
A more recent article on common intestinal parasites is available.
Am Fam Physician. 2004;69(5):1161-1169
Intestinal parasites cause significant morbidity and mortality. Diseases caused by Enterobius vermicularis, Giardia lamblia, Ancylostoma duodenale, Necator americanus, and Entamoeba histolytica occur in the United States. E. vermicularis, or pinworm, causes irritation and sleep disturbances. Diagnosis can be made using the “cellophane tape test.” Treatment includes mebendazole and household sanitation. Giardia causes nausea, vomiting, malabsorption, diarrhea, and weight loss. Stool ova and parasite studies are diagnostic. Treatment includes metronidazole. Sewage treatment, proper handwashing, and consumption of bottled water can be preventive. A. duodenale and N. americanus are hookworms that cause blood loss, anemia, pica, and wasting. Finding eggs in the feces is diagnostic. Treatments include albendazole, mebendazole, pyrantel pamoate, iron supplementation, and blood transfusion. Preventive measures include wearing shoes and treating sewage. E. histolytica can cause intestinal ulcerations, bloody diarrhea, weight loss, fever, gastrointestinal obstruction, and peritonitis. Amebas can cause abscesses in the liver that may rupture into the pleural space, peritoneum, or pericardium. Stool and serologic assays, biopsy, barium studies, and liver imaging have diagnostic merit. Therapy includes luminal and tissue amebicides to attack both life-cycle stages. Metronidazole, chloroquine, and aspiration are treatments for liver abscess. Careful sanitation and use of peeled foods and bottled water are preventive.
Intestinal parasites cause significant morbidity and mortality throughout the world, particularly in undeveloped countries and in persons with comorbidities. Intestinal parasites that remain prevalent in the United States include Enterobius vermicularis, Giardia lamblia, Ancylostoma duodenale, Necator americanus, and Entamoeba histolytica.
E. vermicularis
E. vermicularis, commonly referred to as the pinworm or seatworm, is a nematode, or roundworm, with the largest geographic range of any helminth.1 It is the most prevalent nematode in the United States. Humans are the only known host, and about 209 million persons worldwide are infected. More than 30 percent of children worldwide are infected.2
Adult worms are quite small; the males measure 2 to 5 mm, and the females measure 8 to 13 mm. The worms live primarily in the cecum of the large intestine, from which the gravid female migrates at night to lay up to 15,000 eggs on the perineum. The eggs can be spread by the fecal-oral route to the original host and new hosts. Eggs on the host's perineum can spread to other persons in the house, possibly resulting in an entire family becoming infected.
Ingested eggs hatch in the duodenum, and larvae mature during their migration to the large intestine. Fortunately, most eggs desiccate within 72 hours. In the absence of host autoinfection, infestation usually lasts only four to six weeks.
Disease secondary to E. vermicularis is relatively innocuous, with egg deposition causing perineal, perianal, and vaginal irritation.3 The patient's constant itching in an attempt to relieve irritation can lead to potentially debilitating sleep disturbance. Rarely, more serious disease can result, including weight loss, urinary tract infection, and appendicitis.4,5
Pinworm infection should be suspected in children who exhibit perianal pruritus and nocturnal restlessness. Direct visualization of the adult worm or microscopic detection of eggs confirms the diagnosis, but only 5 percent of infected persons have eggs in their stool. The “cellophane tape test” (Figure 1) can serve as a quick way to clinch the diagnosis.6,7
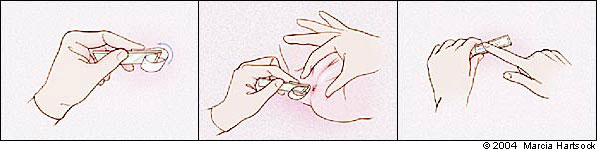
This test consists of touching tape to the perianal area several times, removing it, and examining the tape under direct microscopy for eggs. The test should be conducted right after awakening on at least three consecutive days. This technique can increase the test's sensitivity to roughly 90 percent.
