
Am Fam Physician. 2010;82(11):1357-1366
Author disclosure: Nothing to disclose.
Sudden cardiac death is a major public health problem, affecting 500,000 patients in the United States annually. An implantable cardioverter-defibrillator (ICD) can terminate malignant ventricular arrhythmias and has been shown to improve survival in high-risk populations. Although sudden cardiac death is a heterogeneous condition, left ventricular ejection fraction of 35 percent or less remains the single best factor to stratify patients for prophylactic ICD implantation, and randomized trials have shown mortality benefit in this population. Therefore, in patients with heart disease, assessment of ejection fraction remains the most important step to identify patients at risk of sudden cardiac death who would benefit from ICD implantation. Physician understanding of each patient's ICD type, indication, etiology of heart disease, and cardiovascular status is essential for optimal care. If the ICD was placed for secondary prevention, the circumstances relating to the index event should be explored. Evaluation of defibrillator shocks merits careful assessment of the patient's cardiovascular status. Consultation with a subspecialist and interrogation of the ICD can determine if shocks were appropriate or inappropriate and can facilitate management.
Sudden cardiac death (SCD) is caused by the abrupt cessation of cardiac function due to cardiac arrest.1 The most common electrical sequence of events is ventricular tachycardia (VT) degenerating into ventricular fibrillation (VF).2–4 In older persons and those with advanced heart failure, bradyarrhythmias or electromechanical dissociation may be the primary electrical event.5,6 SCD may also be the final common pathway of noncardiac conditions, such as pulmonary embolism.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Risk stratification | ||
| In patients with coronary artery disease, prior myocardial infarction, or heart failure, LVEF should be measured by echocardiography or radionuclide ventriculography to assess risk of sudden cardiac death. Assessment of LVEF may be repeated annually. | A | 1, 20, 40 |
| Patients with LVEF < 35 percent, nonsustained VT, sustained VT, syncope of undetermined origin, inherited ion channel abnormalities, inherited heart failure syndromes, or structural heart disease should be assessed by a cardiac electrophysiologist or cardiologist for risk stratification for VT/VF and sudden cardiac death. | A | 1, 20, 41 |
| Patients with existing devices | ||
| Patients presenting for primary care should be asked whether they have an implantable cardiac device to ensure appropriate care. The type of device (e.g., pacemaker, defibrillator, cardiac resynchronization therapy with or without defibrillation) and indication (e.g., primary prevention of sudden cardiac death, VT, hypertrophic cardiomyopathy) should be documented for outpatient and perioperative care. | C | 50 |
| Follow-up by a cardiac electrophysiologist or cardiologist should be done every three to 12 months for device interrogation and management. | C | 1, 20 |
| Patients who experience an ICD shock should be evaluated for a change in clinical status in conjunction with device interrogation and consultation with a cardiac electrophysiologist or cardiologist. Repeated shocks require urgent evaluation and consultation. | C | 1, 20 |
