Am Fam Physician. 2013;88(7):441-450
Related: Editor's Note
Related letter: Clarification Regarding AFP's Conflict of Interest Policy
Author disclosure: Dr. Marghoob reports receiving dermoscope prototypes for testing from the four major manufacturers of this device; receiving honoraria for speaking on the topic of dermoscopy; and participating in Institutional Review Board–approved research projects funded by the National Institutes of Health and Melanoma Research Alliance, some of which partnered with companies that produce dermoscopes. Drs. Usatine and Jaimes report no financial affiliations.
Dermoscopy has been shown to increase the clinician's diagnostic accuracy when evaluating cutaneous neoplasms. Two types of dermatoscopes are currently used: nonpolarized, which requires direct skin contact, and polarized, which does not require direct skin contact. A two-step algorithm for dermoscopic evaluation of skin lesions guides the clinician through the diagnosis and management decision-making process.
Noninvasive in vivo imaging techniques have become an important diagnostic aid for skin cancer detection. Dermoscopy, also known as dermatoscopy, epiluminescence microscopy, incident light microscopy, or skin surface microscopy, is performed using a handheld instrument called a dermatoscope or dermoscope, which has a transilluminating light source and standard magnifying optics (10×). The dermatoscope facilitates visualization of subsurface skin structures located within the epidermis, dermoepidermal junction, and papillary dermis, which are otherwise not visible to the unaided eye.1,2
Traditionally, dermoscopy has been taught to and used by dermatologists; however, it is gaining popularity in other fields of medicine, including primary care.3–5 Because many patients are seen routinely in the primary care setting, family physicians play an important role in screening for skin cancer and early detection of skin cancer. Dermoscopy has been shown to improve diagnostic accuracy, sensitivity, and specificity for skin cancer diagnosis by dermatologists.6 Once thought to be a subspecialist tool, the literature has shown that family physicians can also improve their diagnostic accuracy and triage ability by using dermoscopy.3–5,7–11
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Dermoscopy aids clinical examination in differentiating melanoma and basal cell carcinoma from benign skin lesions. | C | 6, 17, 20 |
| The first step in the two-step algorithm for dermoscopy is intended to help differentiate melanocytic lesions from nonmelanocytic lesions; however, its main objective is to prevent clinicians from missing melanomas. | C | 36, 39 |
| The second step in the two-step algorithm for dermoscopy is intended to help differentiate nevi from melanoma. | C | 36 |
Techniques
During naked eye examinations of the skin, much of the light transmitted toward the skin is reflected off the stratum corneum, thus precluding the observer from seeing structures located below this layer. Dermatoscopes illuminate the skin via the use of light-emitting diode bulbs, with or without the use of polarizing filters. Dermatoscopes using nonpolarized light require direct contact between the skin and the scope, and require a liquid interface, such as ultrasound gel or alcohol, to be placed between the skin and glass plate of the dermatoscope.12
Once the liquid interface has been applied to the skin, the scope can be placed on top of the lesion and gently pressed against the skin; it is imperative that enough pressure be applied to eliminate air bubbles. The liquid interface prevents light from being reflected off the stratum corneum and improves refraction, thereby allowing the visualization of structures below the stratum corneum. Dermatoscopes equipped with cross-polarized filters obviate the need for direct skin contact and a liquid interface.13 Cross-polarized filters eliminate the glare off the skin surface and increase light refraction, allowing the observer to visualize deeper skin structures.
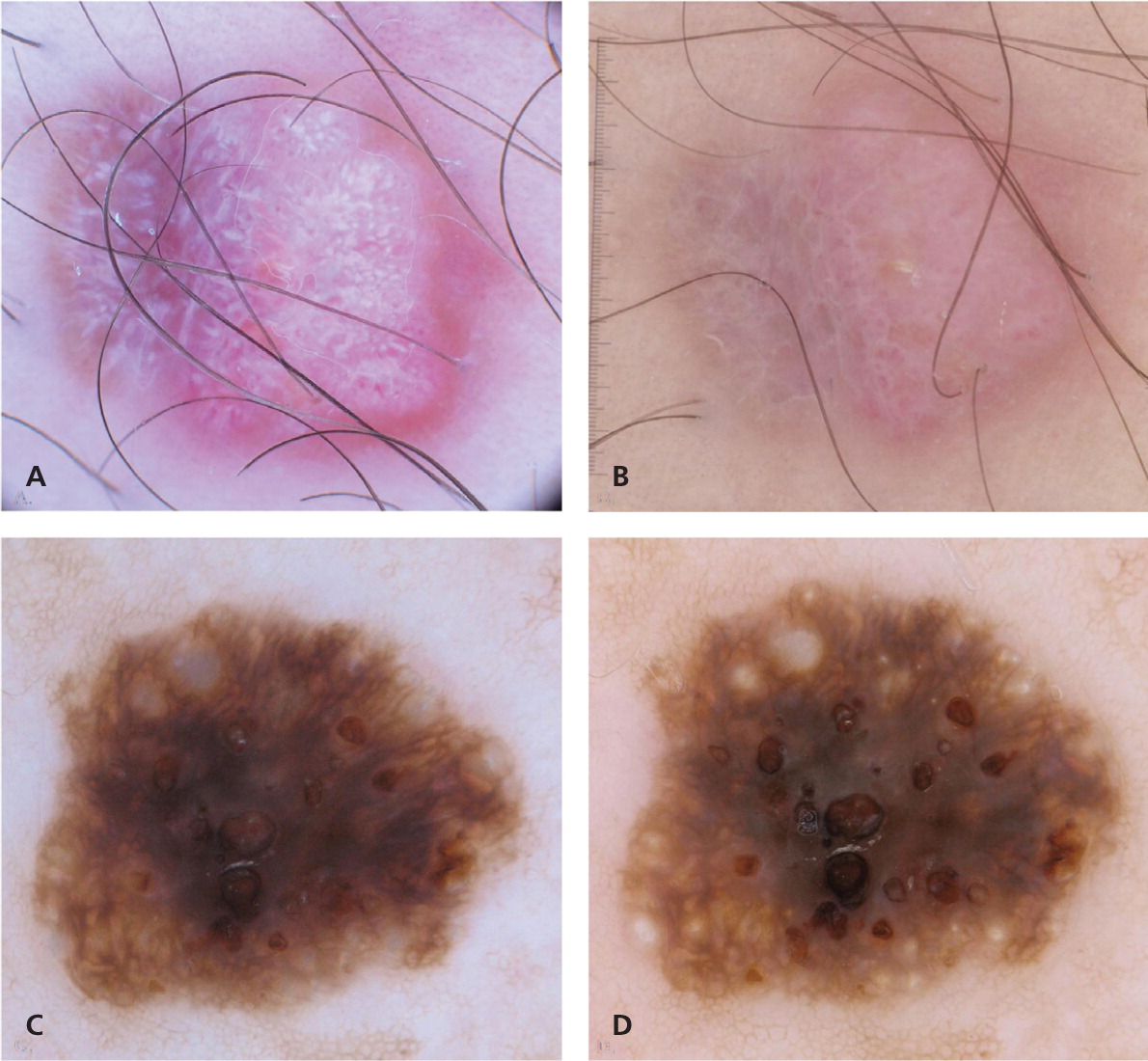
Three types of dermoscopic techniques are currently used: (1) classic or standard contact; (2) polarized contact; and (3) polarized noncontact (Table 12,13 ). Studies have demonstrated that polarized and nonpolarized dermoscopy provide complementary information.14,15 Polarized dermoscopy may have a higher sensitivity for detecting skin cancer based on its ability to enhance the visualization of vascular and crystalline structures, both of which are commonly seen in skin cancer15,16 (Figure 1). Nonpolarized dermoscopy improves specificity because it permits easier visualization of other structures commonly seen in benign lesions, such as milia-like cysts in seborrheic keratosis.