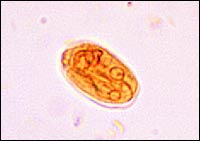
G. lamblia
G. lamblia is a pear-shaped, flagellated protozoan (Figure 2) that causes a wide variety of gastrointestinal complaints. Giardia is arguably the most common parasite infection of humans worldwide, and the second most common in the United States after pin-worm.8,9 Between 1992 and 1997, the Centers for Disease Control and Prevention (CDC) estimated that more than 2.5 million cases of giardiasis occur annually.10
Because giardiasis is spread by fecal-oral contamination, the prevalence is higher in populations with poor sanitation, close contact, and oral-anal sexual practices. The disease is commonly water-borne because Giardia is resistant to the chlorine levels in normal tap water and survives well in cold mountain streams. Because giardiasis frequently infects persons who spend a lot of time camping, backpacking, or hunting, it has gained the nicknames of “backpacker's diarrhea” and “beaver fever.”11
Food-borne transmission is rare but can occur with ingestion of raw or undercooked foods. Giardiasis is a zoonosis, and cross-infectivity among beaver, cattle, dogs, rodents, and bighorn sheep ensures a constant reservoir.12
The life cycle of Giardia consists of two stages: the fecal-orally transmitted cyst and the disease-causing trophozoite. Cysts are passed in a host's feces, remaining viable in a moist environment for months. Ingestion of at least 10 to 25 cysts can cause infection in humans.8,9 When a new host consumes a cyst, the host's acidic stomach environment stimulates excystation. Each cyst produces two trophozoites. These trophozoites migrate to the duodenum and proximal jejunum, where they attach to the mucosal wall by means of a ventral adhesive disk and replicate by binary fission.
Giardia growth in the small intestine is stimulated by bile, carbohydrates, and low oxygen tension.7 It can cause dyspepsia, mal-absorption, and diarrhea. A recent theory suggests that the symptoms are the result of a brush border enzyme deficiency rather than invasion of the intestinal wall.9 Some trophozoites transform to cysts and pass in the feces.
Clinical presentations of giardiasis vary greatly. After an incubation period of one to two weeks, symptoms of gastrointestinal distress may develop, including nausea, vomiting, malaise, flatulence, cramping, diarrhea, steatorrhea, and weight loss. A history of gradual onset of a mild diarrhea helps differentiate giardiasis or other parasite infections from bacterial etiologies. Symptoms lasting two to four weeks and significant weight loss are key findings that indicate giardiasis.
Chronic giardiasis may follow an acute syndrome or present without severe antecedent symptoms. Chronic signs and symptoms such as loose stool, steatorrhea, a 10 to 20 percent loss in weight, malabsorption, malaise, fatigue, and depression may wax and wane over many months if the condition is not treated.
Rarely, patients with giardiasis also present with reactive arthritis or asymmetric synovitis, usually of the lower extremities.13 Rashes and urticaria may be present as part of a hypersensitivity reaction.
Cyst excretion occurs intermittently in both formed and loose stools, while trophozoites are almost only found in diarrhea. Stool studies for ova and parasites (O&P) continue to be a mainstay of diagnosis despite only low to moderate sensitivity. Examination of a single stool specimen has a sensitivity of 50 to 70 percent; the sensitivity increases to 85 to 90 percent with three serial specimens.8,10 Because Giardia is not invasive, eosinophilia, and peripheral or fecal leukocytosis do not occur.
Antigen assays use enzyme-linked immunosorbent assay (ELISA) or immunofluo-rescence to detect antibodies to trophozoites or cysts. Sensitivities range from 90 to 99 percent, with specificities of 95 to 100 percent compared with stool O&P.9 Despite the high yield of these studies, direct microscopy is still important, because multiple diarrhea-causing infectious etiologies can be present simultaneously.
