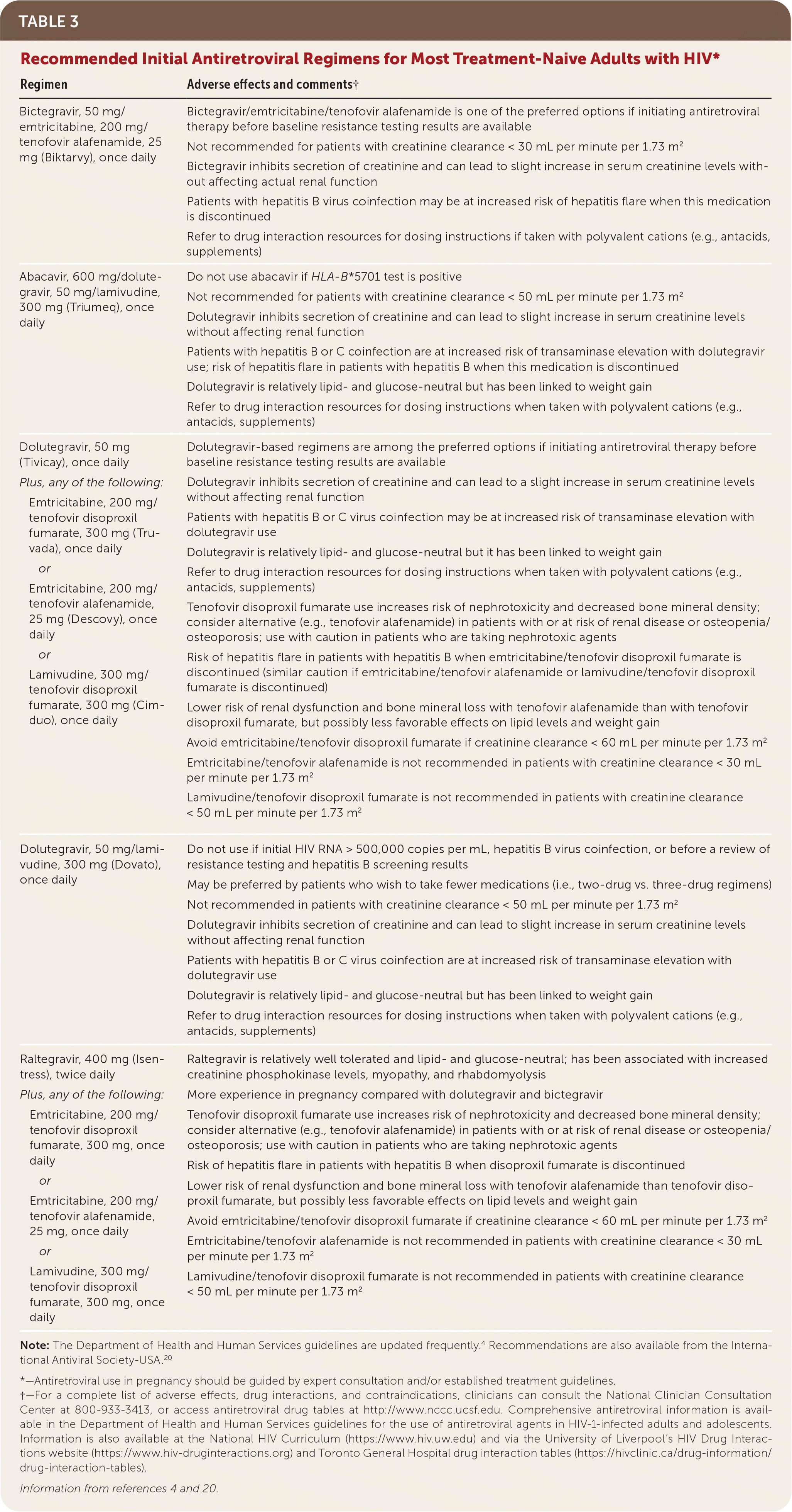
| Regimen | Adverse effects and comments† |
|---|---|
| Bictegravir, 50 mg/emtricitabine, 200 mg/tenofovir alafenamide, 25 mg (Biktarvy), once daily | Bictegravir/emtricitabine/tenofovir alafenamide is one of the preferred options if initiating antiretroviral therapy before baseline resistance testing results are available Not recommended for patients with creatinine clearance < 30 mL per minute per 1.73 m2 Bictegravir inhibits secretion of creatinine and can lead to slight increase in serum creatinine levels without affecting actual renal function Patients with hepatitis B virus coinfection may be at increased risk of hepatitis flare when this medication is discontinued Refer to drug interaction resources for dosing instructions if taken with polyvalent cations (e.g., antacids, supplements) |
| Abacavir, 600 mg/dolute-gravir, 50 mg/lamivudine, 300 mg (Triumeq), once daily | Do not use abacavir if HLA-B*5701 test is positive Not recommended for patients with creatinine clearance < 50 mL per minute per 1.73 m2 Dolutegravir inhibits secretion of creatinine and can lead to slight increase in serum creatinine levels without affecting renal function Patients with hepatitis B or C coinfection are at increased risk of transaminase elevation with dolutegravir use; risk of hepatitis flare in patients with hepatitis B when this medication is discontinued Dolutegravir is relatively lipid- and glucose-neutral but has been linked to weight gain Refer to drug interaction resources for dosing instructions when taken with polyvalent cations (e.g., antacids, supplements) |
| Dolutegravir, 50 mg (Tivicay), once daily Plus, any of the following: Emtricitabine, 200 mg/tenofovir disoproxilfumarate, 300 mg (Truvada), once daily or Emtricitabine, 200 mg/tenofovir alafenamide, 25 mg (Descovy), once daily or Lamivudine, 300 mg/tenofovir disoproxil fumarate, 300 mg (Cimduo), once daily | Dolutegravir-based regimens are among the preferred options if initiating antiretroviral therapy before baseline resistance testing results are available Dolutegravir inhibits secretion of creatinine and can lead to a slight increase in serum creatinine levels without affecting renal function Patients with hepatitis B or C virus coinfection may be at increased risk of transaminase elevation with dolutegravir use Dolutegravir is relatively lipid- and glucose-neutral but it has been linked to weight gain Refer to drug interaction resources for dosing instructions when taken with polyvalent cations (e.g., antacids, supplements) Tenofovir disoproxil fumarate use increases risk of nephrotoxicity and decreased bone mineral density; consider alternative (e.g., tenofovir alafenamide) in patients with or at risk of renal disease or osteopenia/osteoporosis; use with caution in patients who are taking nephrotoxic agents Risk of hepatitis flare in patients with hepatitis B when emtricitabine/tenofovir disoproxil fumarate is discontinued (similar caution if emtricitabine/tenofovir alafenamide or lamivudine/tenofovir disoproxil fumarate is discontinued) Lower risk of renal dysfunction and bone mineral loss with tenofovir alafenamide than with tenofovir disoproxil fumarate, but possibly less favorable effects on lipid levels and weight gain Avoid emtricitabine/tenofovir disoproxil fumarate if creatinine clearance < 60 mL per minute per 1.73 m2 Emtricitabine/tenofovir alafenamide is not recommended in patients with creatinine clearance < 30 mL per minute per 1.73 m2 Lamivudine/tenofovir disoproxil fumarate is not recommended in patients with creatinine clearance < 50 mL per minute per 1.73 m2 |