| ICD implantation—primary prevention | ||
| ICD implantation is indicated in patients with ischemic cardiomyopathy due to prior myocardial infarction who are at least 40 days post-myocardial infarction who have either LVEF < 30 percent and are NYHA functional class I, or LVEF < 35 percent and are NYHA functional class II. | A | 1, 20, 23, 24 |
| ICD implantation is indicated in patients with nonischemic dilated cardiomyopathy and LVEF ≤ 35 percent who are NYHA functional class II or III. | A | 1, 20, 24, 26, 29 |
| ICD implantation may be considered in patients with nonischemic dilated cardiomyopathy and LVEF ≤ 35 percent who are NYHA functional class I. | B | 1, 20 |
| ICD implantation—secondary prevention | ||
| ICD implantation is indicated in survivors of cardiac arrest due to VF or hemodynamically unstable VT after excluding completely reversible causes. | A | 1, 20 |
| ICD implantation is indicated in patients with structural heart disease and spontaneous sustained or nonsustained VT. | B | 20 |
| Situations in which ICD is not recommended | ||
| ICD implantation is not recommended in patients who do not have a reasonable expectation of survival with an acceptable functional status for at least one year, even if they meet other criteria. | C | 20 |
| ICD implantation is not recommended when certain forms of VT and VF are amenable to catheter ablation in the absence of structural heart disease (i.e., atrial arrhythmias with Wolff-Parkinson-White syndrome, idiopathic VT, outflow tract VT, or fascicular VT). | C | 20 |
Epidemiology
In the United States each year, SCD affects 500,000 persons, accounting for more deaths than stroke, lung cancer, and breast cancer combined.7,8 Worldwide, SCD comprises 50 percent of overall cardiac mortality.2 SCD is the most common and often the first manifestation of coronary artery disease, with 60 percent of cases occurring outside of the hospital setting.9 Coronary artery disease is present in two-thirds of all persons with SCD.10 The index cardiac arrest event is usually fatal; only 10 to 15 percent of SCDs occur after recurrent arrhythmic events.11 Men have a 50 percent higher age-adjusted relative risk of SCD than women, with 75 percent of all SCDs occurring in men.2
Major risk factors for SCD are listed in Table 1. The most common risk factors are reduced left ventricular ejection fraction, acute or prior myocardial infarction, prior ventricular arrhythmias, and congestive heart failure.4,12 Although these well-studied risk factors significantly increase the relative risk of SCD, most sudden deaths in the United States occur in persons without known heart disease or in those with a high coronary risk profile.4,12,13 Other important causes of SCD include structural and electrical disease.1,12

| Common | |
| CAD | |
| Acute MI | |
| Prior MI | |
| CAD or risk factors | |
| Heart failure | |
| Reduced left ventricular ejection fraction due to ischemic or nonischemic (dilated) cardiomyopathy | |
| Arrhythmias | |
| Prior sustained or nonsustained ventricular arrhythmias | |
| Less common | |
| Structural heart disease | |
| Hypertrophic cardiomyopathy | |
| Arrhythmogenic right ventricular dysplasia/cardiomyopathy | |