Duodenal aspirates and biopsies give a higher yield than stool studies but are invasive and usually not necessary for diagnosis. Serology and stool cultures are generally unnecessary. Polymerase chain reaction (PCR) analysis, while only experimental, may be effective for screening water supplies.9
A. duodenale and N. americanus
Two species of hookworm, A. duodenale and N. americanus, are found exclusively in humans. A. duodenale, or “Old World” hookworm, is found in Europe, Africa, China, Japan, India, and the Pacific islands. N. americanus, the “New World” hookworm, is found in the Americas and the Caribbean, and has recently been reported in Africa, Asia, and the Pacific.
Until the early 1900s, N. americanus infestation was endemic in the southern United States and was only controlled after the widespread use of modern plumbing and footwear. Even though the prevalence of these parasites has drastically decreased in the general population, the CDC reports that in the United States, hookworm infection is the second most common helminthic infection identified in stool studies.14
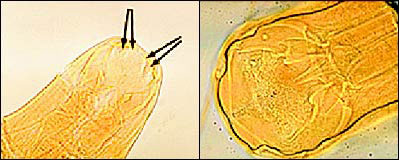
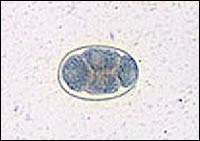
N. americanus ranges from 10 to 12 mm in length for females and 6 to 8 mm for males. It is distinguished from its slightly larger European cousin by its semilunar dorsal and ventral cutting plates at the buccal cavity compared with A. duodenale's two pairs of ventral cutting teeth (Figure 3). The eggs of both worms are 60 to 70 μm in length and bounded by an ovoid transparent hyaline membrane; they contain two to eight cell divisions (Figure 4).
Both species share a common life cycle. Eggs hatch into rhabditiform larvae, feed on bacteria in soil, and molt into the infective filariform larvae. Enabled by moist climates and poor hygiene, filariform larvae enter their hosts through pores, hair follicles, and even intact skin. Maturing larvae travel through the circulation system until they reach alveolar capillaries. Breaking into lung parenchyma, the larvae climb the bronchial tree and are swallowed with secretions. Six weeks after the initial infection, mature worms have attached to the wall of the small intestine to feed, and egg production begins.
While larvae occasionally cause pruritic erythema or pulmonary symptoms during their migration to the gut,15 hookworm infection rarely is symptomatic until a significant intestinal worm burden is established. A transient gastroenteritis-like syndrome can occur because mature worms attach to the intestinal mucosa.
The greatest concern from infection is blood loss. Aided by an organic anticoagulant, a hookworm consumes about 0.25 mL of host blood per day. The blood loss caused by hookworms can produce a microcytic hypochromic anemia.16 Compensatory volume expansion contributes to hypoproteinemia, edema, pica, and wasting. The infection may result in physical and mental retardation in children. Eosinophilia has been noted in 30 to 60 percent of infected patients.
While clinical history, hygiene status, and recent travel to endemic areas can give important clues, definitive diagnosis rests on microscopic visualization of eggs in the stool.
E. histolytica
Amebiasis is caused by E. histolytica, a protozoan that is 10 to 60 μm in length and moves through the extension of finger-like pseudo-pods.1 Spreading occurs via the fecal-oral route, usually by poor hygiene during food preparation or by the use of “night soil” (crop fertilization with human waste), as well as by oral-anal sexual practices. Spreading is frequent in persons who have a deficient immune system. Crowding and poor sanitation contribute to its prevalence in Asia, Africa, and Latin America. Approximately 10 percent of the world's population is infected, yet 90 percent of infected persons are asymptomatic.17 Of the roughly 50 million symptomatic cases occurring each year, up to 100,000 are fatal.18 The stable reservoir of infective cases complicates eradication. After malaria, it is likely that E. histolytica is the world's second leading protozoan cause of death.19
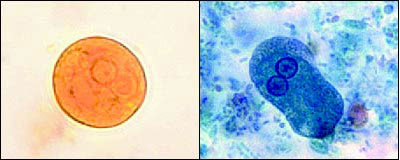
Much like Giardia, the two stages in the E. histolytica life cycle are cysts and trophozoites (Figure 5). Infective cysts are spheres of about 12 μm in diameter that have one to four nuclei and can be spread via the fecal-oral route by contaminated food and water or oral-anal sexual practices. The pseudopodal trophozoite is about 25 μm across, has a single nucleus, and may contain red blood cells of the host in various stages of digestion. Ingested cysts hatch into trophozoites in the small intestine and continue moving down the digestive tract to the colon. Also like Giardia, some ameba trophozoites become cysts that are passed in the stool and can survive for weeks in a moist environment. However, trophozoites can invade the intestinal mucosa and spread in the bloodstream to the liver, lung, and brain.