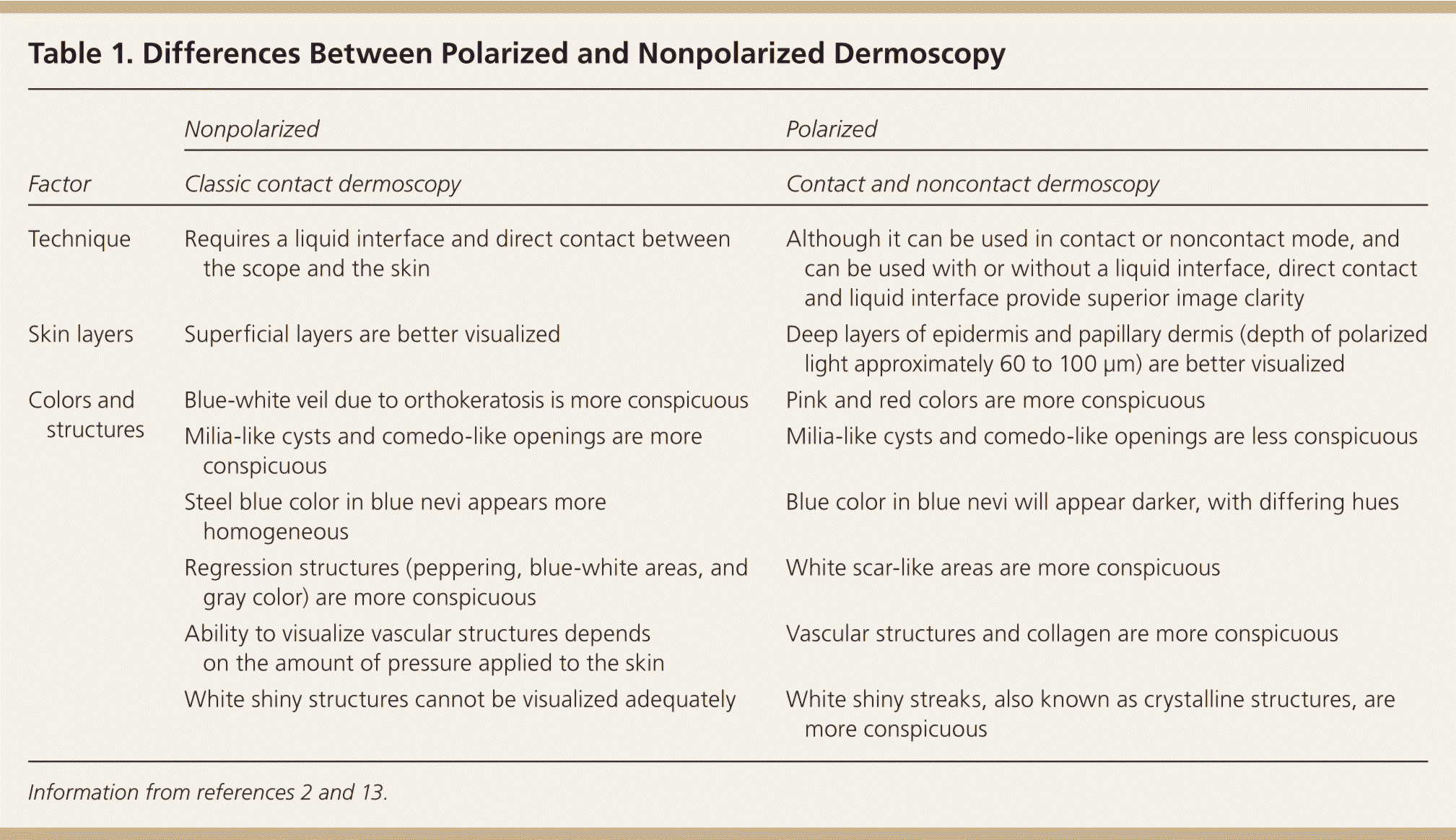
| Factor | Nonpolarized | Polarized |
|---|---|---|
| Classic contact dermoscopy | Contact and noncontact dermoscopy | |
| Technique | Requires a liquid interface and direct contact between the scope and the skin | Although it can be used in contact or noncontact mode, and can be used with or without a liquid interface, direct contact and liquid interface provide superior image clarity |
| Skin layers | Superficial layers are better visualized | Deep layers of epidermis and papillary dermis (depth of polarized light approximately 60 to 100 μm) are better visualized |
| Colors and structures | Blue-white veil due to orthokeratosis is more conspicuous | Pink and red colors are more conspicuous |
| Milia-like cysts and comedo-like openings are more conspicuous | Milia-like cysts and comedo-like openings are less conspicuous | |
| Steel blue color in blue nevi appears more homogeneous | Blue color in blue nevi will appear darker, with differing hues | |
| Regression structures (peppering, blue-white areas, and gray color) are more conspicuous | White scar-like areas are more conspicuous | |
| Ability to visualize vascular structures depends on the amount of pressure applied to the skin | Vascular structures and collagen are more conspicuous | |
| White shiny structures cannot be visualized adequately | White shiny streaks, also known as crystalline structures, are more conspicuous |
Clinical Impact
Dermoscopy can enhance a clinician's ability to detect skin cancer. However, improvement in diagnostic accuracy is contingent on acquiring dermoscopy training. Without formal training, the use of dermoscopy may result in poorer performance compared with naked eye examination.17 Primary care physicians trained in dermoscopy can reduce their referral rate or benign-to-malignant excision ratio (from 9.5 to 3.5).5 In addition, primary care physicians trained in dermoscopy improve their ability to identify skin lesions suggestive of skin cancer compared with naked eye examination alone (76% to 79% vs. 54%).3,4 In other words, in primary care, dermoscopy improves sensitivity for the diagnosis of melanoma and improves the ability to recognize suspicious lesions that require biopsy, without significantly changing specificity (71%).3–5 Dermoscopy also has been shown to improve the diagnostic accuracy for nonmelanocytic lesions, such as basal cell carcinoma.18 The indications for dermoscopy are listed in Table 2,6,16,19,20 and the advantages and limitations are listed in Table 3.3,6,14,20–32
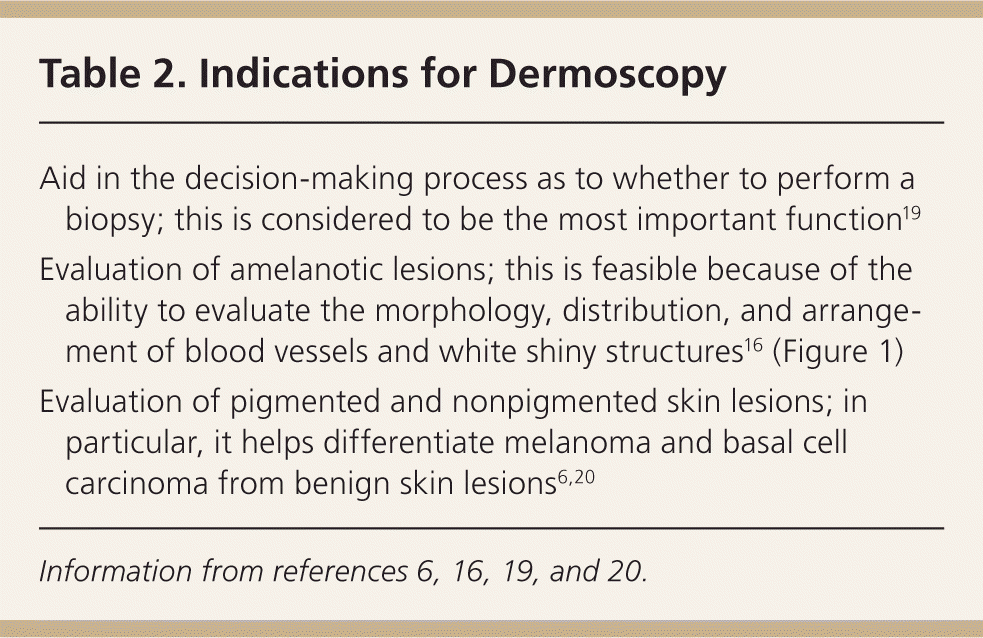
| Aid in the decision-making process as to whether to perform a biopsy; this is considered to be the most important function19 |
| Evaluation of amelanotic lesions; this is feasible because of the ability to evaluate the morphology, distribution, and arrangement of blood vessels and white shiny structures16 (Figure 1) |
| Evaluation of pigmented and nonpigmented skin lesions; in particular, it helps differentiate melanoma and basal cell carcinoma from benign skin lesions6,20 |

| Advantages |
| Aids in diagnosis and differentiation of tumoral pathologies (i.e., basal cell carcinoma, dermatofibroma, seborrheic keratosis, hemangioma, melanoma, Spitz nevi, and clear cell acanthoma),14,21,22 and in the assessment of borders in some tumoral pathologies (e.g., lentigo maligna, basal cell carcinoma)23,24 |
| Allows digital surveillance and monitoring of melanocytic lesions25,26 |
| Allows clinician to formulate a more precise differential diagnosis (clinical-dermoscopy concordance) |
| Enhances confidence in the clinical diagnosis |
| Improves the diagnostic accuracy, sensitivity, and specificity for the diagnosis of melanoma6,20 |
| Isolates suspicious areas within a lesion to help guide step-sectioning, which is performed by the pathologist |
| Reassures patients and physicians27 |
| Reduces the number of unnecessary biopsies3,27,28 |
| Limitations |
| Anchoring bias (relying too heavily on one piece of information) and search satisfaction (basing a diagnosis on incomplete information and ending the search) |
| Can result in lower diagnostic accuracy if the physician does not recognize or correctly interpret the significance of structures29 |
| May not detect early melanomas that have not yet developed any specific dermoscopic criteria30 |
| Lower diagnostic accuracy when lesions are diagnosed using dermoscopy alone, without clinical context31,32 |
Essentials of Dermoscopy: Colors and Structures
The visualization of structures within the epidermis and papillary dermis using dermoscopy has generated new terminology and clinical criteria for cutaneous lesions.33 It is imperative that physicians using dermoscopy learn and become proficient at identifying the colors and structures, because they are required in generating a correct diagnosis.