| Severe left ventricular hypertrophy | |
| Congenital heart disease | |
| Coronary circulatory anomalies | |
| Myocarditis | |
| Electrical disease | |
| Long QT syndrome | |
| Brugada syndrome | |
| Catecholaminergic polymorphic ventricular tachycardia | |
| Primary electrical disease (idiopathic ventricular fibrillation) | |
| Preexcitation syndrome | |
| Chest wall trauma (commotio cordis) | |
| Complete heart block | |
| Drug-induced QT prolongation and polymorphic ventricular tachycardia (torsades de pointes) | |
Implantable Cardioverter-Defibrillators
Implantable cardioverter-defibrillators (ICDs) are designed to detect and treat life-threatening arrhythmias. An ICD consists of a pulse generator and lead, analogous to a pacemaker but with a few notable differences. The ICD generator is larger because it houses a largecapacity battery, a high-voltage capacitor, and a more complex microprocessor. The ICD lead, which is placed in the right ventricular apex, consists of a pacing tip electrode and one or two integrated defibrillator or shock coils that are positioned in the right ventricle and superior vena cava. Biventricular ICDs, or cardiac resynchronization therapy defibrillators, have an additional lead placed in a branch of the coronary sinus via the right atrium for left ventricular pacing. A chest radiograph can help differentiate among these devices (Figure 1).
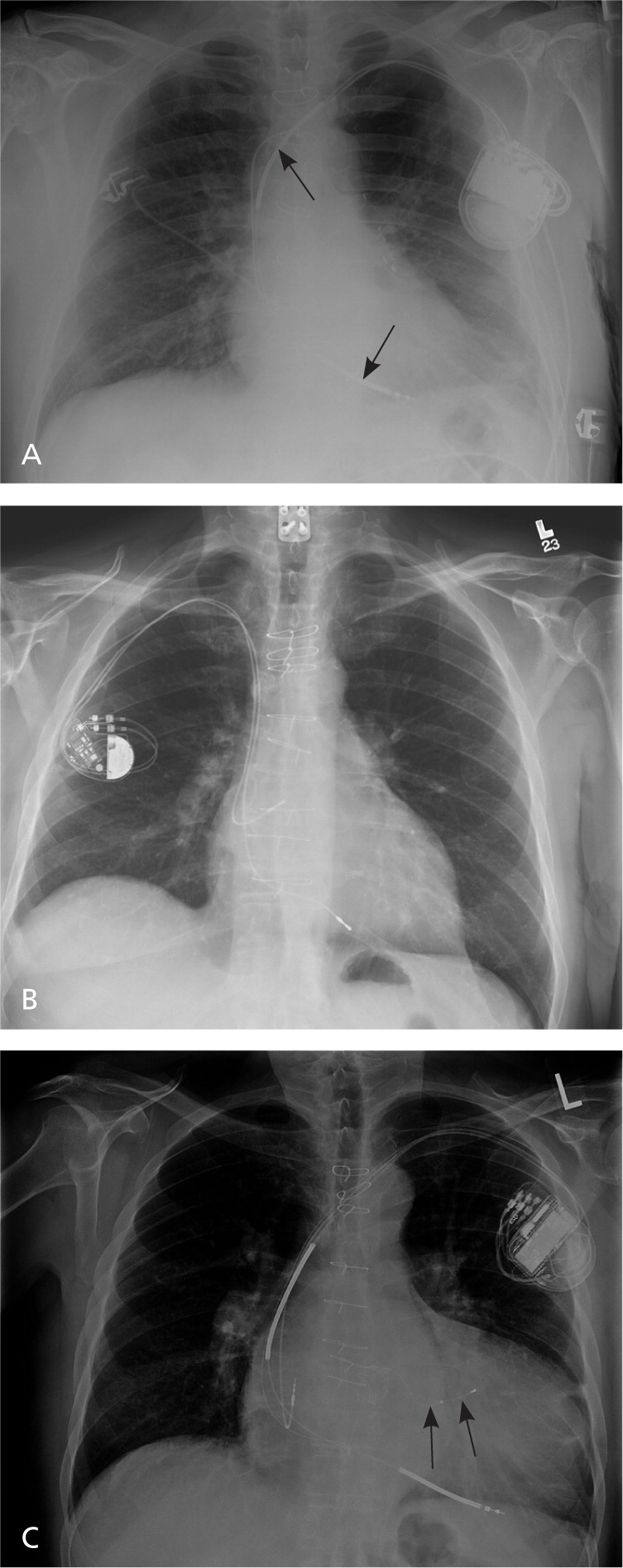
Although ICDs have pacing capabilities nearly identical to conventional pacemakers, ICDs are generally programmed to minimize ventricular pacing. In contrast, cardiac resynchronization therapy defibrillators are intended to always pace the right and left ventricles to prevent ventricular dyssynchrony from underlying intraventricular conduction delay or bundle branch block. Modern defibrillator systems are implanted via transvenous access from an incision below the clavicle and medial to the deltopectoral groove, similar to a pacemaker. Defibrillation threshold testing is usually performed at implantation to test device integrity and ensure successful internal defibrillation. Experienced operators can perform the procedure in one to two hours. The most common complications are hematoma (1.1 percent of implantations), lead dislodgement (1.0 percent), and pneumothorax (0.5 percent); the cumulative major complication rate is 1.5 percent.14 The procedure is performed by cardiac electrophysiologists, cardiologists, and cardiac surgeons. Implantation by a board-certified cardiac electrophysiologist is associated with a lower risk of complications.14
An ICD will continuously scan the patient's intrinsic heart rhythm in real time to detect tachycardia. If detection criteria are met for VT or VF, usually based on ventricular rate, the device can terminate the ventricular arrhythmia with overdrive pacing, cardioversion, or defibrillation. The device rate zones can be programmed to allow for different types of therapies based on ventricular rate (Table 2). ICDs are believed to terminate 97 percent of ventricular arrhythmias.15 Modern ICD generators store intracardiac electrograms of detected arrhythmias and delivered therapies, which can be downloaded via in-person interrogation or remotely via transtelephonic monitoring from the patient's home.