Amebiasis can cause both intraluminal and disseminated disease. In the intestinal lumen, E. histolytica can disrupt the protective mucus layer overlying the colonic mucosa. The resulting epithelial ulcerations can bleed and cause colitis,20 usually two to six weeks after initial infection. Acute progression to malaise, weight loss, severe abdominal pain, profuse bloody diarrhea, and fever can occur, often leading to a misdiagnosis of appendicitis, especially in children. In chronic smoldering cases, inflammatory bowel disease can be misdiagnosed, and treatment with steroids only exacerbates the infection.
Tissue penetration and dissemination are possible. Trophozoites that penetrate the intestinal wall spread through the body via the portal circulation. Amebas are chemotactic, attracting neutrophils in the circulation. Amebic liver abscesses form because of toxin release and hepatocyte damage, and usually develop within five months after infection. Symptoms of a developing abscess include fever, dull pleuritic right upper quadrant pain radiating to the right shoulder, and pleural effusions. Diarrhea is present in only one of three patients with abscess. Fever is the presenting symptom in 10 to 15 percent of patients, and therefore amebic abscess should be considered in patients with a fever of unknown origin. Abscesses may rupture into the pleural space, peritoneum, or pericardium, requiring emergency drainage.
Traditional O&P stool testing for amebiasis should use at least three fresh samples to increase sensitivity. However, this test has recently fallen out of favor18 because an E. histolytica stool antigen test with a sensitivity of 87 percent and a specificity of more than 90 percent has become available.19 Stool culture and PCR testing modalities used in research are not yet sufficiently widespread to be clinically useful. Positive stool samples are likely to be heme positive and to have low neutrophils but may contain Charcot-Leyden crystals, indicating the presence of eosinophils. Biopsy of colonic ulcer edges may yield intramural trophozoites but carries with it the risk of perforation.
Serologic tests such as ELISA and agar gel diffusion are more than 90 percent sensitive, but these tests often become negative within a year of initial infection. Approximately 75 percent of infected patients have leukocytosis, but mucosal invasion does not cause eosinophilia. Liver function tests usually are normal but may show minimal elevation of alkaline phosphatase, even in the presence of large abscesses. To avoid misdiagnosis, patients with suspected ulcerative colitis must be tested for E. histolytica antibodies before starting steroid therapy.17
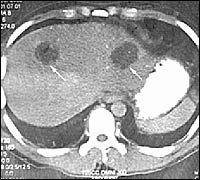
Intestinal barium studies may be useful in identifying possible amebomas, but biopsy is required to confirm the diagnosis and rule out neoplasia. Liver imaging studies, such as ultra-sonography, computed tomography (Figure 6), magnetic resonance imaging, and nuclear medicine scans, can reveal abscesses as oval or round hypoechoic cysts, usually in the right lobe of the liver.21
Risk of complications increases with cysts of more than 10 cm, multiple cysts, superior right lobe involvement, or any left lobe involvement. Repeat studies may be confusing by showing larger abscesses in improving patients. Although two thirds of abscesses resolve within six months, approximately 10 percent of abscesses persist for more than a year.17
| Parasite | Treatment | Prevention | |||
|---|---|---|---|---|---|
| Enterobius vermicularis | Primary: Mebendazole (Vermox), 100 mg orally once Secondary: Pyrantel pamoate (Pin-Rid), 11 mg per kg (maximum of 1 g) orally once; or albendazole (Valbazen), 400 mg orally once If persistent, repeat treatment in two weeks. Do not give to children younger than two years. | Treat household contacts. Clean bedrooms, bedding. | |||