Colors visible under dermoscopy depend on the quantity and location of keratin, blood, collagen, and melanin. Accordingly, colors visualized include yellow, red, and white for keratin, blood, and collagen, respectively. Colors for melanin range from black and brown to gray and blue, depending on the location of melanin, in terms of depth, within the skin layers.34
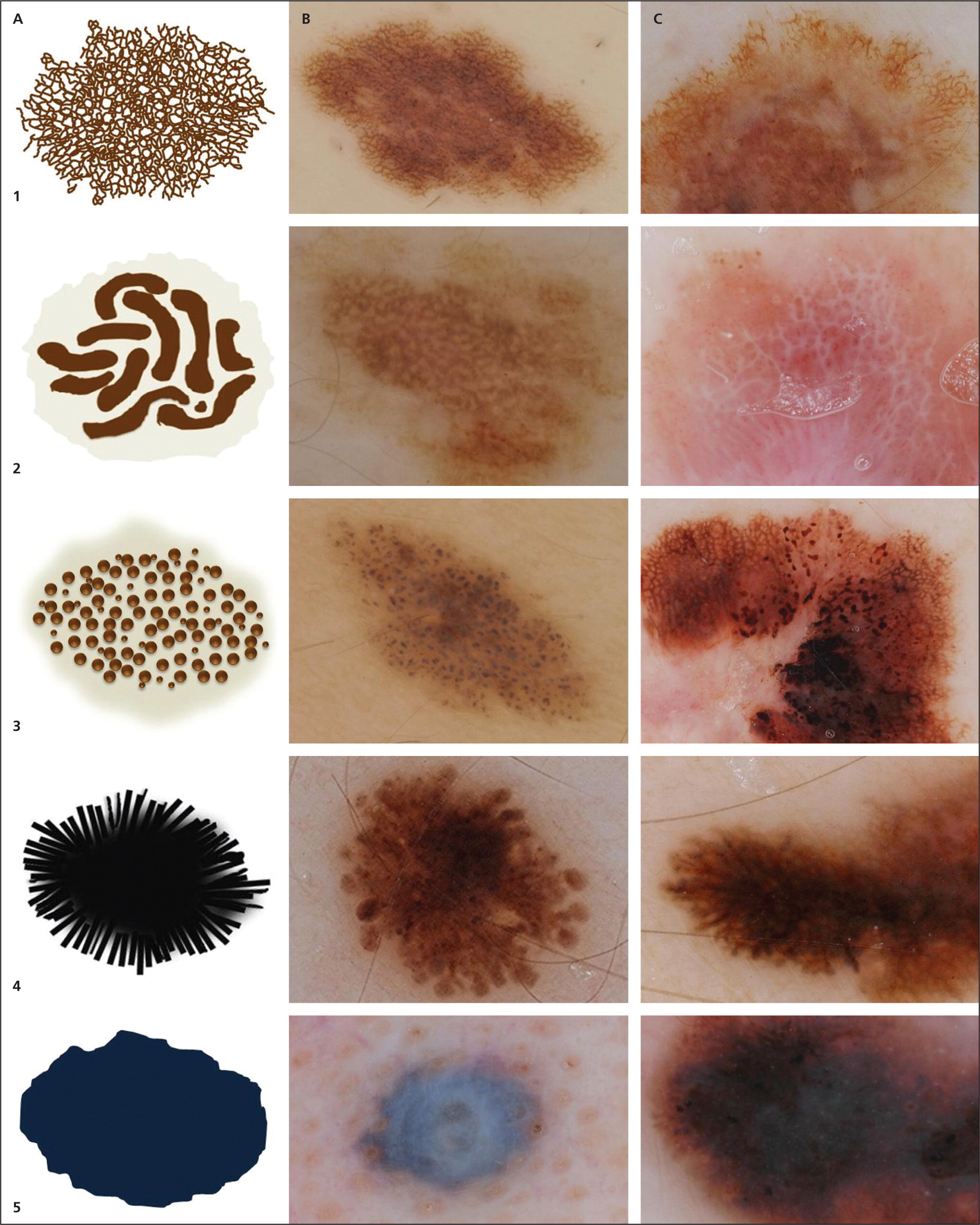
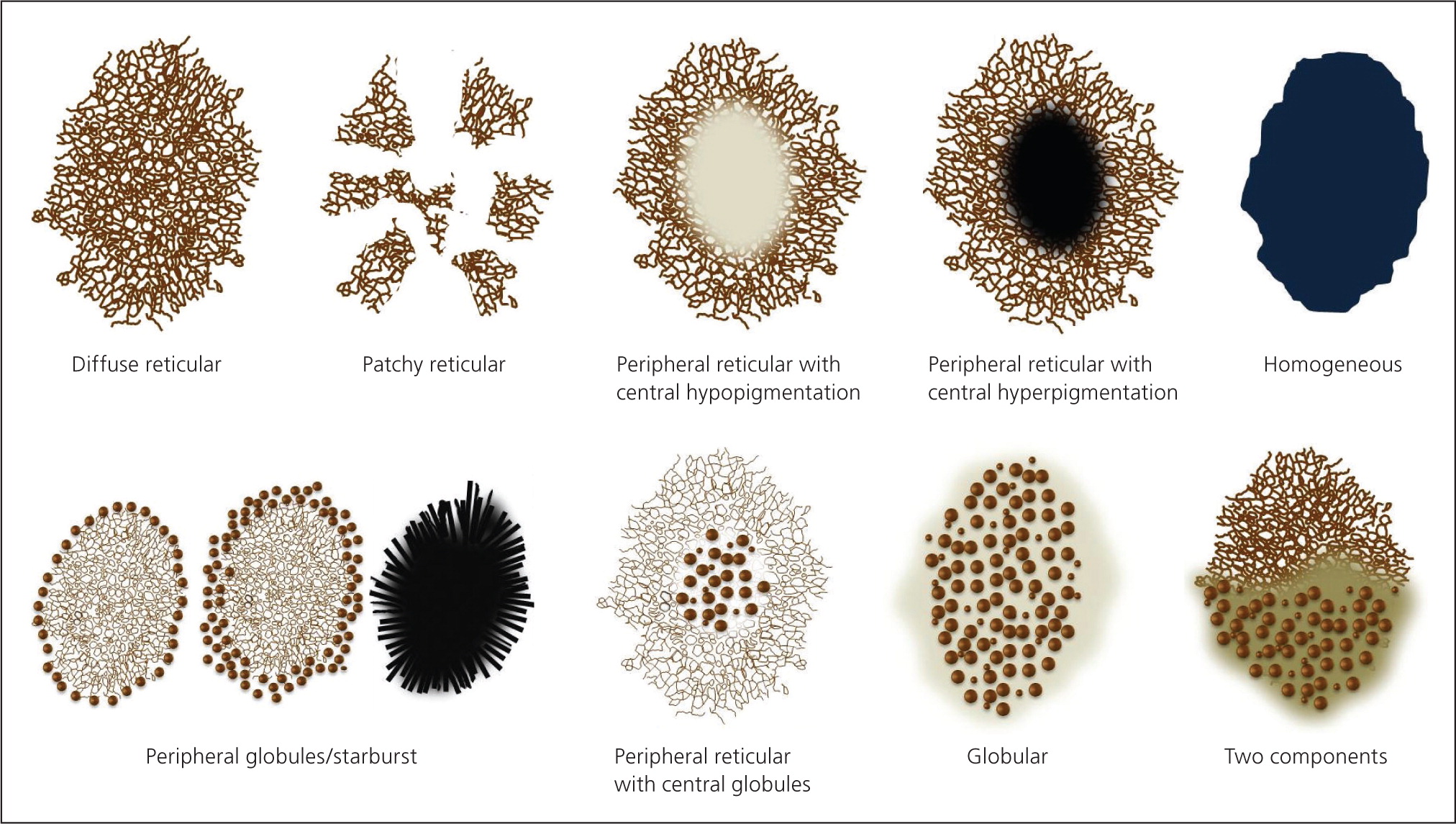
Structures visible under dermoscopy have histopathologic correlates (see Appendix below). The presence of specific structures permits the classification of a lesion as a melanocytic tumor (i.e., melanocytic nevus or melanoma). Common structures visualized in melanocytic lesions are provided in Table 421,22,35–39 and Figure 2. When a lesion has been determined to be melanocytic, the next step is to determine if it is a nevus or a melanoma. Melanocytic nevi tend to manifest one of the symmetric patterns depicted in Figure 3, and melanomas reveal at least one of the 10 melanoma-specific structures provided in Table 5.