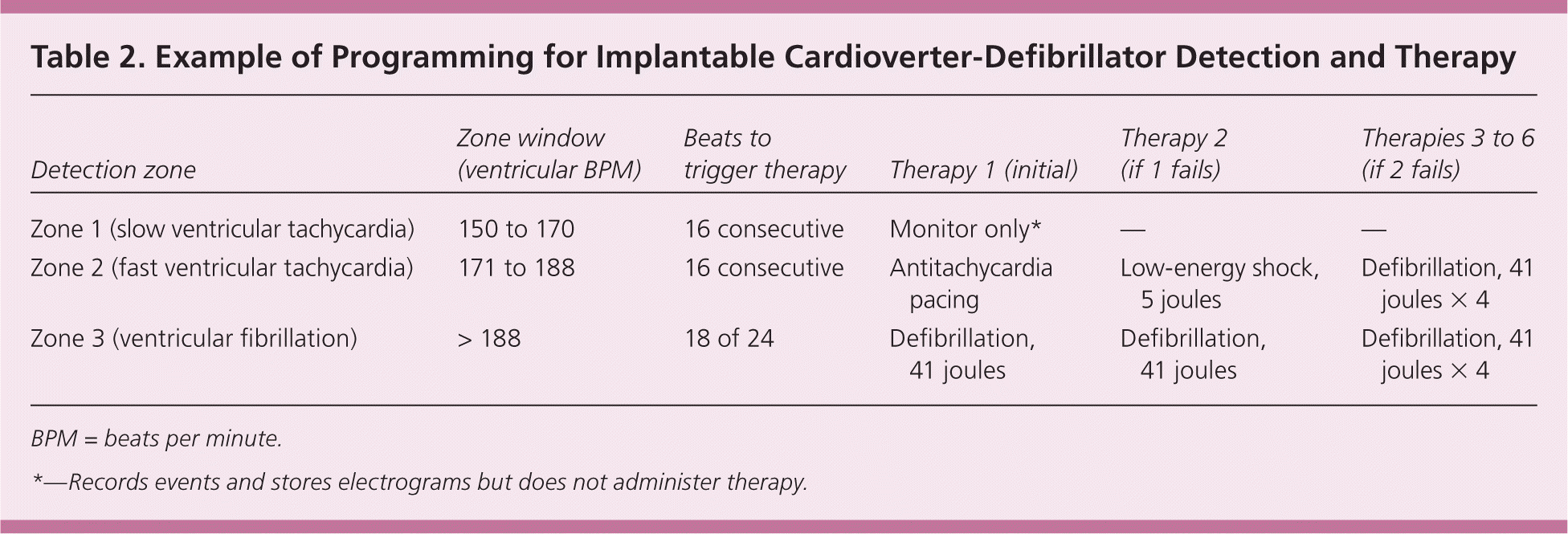
| Detection zone | Zone window (ventricular BPM) | Beats to trigger therapy | Therapy 1 (initial) | Therapy 2 (if 1 fails) | Therapies 3 to 6 (if 2 fails) |
|---|---|---|---|---|---|
| Zone 1 (slow ventricular tachycardia) | 150 to 170 | 16 consecutive | Monitor only* | — | — |
| Zone 2 (fast ventricular tachycardia) | 171 to 188 | 16 consecutive | Antitachycardia pacing | Low-energy shock, 5 joules | Defibrillation, 41 joules × 4 |
| Zone 3 (ventricular fibrillation) | > 188 | 18 of 24 | Defibrillation, 41 joules | Defibrillation, 41 joules | Defibrillation, 41 joules × 4 |
Secondary Prevention
The first randomized trial of ICDs was not published until 17 years after the first ICD was implanted.16 Three secondary prevention trials have demonstrated that ICDs reduce mortality in persons with aborted SCD or documented VT or VF, compared with antiarrhythmic drug therapy.17–19 Therefore, patients with recent or remote VT, VF, or aborted sudden death should be promptly referred for risk stratification.1,20 It should be noted that some forms of VT or triggers of VT are amenable to catheter ablation, and in the absence of structural heart disease, ICD may not be required after successful ablation.20
Primary Prevention
Because the index arrhythmic event is usually fatal in persons with SCD, identifying high-risk persons is critical. Almost all tests and clinical features lack adequate sensitivity, specificity, and reproducibility to identify vulnerable patients; therefore, criteria for selecting patients for primary prevention are difficult to define. Studies show that reduced left ventricular ejection fraction predicts sudden death in ischemic and nonischemic dilated cardiomyopathy.16 Five randomized trials have subsequently evaluated ICDs for primary prevention in patients with reduced ejection fraction (less than 30 to 40 percent) and accompanying risk factors, including prior myocardial infarction, nonsustained VT, symptomatic heart failure, and inducible VT on invasive electrophysiologic testing.21–25
In the second landmark Multicenter Automated Defibrillator Implantation Trial (MADIT-II), 1,232 patients with prior myocardial infarction and an ejection fraction of 30 percent or less received an ICD or drug therapy.23 The trial was stopped at 20 months because the ICD arm had a 6 percent reduction in absolute mortality and a 31 percent reduction in relative mortality. In the Sudden Cardiac Death in Heart Failure Trial (SCDHeFT), 2,500 patients with stable New York Heart Association class II or III heart failure and an ejection fraction of 35 percent or less received an ICD, amiodarone (Cordarone), or placebo.24 At four years, the ICD arm had a 7 percent reduction in absolute mortality and a 23 percent reduction in relative mortality, with no difference between the amiodarone and placebo arms. The benefit of ICDs was comparable in patients with ischemic and nonischemic cardiomyopathy. Based on these and other data, the American College of Cardiology, American Heart Association, and Heart Rhythm Society published joint guidelines recommending ICDs for primary prevention in patients with an ejection fraction of less than 30 or 35 percent, depending on heart failure etiology and severity. These guidelines for ICD placement are summarized in Table 3.20 Therefore, in patients with heart disease, assessment of ejection fraction remains the most important step to identify those who are at risk of SCD and would benefit from ICD implantation.