| Dolutegravir, 50 mg/lamivudine, 300 mg (Dovato), once daily | Do not use if initial HIV RNA > 500,000 copies per mL, hepatitis B virus coinfection, or before a review of resistance testing and hepatitis B screening results May be preferred by patients who wish to take fewer medications (i.e., two-drug vs. three-drug regimens) Not recommended in patients with creatinine clearance < 50 mL per minute per 1.73 m2 Dolutegravir inhibits secretion of creatinine and can lead to slight increase in serum creatinine levels without affecting renal function Patients with hepatitis B or C virus coinfection are at increased risk of transaminase elevation with dolutegravir use Dolutegravir is relatively lipid- and glucose-neutral but has been linked to weight gain Refer to drug interaction resources for dosing instructions when taken with polyvalent cations (e.g., antacids, supplements) |
| Raltegravir, 400 mg (Isentress), twice daily Plus, any of the following: Emtricitabine, 200 mg/tenofovir disoproxil fumarate, 300 mg, once daily or Emtricitabine, 200 mg/tenofovir alafenamide, 25 mg, once daily or Lamivudine, 300 mg/tenofovir disoproxil fumarate, 300 mg, once daily | Raltegravir is relatively well tolerated and lipid- and glucose-neutral; has been associated with increased creatinine phosphokinase levels, myopathy, and rhabdomyolysis More experience in pregnancy compared with dolutegravir and bictegravir Tenofovir disoproxil fumarate use increases risk of nephrotoxicity and decreased bone mineral density; consider alternative (e.g., tenofovir alafenamide) in patients with or at risk of renal disease or osteopenia/osteoporosis; use with caution in patients who are taking nephrotoxic agents Risk of hepatitis flare in patients with hepatitis B when disoproxil fumarate is discontinued Lower risk of renal dysfunction and bone mineral loss with tenofovir alafenamide than tenofovir disoproxil fumarate, but possibly less favorable effects on lipid levels and weight gain Avoid emtricitabine/tenofovir disoproxil fumarate if creatinine clearance < 60 mL per minute per 1.73 m2 Emtricitabine/tenofovir alafenamide is not recommended in patients with creatinine clearance < 30 mL per minute per 1.73 m2 Lamivudine/tenofovir disoproxil fumarate is not recommended in patients with creatinine clearance < 50 mL per minute per 1.73 m2 |
ART response is monitored by sequential measurement of viral load and CD4 counts. Optimal virologic suppression is a confirmed HIV RNA level persistently below the lower limit of detection (undetectable), which varies by assay. Undetectable levels are typically achieved in eight to 24 weeks, depending on pretreatment viral load and ART selected. Failure to achieve sustained virologic suppression by 24 weeks should prompt an assessment of adherence and ART drug absorption or interactions. CD4 count is less helpful for assessing initial response to ART because changes in CD4 count predictably follow changes in viral load. Table 2 provides recommended intervals for laboratory monitoring.4,20 Patients with low CD4 counts starting or resuming ART should be monitored closely for immune reconstitution inflammatory syndrome during the first weeks to months after treatment initiation.4,20
OPPORTUNISTIC INFECTIONS