| Level | Type of lesion | Dermoscopic criteria |
|---|---|---|
| 1 | Melanocytic lesions | Pigment network* |
| Negative pigment network | ||
| Aggregated globules | ||
| Streaks (pseudopods and radial streaming) | ||
| Homogeneous blue pigmentation | ||
| Pseudonetwork (facial skin) | ||
| Parallel pigment pattern (acral lesions on palms and soles) | ||
| 2 | Basal cell carcinoma†35 | Arborizing blood vessels |
| Leaf-like structures | ||
| Large blue-gray ovoid nests | ||
| Multiple blue-gray nonaggregated globules | ||
| Spoke wheel–like structures/concentric structures | ||
| 3 | Seborrheic keratosis‡36,37 | Multiple (thee or more) milia-like cysts |
| Comedo-like opening | ||
| Moth-eaten borders | ||
| Gyri (ridges) and sulci (fissures) | ||
| Fingerprint-like structures | ||
| 4 | Hemangioma/angiokeratoma36 | Red, maroon, or black lacunae |
| 5 | Blood vessels seen in nonmelanocytic tumors22,36 | Arborizing, glomerular, hairpin, crown, and serpiginous vessels |
| 6 | Blood vessels seen in melanocytic tumors21,36 | Comma-shaped, dotted, serpentine or irregular linear, polymorphous, and corkscrew vessels; milky-red globules/vascular blush |
| 7 | Structureless lesions36 | No structures or blood vessels noted |
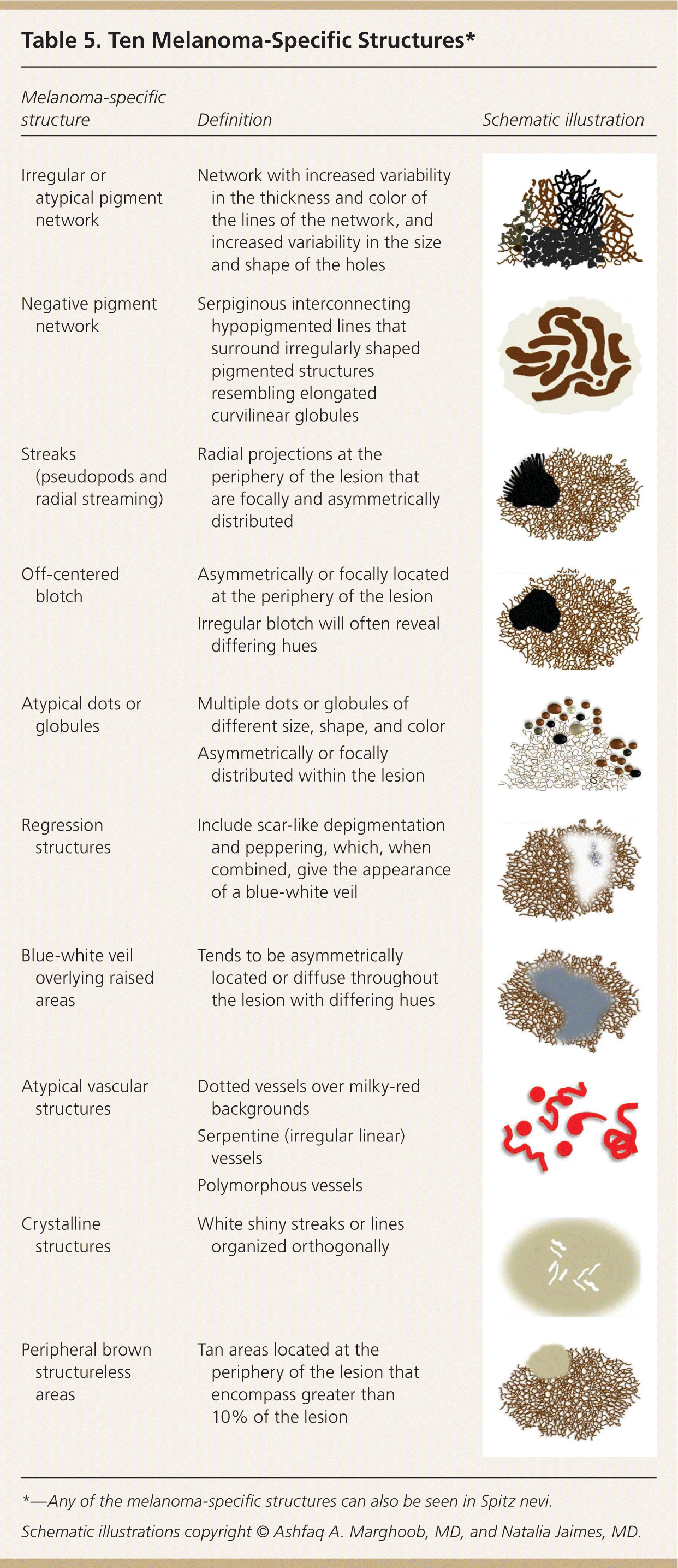
| Melanoma-specific structure | Definition | Schematic illustration |
|---|---|---|
| Irregular or atypical pigment network | Network with increased variability in the thickness and color of the lines of the network, and increased variability in the size and shape of the holes | |
| Negative pigment network | Serpiginous interconnecting hypopigmented lines that surround irregularly shaped pigmented structures resembling elongated curvilinear globules | |
| Streaks (pseudopods and radial streaming) | Radial projections at the periphery of the lesion that are focally and asymmetrically distributed | |
| Off-centered blotch | Asymmetrically or focally located at the periphery of the lesion | |
| Irregular blotch will often reveal differing hues | ||
| Atypical dots or globules | Multiple dots or globules of different size, shape, and color | |
| Asymmetrically or focally distributed within the lesion | ||
| Regression structures | Include scar-like depigmentation and peppering, which, when combined, give the appearance of a blue-white veil | |
| Blue-white veil overlying raised areas | Tends to be asymmetrically located or diffuse throughout the lesion with differing hues | |
| Atypical vascular structures | Dotted vessels over milky-red backgrounds | |
| Serpentine (irregular linear) vessels | ||
| Polymorphous vessels | ||
| Crystalline structures | White shiny streaks or lines organized orthogonally | |
| Peripheral brown structureless areas | Tan areas located at the periphery of the lesion that encompass greater than 10% of the lesion |
Nonmelanocytic lesions, including basal cell carcinoma, seborrheic keratosis, hemangioma, and angiokeratoma, also demonstrate specific structures under dermoscopy. Pigmented and nonpigmented basal cell carcinomas can reveal vascular structures such as arborizing blood vessels36,38 (Table 421,22,35–39 ; Figure 4). In addition, pigmented basal cell carcinoma can demonstrate pigmented structures, including leaf-like structures, spoke wheel–like structures, ovoid nests, and nonaggregated blue-gray globules.
Seborrheic keratosis is one of the most common benign epidermal tumors. Clinically, these lesions are characterized by well-circumscribed plaques or papules with pseudohorn cysts. They range from light brown to dark brown or black. Dermoscopically, seborrheic keratoses display multiple milia-like cysts, comedo-like openings, gyri and sulci, fingerprint-like structures, and moth-eaten borders (Table 421,22,35–39; Figure 5).
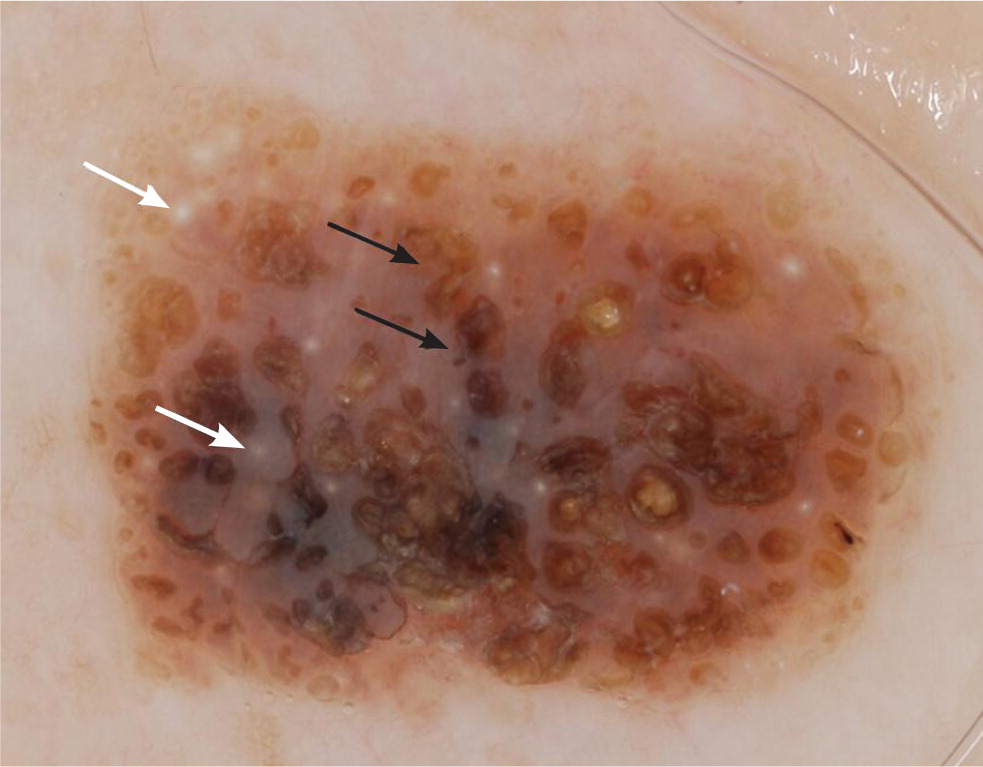
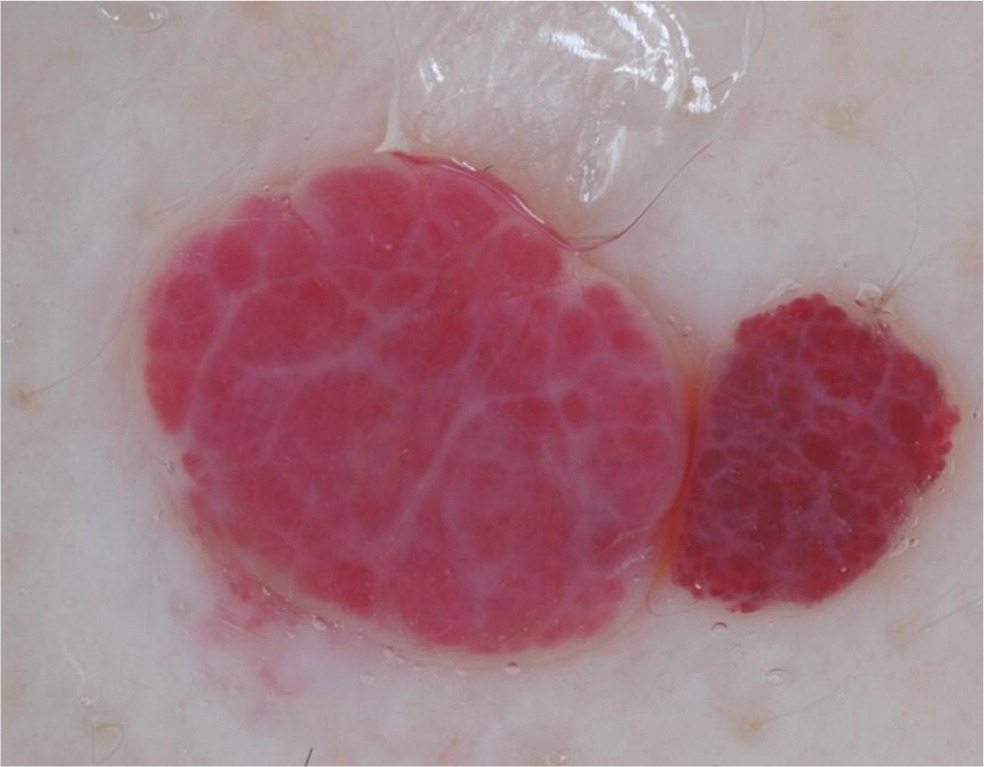
Cherry hemangiomas are bright red to violaceous papules that consist of dilated capillaries. Similarly, angiokeratomas are dark violaceous to black papules that are often keratotic or firm on palpation. Dermoscopically, hemangiomas and angiokeratomas are distinguished by the presence of well-demarcated lacunae that can be red, maroon, blue, or black 36 (Table 421,22,35–39; Figure 6).