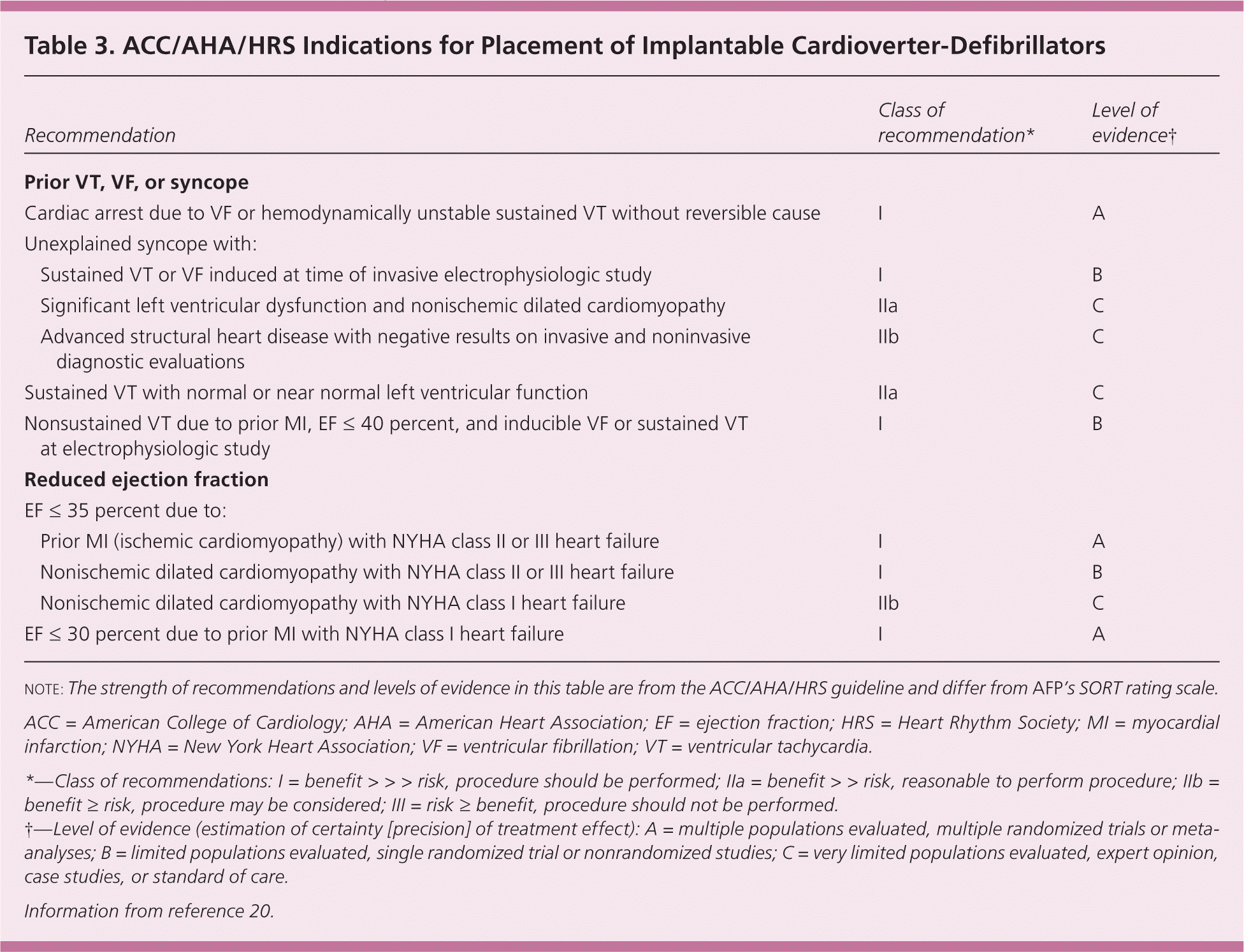
| Recommendation | Class of recommendation* | Level of evidence† | |
|---|---|---|---|
| Prior VT, VF, or syncope | |||
| Cardiac arrest due to VF or hemodynamically unstable sustained VT without reversible cause | I | A | |
| Unexplained syncope with: | |||
| Sustained VT or VF induced at time of invasive electrophysiologic study | I | B | |
| Significant left ventricular dysfunction and nonischemic dilated cardiomyopathy | IIa | C | |
| Advanced structural heart disease with negative results on invasive and noninvasive diagnostic evaluations | IIb | C | |
| Sustained VT with normal or near normal left ventricular function | IIa | C | |
| Nonsustained VT due to prior MI, EF ≤ 40 percent, and inducible VF or sustained VT at electrophysiologic study | I | B | |
| Reduced ejection fraction | |||
| EF ≤ 35 percent due to: | |||
| Prior MI (ischemic cardiomyopathy) with NYHA class II or III heart failure | I | A | |
| Nonischemic dilated cardiomyopathy with NYHA class II or III heart failure | I | B | |
| Nonischemic dilated cardiomyopathy with NYHA class I heart failure | IIb | C | |
| EF ≤ 30 percent due to prior MI with NYHA class I heart failure | I | A | |
Although these trials demonstrated early and sustained benefit of ICDs in patients with reduced ejection fraction, the absolute risk reductions were varied and, in a few cases, only modest.26–29 The Centers for Medicare and Medicaid Services covers guideline-supported indications for primary prevention ICDs,30 but the cost is substantial at an estimated $30,000 to $50,000 per implant, not including costs of follow-up and device replacement. ICD placement is considered cost-effective if the patient survival is at least seven years with the device (incremental cost-effectiveness ratio less than $100,000 per quality-adjusted life-year).31 However, registry data tracking real-world cases have shown that many U.S. patients receiving ICDs are older and have more comorbidities compared with those enrolled in randomized trials.20 Benefit is also diminished in patients with chronic kidney disease32 and in those who are older.33,34 As a result, ICDs are generally not recommended in patients who do not have a reasonable expectation of survival with an acceptable functional status beyond one year.20 Additional studies to improve the specificity of risk prediction are ongoing,35,36 and novel Bayesian methods to characterize the strength of evidence of ICD guidelines based on pretest risk have been proposed.37