Primary chemoprophylaxis against P. jiroveci pneumonia is recommended for people with CD4 counts less than 200 cells per μL and can be discontinued when CD4 counts increase with treatment to greater than 200 cells per μL for at least three months.23 Patients with CD4 counts less than 100 cells per μL (0.10 × 109 per L) who are Toxoplasma seropositive require chemoprophylaxis against Toxoplasma gondii23; trimethoprim/sulfamethoxazole or atovaquone (Mepron) offers sufficient prophylaxis against both. Primary prophylaxis against disseminated Mycobacterium avium is unnecessary for people with HIV who immediately initiate ART, but it is recommended for people who have HIV with CD4 counts less than 50 cells per μL (0.05 × 109 per L) who are not receiving ART.
IMMUNIZATIONS
Table 4 lists recommended immunizations for certain vaccine-preventable conditions (e.g., community-acquired pneumonia, hepatitis B).23,24 Live vaccines should be avoided in people with CD4 counts less than 200 cells per μL. Additionally, people who have HIV with low CD4 counts may have suboptimal vaccination responses. Updated guidance on vaccination management for people who have HIV with low CD4 counts is available.24

| Vaccine | Indication |
|---|---|
| Hepatitis A | All susceptible people with HIV; single preparation (Twinrix) available if administering with a hepatitis B vaccine |
| Hepatitis B* | All susceptible people with HIV Not required if: hepatitis B surface antibody and core antibodies are both positive or Hepatitis B surface antibody is positive after a previous complete vaccine series See guidelines for serologic scenarios, immunization schedules, and preparations (e.g., two- or three-dose series)† |
| Herpes zoster | Recombinant zoster vaccine (Shingrix) recommended for people ≥ 50 years |
| Human papillomavirus | Standard schedule; if not fully immunized, a catch-up immunization is indicated through 26 years of age; shared decision-making for 27 to 45 years of age |
| Influenza (inactivated only) | Standard schedule; high dose usually recommended; live attenuated influenza vaccine contraindicated |
| Meningococcal | All people with HIV |
| Pneumococcal | All people with HIV‡ |
| Tetanus toxoid, reduced diphtheria toxoid, acellular pertussis (Tdap) | Standard schedule |
PSYCHOSOCIAL ASPECTS OF HIV PRIMARY CARE
Psychosocial factors can be central to developing therapeutic relationships with patients and identifying strategies to provide long-term, person-centered care. Psychosocial factors include consideration of the patient's social, family, and community support networks; housing, financial, and employment status; access to health insurance and medication coverage; concerns about stigma and unwanted disclosure; a history of trauma or interpersonal violence; and impact of racial and gender bias on their health and health care.25–27 Assessing how patients self-identify (he/she/they) and knowing whether and how to share that information with family members or associates can be extremely beneficial in developing a therapeutic relationship.
IMPORTANT CO-OCCURRING CONDITIONS
Coinfection with hepatitis B or C should be treated per established guidelines, with attention to drug-drug interactions.28,29 Some HIV antiretroviral drugs are also active against hepatitis B; if these medications are discontinued, a hepatitis B flare can occur with significant transaminase elevation. Standard hepatocellular carcinoma screening should be performed if indicated.30
Substance use can impact treatment adherence and engagement in care. Because substance use disproportionately affects people with HIV, all patients should be screened and treated for substance use disorders.