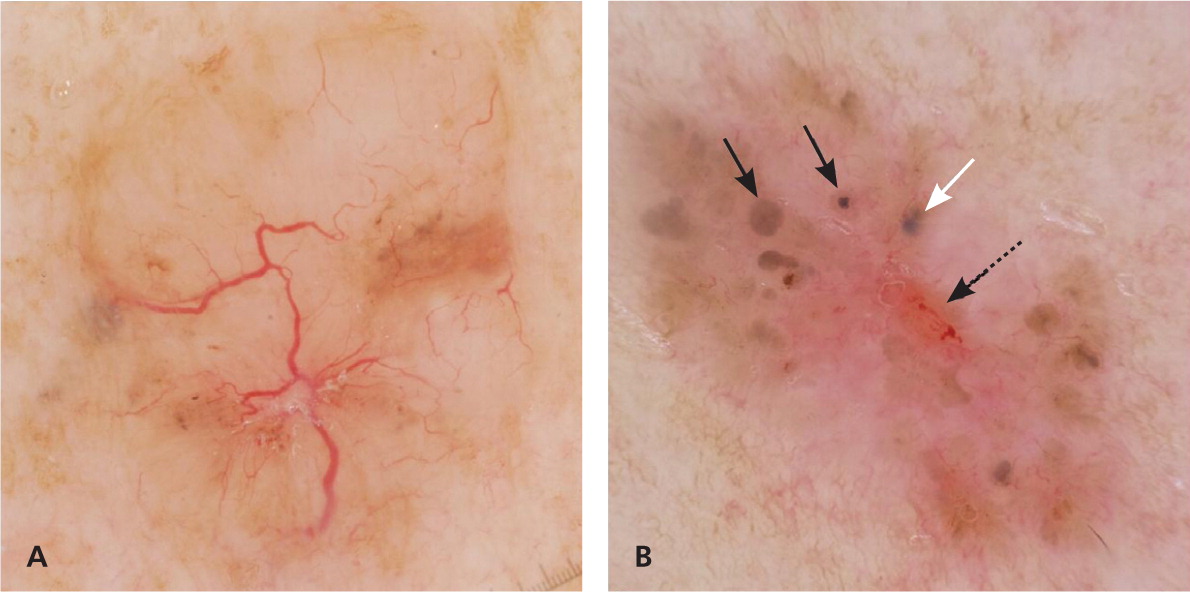
Some lesions, particularly amelanotic or hypomelanotic tumors, cannot be classified based on the aforementioned features. It is important to search for the presence of blood vessels in these lesions. If present, the blood vessel morphology can assist in making the correct diagnosis. In most amelanotic and hypomelanotic lesions, the only clue to the diagnosis depends on evaluating the blood vessel morphology and distribution, whereas in pigmented lesions (as previously mentioned), the analysis of vascular structures provides secondary clues to the diagnosis.21,40
The easiest way to visualize blood vessels is to use a noncontact polarized dermatoscope. If a contact dermatoscope is used, it is beneficial to use ultrasound gel as the liquid interface, because the gel acts as a cushion that minimizes the amount of pressure applied to the skin, which in turn prevents blanching of the vessels.21 Although it is important to analyze blood vessel morphology, especially in amelanotic lesions, it is beyond the scope of this article to review all the vessel morphologies; readers should refer to the referenced articles for more information.21,22
Interpretation of Dermoscopic Structures: The Two-Step Algorithm
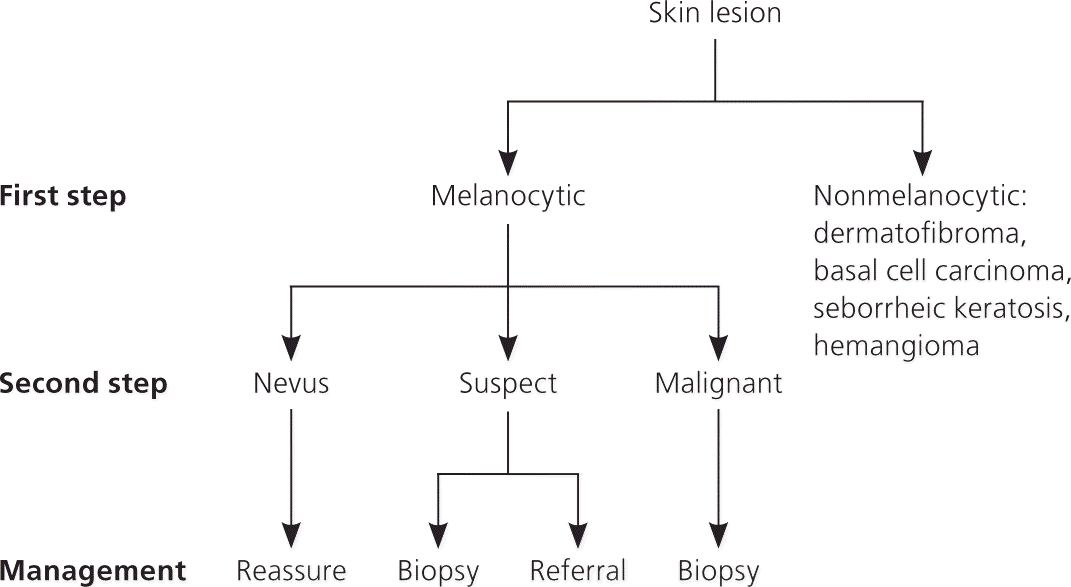
FIRST STEP: MELANOCYTIC VS. NONMELANOCYTIC LESIONS
The first step of the algorithm is based on a seven-level criterion ladder, which is intended to help differentiate melanocytic lesions from the following nonmelanocytic lesions: dermatofibroma, basal cell carcinoma, seborrheic keratosis, and hemangioma36 (Table 421,22,35–39 ; Figure 7). Even if the two-step algorithm cannot reliably differentiate between melanocytic and nonmelanocytic lesions, it has been structured to ensure that cutaneous malignancies will not be missed.36,39 For example, occasionally basal cell carcinoma and melanoma can manifest overlapping features that may lead to the incorrect classification of some basal cell carcinomas as melanocytic (i.e., melanoma) and some melanomas as nonmelanocytic (i.e., basal cell carcinoma). Despite this potential error, the concern for a cutaneous malignancy will remain high, and the management decision to perform a biopsy will not be altered.
SECOND STEP: BENIGN VS. MALIGNANT MELANOCYTIC LESIONS
The second step of the algorithm is intended only for the evaluation of melanocytic lesions, including common acquired melanocytic nevi, blue nevi, Spitz nevi, atypical nevi, and melanoma. In essence, the second step is intended to help differentiate nevi from melanoma.36 To accomplish this, numerous quantitative and qualitative methods have been created.34,41–45 In particular, scoring systems have proven to be relatively simple, accurate, and reproducible methods for diagnosing melanoma.6,19,44
The three-point checklist is considered the simplest method to learn and use, and has the highest sensitivity for identifying melanoma36 (Figure 8). It is intended as a screening algorithm for detecting skin cancer (melanoma and pigmented basal cell carcinoma) and applies only to pigmented skin lesions. One point is assigned to each of the following criteria present in the lesion43:
Asymmetry in distribution of dermoscopic color and/or structures in one or two perpendicular axes. The contour or silhouette of the lesion does not factor into whether the lesion is symmetric or not.
Irregular or atypical pigment network consisting of thick lines and irregular holes.
Blue-white veil and/or white scar-like depigmentation and/or blue pepper-like granules.