| Giardia lamblia | Adults: Metronidazole (Flagyl), 250 mg orally three times daily for five to seven days Pregnant women with mild symptoms: consider deferring treatment until after delivery. Pregnant women with severe symptoms: paromomycin (Humatin), 500 mg orally four times daily for seven to 10 days; metronidazole is acceptable. Children: albendazole, 400 mg orally for five days Asymptomatic carriers in developed countries: treat using regimen for adults or children. Asymptomatic carriers in developing countries: not cost-effective to treat because of high reinfection rate. | Use proper sewage disposal and water treatment (flocculation, sedimentation, filtration, and chlorination). Consume only bottled water in endemic areas. Water treatment options: Boil water for one minute Heat water to 70 C (158 F) for 10 minutes Portable camping filter Iodine purification tablets for eight hours Daycare centers: Proper disposal of diapers Proper and frequent handwashing | |||
| Ancylostoma duodenale, Necator americanus | Albendazole, 400 mg orally once Mebendazole, 100 mg orally twice daily for three days Pyrantel pamoate, 11 mg per kg (maximum of 1 g) once Iron supplementation is beneficial even before diagnosis or treatment initiation. Packed red blood cells (as needed) can minimize risk of volume overload in severely hypoproteinemic patients. Confirm eradication with follow-up stool examination two weeks after discontinuation of treatment. | Use proper and continued shoe wear. Use proper sewage disposal. | |||
| Entamoeba histolytica | Intestinal disease: use both luminal amebicide (for cysts) and tissue amebicide (for trophozoites) | Use proper sanitation to eradicate cyst carriage. Avoid eating unpeeled fruits and vegetables. Drink bottled water. Use iodine disinfection of nonbottled water. | |||
| Luminal: | |||||
| Iodoquinol (Yodoxin), 650 mg orally three times daily for 20 days | |||||
| or | |||||
| Paromomycin, 500 mg orally three times daily for seven days | |||||
| or | |||||
| Diloxanide furoate (Furamide), 500 mg orally three times daily for 10 days (available from CDC) | |||||
| Tissue: | |||||
| Metronidazole, 750 mg orally three times daily for 10 days | |||||
| Liver abscess: | |||||
| Metronidazole, 750 mg orally three times daily for five days, then paromomycin, 500 mg three times daily for seven days | |||||
| or | |||||
| Chloroquine (Aralen), 600 mg orally per day for two days, then 200 mg orally per day for two to three weeks (higher relapse rates) | |||||
| Aspirate if: | |||||
| Pyogenic abscess is ruled out; there is no response to treatment in three to five days; rupture is imminent; pericardial spread is imminent | |||||
Treatment and Prevention
| Amebicidal agent | Advantages | Disadvantages | |
|---|---|---|---|
| Luminal amebicides | |||
| Paromomycin (Humatin) | Seven-day treatment course; may be useful during pregnancy | Frequent GI disturbances; rare ototoxicity and nephrotoxicity; expensive | |
| Iodoquinol (Yodoxin) | Inexpensive and effective | 20-day treatment course; contains iodine; rare optic neuritis and atrophy with prolonged use | |
| Diloxanide furoate (Furamide) | Alternative to paromomycin if unable to tolerate | Available in United States only from the CDC; frequent GI disturbances; rare diplopia; contraindicated in pregnant women | |
| For invasive intestinal disease only | |||
| Tetracycline, erythromycin | Alternative to metronidazole (Flagyl) if unable to tolerate | Not active for liver abscesses; frequent GI disturbances; tetracycline should not be administered to children or pregnant women; must be used with luminal agent | |
| For invasive intestinal and extraintestinal amebiasis | |||
| Metronidazole | Drug of choice for amebic colitis and liver abscess | Anorexia, nausea, vomiting, and metallic taste in nearly one third of patients at dosages used; disulfiram-like reaction with alcohol; rare seizures | |
| Chloroquine (Aralen) | Useful only for amebic liver abscess | Occasional headache, pruritus, nausea, alopecia, and myalgias; rare heart block and irreversible retinal injury | |