HIV infection itself, and some antiretroviral medications, can impair multiple organ systems and lead to renal and hepatic disease; cognitive, motor, sensory, and neurologic deficits; osteopenia or osteoporosis; metabolic complications including diabetes mellitus and dyslipidemia; and chronic cardiopulmonary disorders.25,31
PREGNANT PEOPLE AND OLDER ADULTS
Perinatal HIV transmission is rare in the United States because of high rates of antenatal HIV screening and effective ART use through pregnancy and delivery. All pregnant people should be screened for HIV.12,14 Pregnant people with HIV and people planning to conceive should maintain continuous, suppressive ART throughout pregnancy to reduce the risk of perinatal transmission.32 Because the postpartum period has unique challenges, clinicians are encouraged to coordinate early with other clinicians and health care team members (e.g., case managers, social workers, adherence counselors) to provide continuing care after delivery.33,34 Perinatally-exposed infants are generally managed in conjunction with pediatric HIV or infectious disease specialists.
Older adults can present for initial care with recently diagnosed HIV or with chronic infection reentering care. Management in older adults is often complicated by neurocognitive deficits, frailty, polypharmacy, multimorbidity, and social isolation. Therefore, older patients with HIV may require increased monitoring for medication adherence and complications. Assessing the family's health concerns can help guide care, including coordination with caregivers to clarify function and goals of care.35
Preventing Transmission
Primary care clinicians are well positioned to effectively prevent HIV transmission by initiating and maintaining effective ART because combination ART-induced viral load suppression dramatically reduces the risk of sexual transmission to partners who are seronegative.5–8 Patients should be counseled on safer sex practices to prevent sexually transmitted infections. For patients who use drugs, information on harm reduction measures and substance use disorder treatment associated with decreased HIV transmission risk, can be invaluable.36–38
Family physicians are able to offer HIV pre- and postexposure prophylaxis for people at risk.15–18 Preexposure prophylaxis is recommended for people in several risk categories, including seronegative partners of people with HIV. The U.S. Preventive Services Task Force recommendation criteria for preexposure prophylaxis have been published in American Family Physician (AFP; https://www.aafp.org/afp/2019/1115/od1.html). Postexposure prophylaxis is recommended as soon as possible and no later than 72 hours after nonoccupational exposure to potentially infectious bodily fluids from a person with HIV. AFP published a summary of the Centers for Disease Control and Prevention guidelines for postexposure prophylaxis (https://www.aafp.org/afp/2016/0901/p392.html). Other clinical resources are listed in Table 5.
| Resource | Contact information | Comments |
|---|---|---|
| AIDS Education and Training Centers Program | https://aidsetc.org/ | Webinars, courses, and training for preventing and treating HIV |
| HIV Info | https://aidsinfo.nih.gov | Department of Health and Human Services practice guidelines |
| International Antiviral Society–USA | https://www.iasusa.org | Webinars, courses |
| National Clinician Consultation Center, HIV/AIDS Management | https://nccc.ucsf.edu 800-933-3413 | Information and guidance for clinicians, available free from experts in HIV prevention, treatment, and HIV comorbidities including viral hepatitis and substance use; most calls are answered live and provide guidance at the point of care |
| National Clinician Consultation Center, Hepatitis C | 844-437-4636 | Managing HIV and hepatitis C coinfections |
| National Clinician Consultation Center, Perinatal HIV/AIDS Consultation | 888-448-8765 | Managing HIV in pregnancy, preventing perinatal transmission, caring for HIV-exposed infants |
| National HIV Curriculum | https://www.hiv.uw.edu | Online modules and summaries of core HIV information |
| Postexposure Prophylaxis Hotline (PEPline) | 888-448-4911 | Managing occupational and nonoccupational exposures |
| Preexposure Prophylaxis Hotline (PrEPline) | 855-448-7737 | Providing preexposure prophylaxis |
| Substance Use Consultation | 855-300-3595 | Managing co-occurring substance use |
This article updates previous articles on this topic by Khalsa,39 Romanelli and Matheny,40 and Goldschmidt, et al.41
Data Sources: A PubMed search was completed in Clinical Queries using the key terms HIV, AIDS, primary care, and guidelines. The search included meta analyses, randomized controlled trials, clinical trials, guidelines, and reviews. We performed specific searches on key guidelines websites, including: https://hivinfo.nih.gov; https://www.cdc.gov; and https://www.cdc.gov/mmwr; the Cochrane database; Essential Evidence Plus; and https://www.aafp.org/journals/afp. Search dates: June 3 to 5, June 27, and July 11 to 20, 2020.
This article was made possible by the AIDS Education and Training Center Program grant award #U1OHA30039 from the Health Resources and Services Administration, U.S. Department of Health and Human Services, with additional funding from the Centers for Disease Control and Prevention. The information presented is the responsibility of the authors and does not necessarily represent the official view of the Health Resources and Services Administration, the AIDS Education and Training Center Program, or the Centers for Disease Control and Prevention.