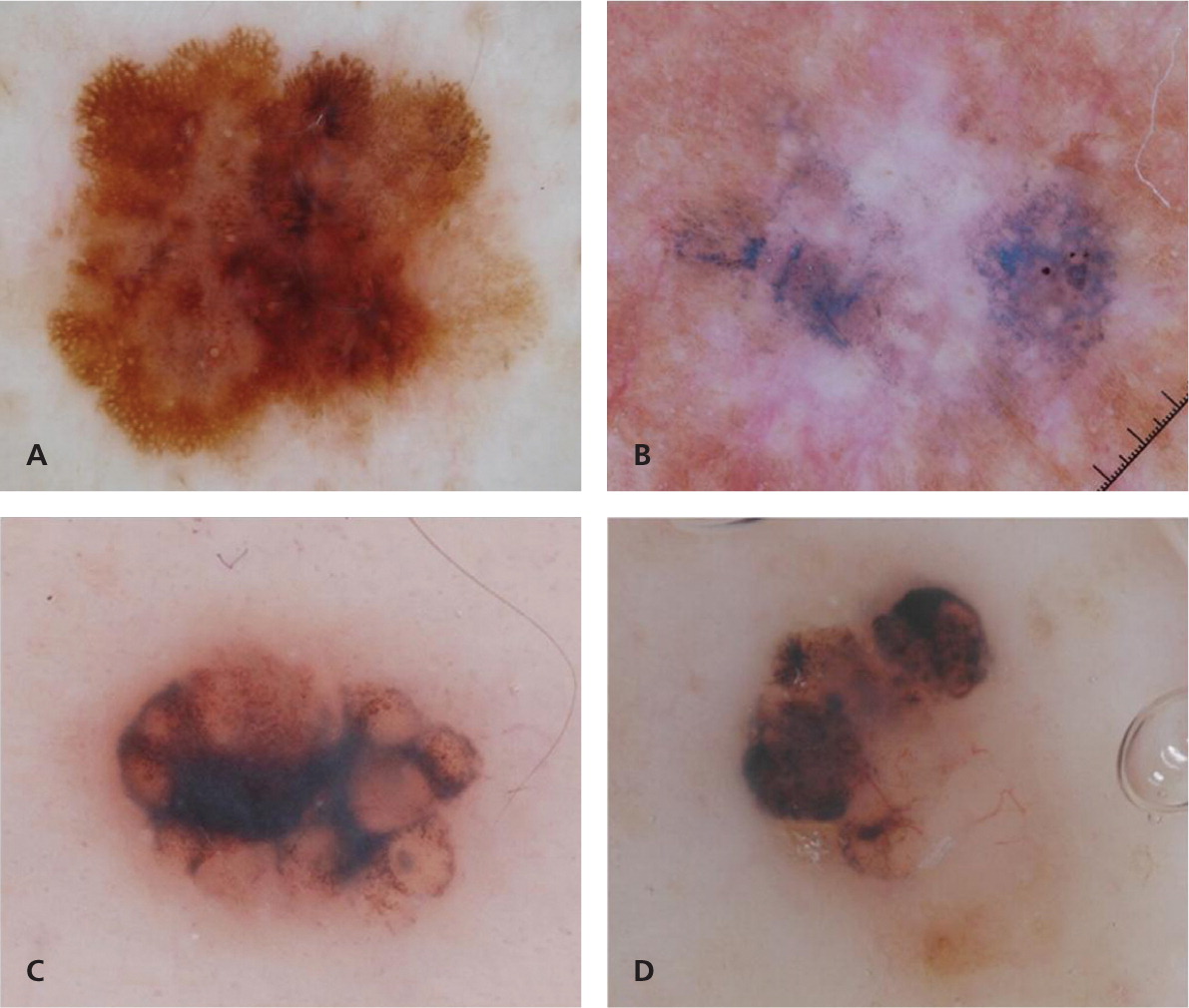
A total score of 2 or 3 is considered positive, and the lesion should be biopsied or the patient referred for further evaluation.43 Given its simplicity and high sensitivity for detecting pigmented skin cancer, the three-point checklist may be ideally suited for clinicians with little experience in dermoscopy and for use as a screening tool in the primary care setting.19 Atypical pigment network and blue-white structures have previously been defined (Figure 2; Table 5; Appendix). Dermoscopic asymmetry requires further clarification, because it differs from the conventional way that clinicians define asymmetry. The conventional view of asymmetry, as in the well-known ABCD mnemonic for melanoma (asymmetry, border irregularities, color variation, diameter [greater than 6 mm]), factors in the contour or shape of the lesion. In contrast, symmetry or asymmetry in dermoscopy does not factor in the contour or shape, but rather the distribution of colors and structures within the lesion (Figure 8). For example, using the conventional definition of symmetry, a heart-shaped lesion that reveals only globules would be considered asymmetric in one axis; however, under dermoscopy, this lesion would be considered completely symmetric in both axes.
Pattern analysis is a qualitative method that evaluates dermoscopic structures and their distribution. It requires the ability to recognize, and experience in recognizing, benign patterns46–48 (Figure 3). It also requires the ability to recognize the 10 melanoma-specific structures listed in Table 5. Any melanocytic lesion that deviates from one of these benign patterns, while also revealing at least one of the 10 melanoma-specific structures, should be biopsied to rule out melanoma.
Dermoscopy as a Guide for Management
If the lesion is considered benign, the patient can be reassured, provided education on skin self-examination, and instructed to return if any changes or new lesions develop.25,50 If the lesion is suspicious for melanoma or basal cell carcinoma, it should be biopsied.51 If the lesion is considered suspicious (i.e., the clinician is uncertain of the diagnosis), it can be biopsied or the patient can be referred for another opinion.
All of the criteria discussed in this article can be applied during the evaluation of any lesion on any area of the skin. However, there are additional criteria that are specific to mucosal, volar, and facial skin lesions. For discussion of the specific features of lesions on these areas, refer to the Atlas of Dermoscopy52 or other online tutorials on dermoscopy, such as http://www.dermoscopy-ids.org/index.php/education/podcasts and http://www.genomel.org/dermoscopy.
Dermoscopy is a useful, easy-to-use, and non–time-consuming technique that increases diagnostic accuracy for pigmented and nonpigmented skin lesions53; in particular, it increases the sensitivity for detecting melanoma and basal cell carcinoma. In addition, dermoscopy has been shown to improve specificity by decreasing the number of biopsies of benign lesions, such as nevi, seborrheic keratosis, and hemangioma, that may clinically mimic malignancy.28
Data Sources: A PubMed search was completed in Clinical Queries using the key terms dermoscopy, dermatoscopy, epiluminescence microscopy, skin surface microscopy, and incident light microscopy. The search included meta-analyses, randomized controlled trials, clinical trials, reviews, and case series.
EDITOR'S NOTE: At the time of initial submission, Dr. Marghoob did not list any disclosures on AFP's Conflict of Interest form, which asks for financial relationships with commercial entities that might have an interest in the topic. During the final stages of production, we discovered that Dr. Marghoob had the following relationships with makers of dermoscopes, which we agreed should be disclosed: (1) receiving dermoscope prototypes for testing from the four major manufacturers, and providing unpaid feedback and advice about these devices; (2) receiving honoraria for speaking on the topic of dermoscopy at meetings funded in part by makers of dermoscopes (however, Dr. Marghoob is not on any speakers' bureaus); and (3) being an investigator in Institutional Review Board–approved research projects funded by the National Institutes of Health and Melanoma Research Alliance, some of which partnered (at least to some extent) with companies that produce dermoscopes (however, Dr. Marghoob does not receive any compensation from this grant funding). Given the stage at which these conflicts came to our attention, we performed an internal review of the manuscript and disclosures, and ultimately decided that the manuscript provided an unbiased and nonpromotional description of this technique. In addition, Dr. Marghoob agreed to not enter into any relationships with a maker of dermoscopes that used his AFP article for any presentations on dermoscopy for at least 12 months after the article's publication. For these reasons, we decided to continue with publication. However, to be clear, relationships like these would generally be disqualifying according to our conflict of interest policy (https://www.aafp.org/journals/afp/authors/guide/coi.html).
Appendix
| Dermoscopic structures | Schematic illustration | Definition | Histopathologic correlation |
|---|---|---|---|
| Pigment network | Grid-like network consisting of pigmented lines and hypopigmented holes | Melanin in keratinocytes or melanocytes along the dermoepidermal junction | |
| Network lines correspond to the rete ridges | |||
| Holes correspond to the suprapapillary plate | |||
| Pseudonetwork | Diffuse pigmentation interrupted by adnexal opening | Pigment in the epidermis or dermis in which the rete ridges are attenuated | |
| Usually seen in facial lesions | |||
| Negative pigment network | Serpiginous interconnecting hypopigmented lines that surround irregularly shaped pigmented structures resembling elongated curvilinear globules | Remains unknown | |
| Presumed to be related to bridging of rete ridges or large melanocytic nests in the papillary dermis, resulting in compression of adjacent rete ridges; these nests may correspond to globules that are not spherical in shape | |||
| Aggregated globules | More than three clustered, well- demarcated, round to oval, symmetric structures, or three or more of these structures aligned at the lesion's perimeter | Nests of melanocytes at the dermoepidermal junction | |
| May be brown, black, or blue | |||
| Diameters are greater than 0.1 mm | |||
| Dots | Small, round structures of less than 0.1 mm in diameter | Aggregates of melanocytes or melanin granules | |
| May be black, brown, or blue-gray | |||
| Streaks (pseudopods and radial streaming) | Streaks are radial projections at the periphery of the lesion, extending from the tumor toward the surrounding normal skin; may be brown or black | Confluent junctional nests of melanocytes | |
| Pseudopods are streaks with finger-like projections with small knobs at the tips | |||
| Radial streaming is streaks without knobs at the tips | |||
| Peppering (or granularity) | Tiny, blue-gray granules | Melanin deposited as intracellular (mostly within melanophages) or extracellular particles in the upper dermis | |
| Structureless areas | Devoid of dermoscopic structures within the lesion and without manife sting any regression structures | Relative decreased concentration of melanin or flattening of rete ridges | |
| Tend to be tan to light brown, but have lighter pigment compared with the rest of the lesion | |||
| Peripheral light brown or tan structureless areas | Structureless areas (as above), located at the periphery of the lesion | Partial or complete flattening of the rete ridges | |
| Increased number of pigmented atypical melanocytes predominantly at the dermoepidermal junction | |||
| Diffuse scattering of melanocytes in the spinous layer of the epidermis | |||
| Regression structures | Dark brown to black homogeneous areas of pigment that obscure visualization of any other structures | Aggregates of melanin in the stratum corneum or throughout all layers of the skin | |
| Blotches | Include scar-like depigmentation (lighter than the surrounding uninvolved normal skin; appear shiny white under polarized dermoscopy) often combined with peppering; combination of scar-like depigmentation and peppering gives the appearance of a blue-white veil | Scar-like changes/white areas: thickened fibrotic papillary dermis Blue areas: correlate with melanosis type of regression | |
| Blue-white veil | Confluent blue pigmentation with an overlying white ground-glass haze | Aggregation of heavily pigmented melanocytes or melanophages in combination with compact orthokeratosis of the stratum corneum | |
| White shiny structures (more conspicuous with polarized dermoscopy) | Rosettes: appear as four white shiny points creating a pattern reminiscent of a four- leaf cloverA4 | Histopathologic correlation has not been fully explained | |
| Crystalline structures: short, white, shiny linear streaks that are often parallel or orthogonal to each otherA5, A6 | Altered collagen or fibrosis in the dermis | ||
| White shiny areas: appear as larger structureless areas of shiny white color | Altered stromal matrix | ||
| Parallel pigment pattern | On volar skin (i.e., palms and soles) | Pigmented melanocytes in the furrows (crista limitants) or ridges (crista intermedia) on skin of palms and soles | |
| Parallel rows of pigmentation following the furrows (as seen in nevi) or ridges (as seen in melanoma) of the dermatoglyphics |
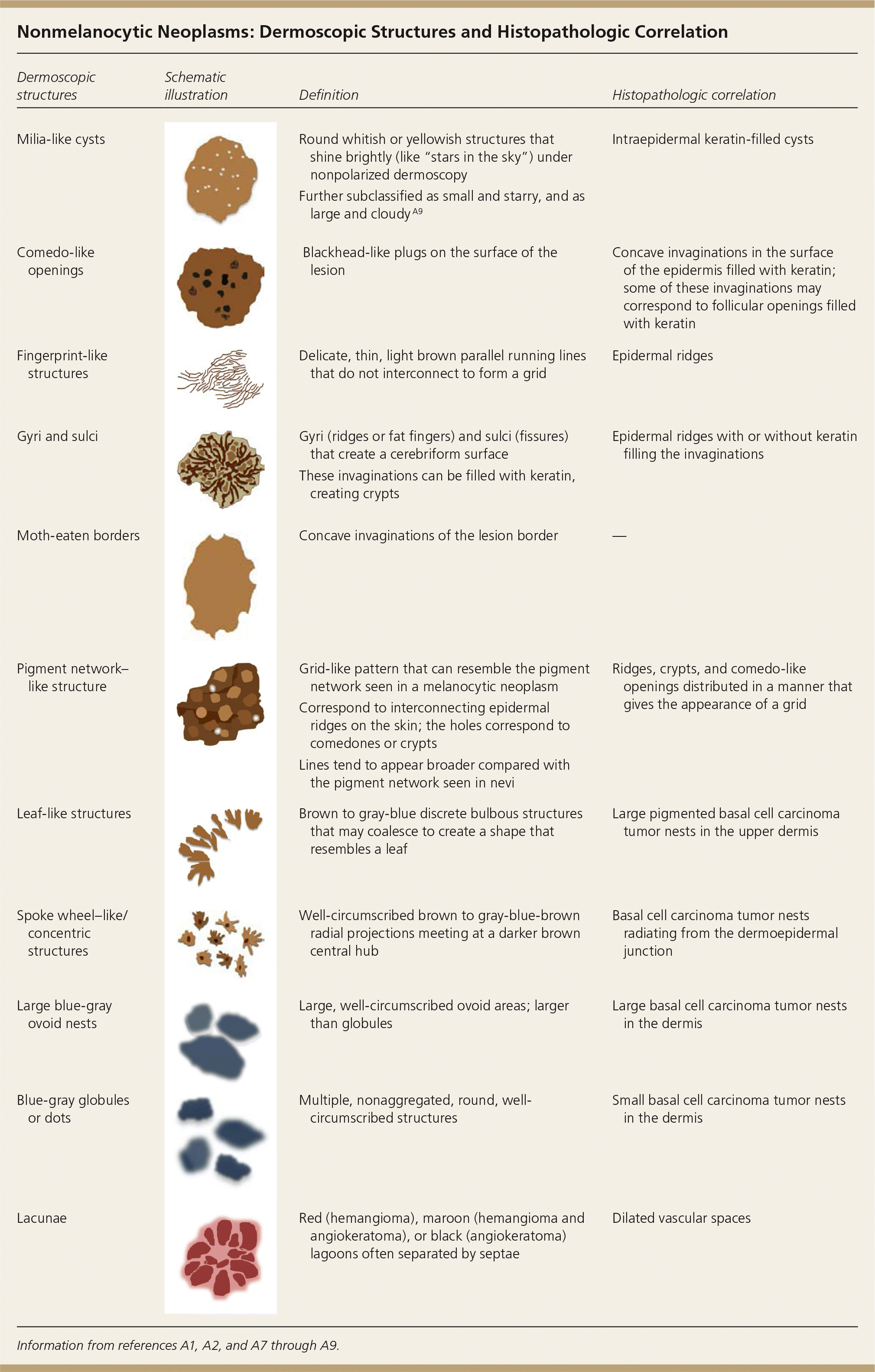
| Dermoscopic structures | Schematic illustration | Definition | Histopathologic correlation |
|---|---|---|---|
| Milia-like cysts | Round whitish or yellowish structures that shine brightly (like “stars in the sky”) under nonpolarized dermoscopy | Intraepidermal keratin-filled cysts | |
| Further subclassified as small and starry, and as large and cloudy A9 | |||
| Comedo-like openings | Blackhead-like plugs on the surface of the lesion | Concave invaginations in the surface of the epidermis filled with keratin; some of these invaginations may correspond to follicular openings filled with keratin | |
| Fingerprint-like structures | Delicate, thin, light brown parallel running lines that do not interconnect to form a grid | Epidermal ridges | |
| Gyri and sulci | Gyri (ridges or fat fingers) and sulci (fissures) that create a cerebriform surface | Epidermal ridges with or without keratin filling the invaginations | |
| These invaginations can be filled with keratin, creating crypts | |||
| Moth-eaten borders | Concave invaginations of the lesion border | — | |
| Pigment network–like structure | Grid-like pattern that can resemble the pigment network seen in a melanocytic neoplasm | Ridges, crypts, and comedo-like openings distributed in a manner that gives the appearance of a grid | |
| Correspond to interconnecting epidermal ridges on the skin; the holes correspond to comedones or crypts | |||
| Lines tend to appear broader compared with the pigment network seen in nevi | |||
| Leaf-like structures | Brown to gray-blue discrete bulbous structures that may coalesce to create a shape that resembles a leaf | Large pigmented basal cell carcinoma tumor nests in the upper dermis | |
| Spoke wheel–like/concentric structures | Well-circumscribed brown to gray-blue-brown radial projections meeting at a darker brown central hub | Basal cell carcinoma tumor nests radiating from the dermoepidermal junction | |
| Large blue-gray ovoid nests | Large, well-circumscribed ovoid areas; larger than globules | Large basal cell carcinoma tumor nests in the dermis | |
| Blue-gray globules or dots | Multiple, nonaggregated, round, well-circumscribed structures | Small basal cell carcinoma tumor nests in the dermis | |
| Lacunae | Red (hemangioma), maroon (hemangioma and angiokeratoma), or black (angiokeratoma) lagoons often separated by septae | Dilated vascular spaces |
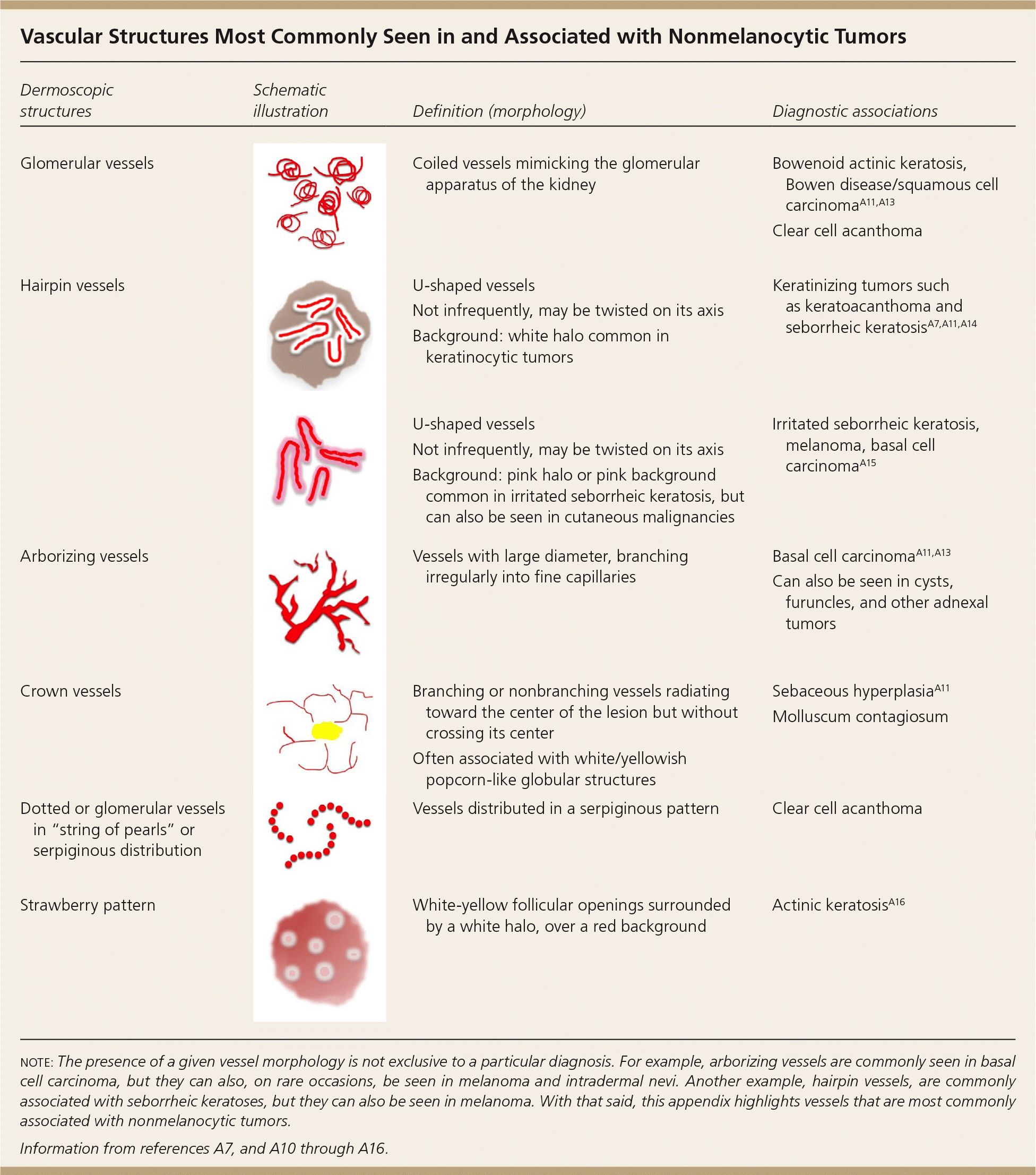
| Dermoscopic structures | Schematic illustration | Definition (morphology) | Diagnostic associations |
|---|---|---|---|
| Glomerular vessels | Coiled vessels mimicking the glomerular apparatus of the kidney | Bowenoid actinic keratosis, Bowen disease/squamous cell carcinomaA11,A13 | |
| Clear cell acanthoma | |||
| Hairpin vessels | U-shaped vessels | Keratinizing tumors such as keratoacanthoma and seborrheic keratosisA7,A11,A14 | |
| Not infrequently, may be twisted on its axis | |||
| Background: white halo common in keratinocytic tumors | |||
| U-shaped vessels | Irritated seborrheic keratosis, melanoma, basal cell carcinomaA15 | ||
| Not infrequently, may be twisted on its axis | |||
| Background: pink halo or pink background common in irritated seborrheic keratosis, but can also be seen in cutaneous malignancies | |||
| Arborizing vessels | Vessels with large diameter, branching irregularly into fine capillaries | Basal cell carcinomaA11, A13 | |
| Can also be seen in cysts, furuncles, and other adnexal tumors | |||
| Crown vessels | Branching or nonbranching vessels radiating toward the center of the lesion but without crossing its center | Sebaceous hyperplasiaA11 | |
| Molluscum contagiosum | |||
| Often associated with white/yellowish popcorn-like globular structures | |||
| Dotted or glomerular vessels in “string of pearls” or serpiginous distribution | Vessels distributed in a serpiginous pattern | Clear cell acanthoma | |
| Strawberry pattern | White-yellow follicular openings surrounded by a white halo, over a red background | Actinic keratosisA16 |
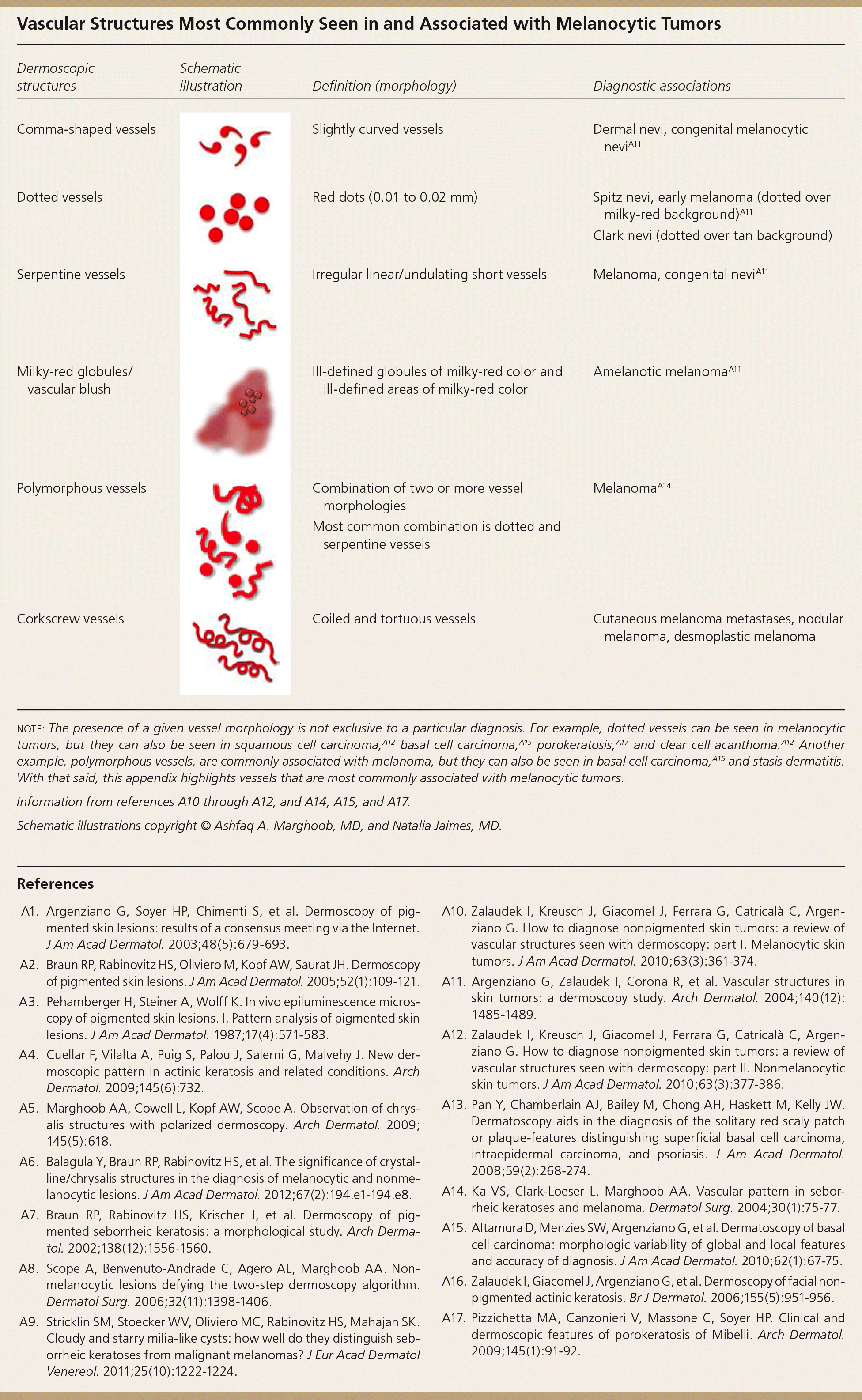
| Dermoscopic structures | Schematic illustration | Definition (morphology) | Diagnostic associations |
|---|---|---|---|
| Comma-shaped vessels | Slightly curved vessels | Dermal nevi, congenital melanocytic neviA11 | |
| Dotted vessels | Red dots (0.01 to 0.02 mm) | Spitz nevi, early melanoma (dotted over milky-red background)A11 | |
| Clark nevi (dotted over tan background) | |||
| Serpentine vessels | Irregular linear/undulating short vessels | Melanoma, congenital neviA11 | |
| Milky-red globules/vascular blush | Ill-defined globules of milky-red color and ill-defined areas of milky-red color | Amelanotic melanomaA11 | |
| Polymorphous vessels | Combination of two or more vessel morphologies | MelanomaA14 | |
| Most common combination is dotted and serpentine vessels | |||
| Corkscrew vessels | Coiled and tortuous vessels | Cutaneous melanoma metastases, nodular melanoma, desmoplastic melanoma |