Still, reduced ejection fraction remains the single best predictor of SCD. A systematic review of 12 randomized trials and 76 observational studies with more than 100,000 patients showed that ICDs are effective in adults with reduced ejection fraction, and that the benefits extend to nontrial populations.38 These findings were validated in a technology assessment by the Agency for Healthcare Research and Quality using similar study selection criteria and methods. 39 Therefore, in patients with a history of myocardial infarction, coronary artery disease, cardiomyopathy, or heart failure, the ejection fraction should first be determined, 40 followed with a more detailed discussion involving the patient, primary care physician, and electrophysiologist or cardiologist. Other risk factors such as nonsustained VT, syncope of undetermined origin, structural heart disease, and inherited heart failure or arrhythmia syndromes may also carry substantial risk of SCD, and consultation with a cardiac electrophysiologist or cardiologist is advised.1,20,41
Evaluation of Patients with ICDs
Due to the increasing prevalence of ICDs in the United States, primary care and emergency department physicians may often encounter patients with these devices. When taking a history in these patients, it is critical to clarify five key elements (Table 4). If the device was placed for primary prevention, then the primary disease state should be determined (e.g., ischemic or nonischemic cardiomyopathy). If the ICD was placed for secondary prevention, then the circumstances relating to the index event should be explored. Pacemaker dependency may be established by confirming a history of complete atrioventricular heart block, severe sinus bradycardia, prior catheter ablation of the atrioventricular node, or an ICD interrogation that demonstrates the absence of a normal underlying rhythm.
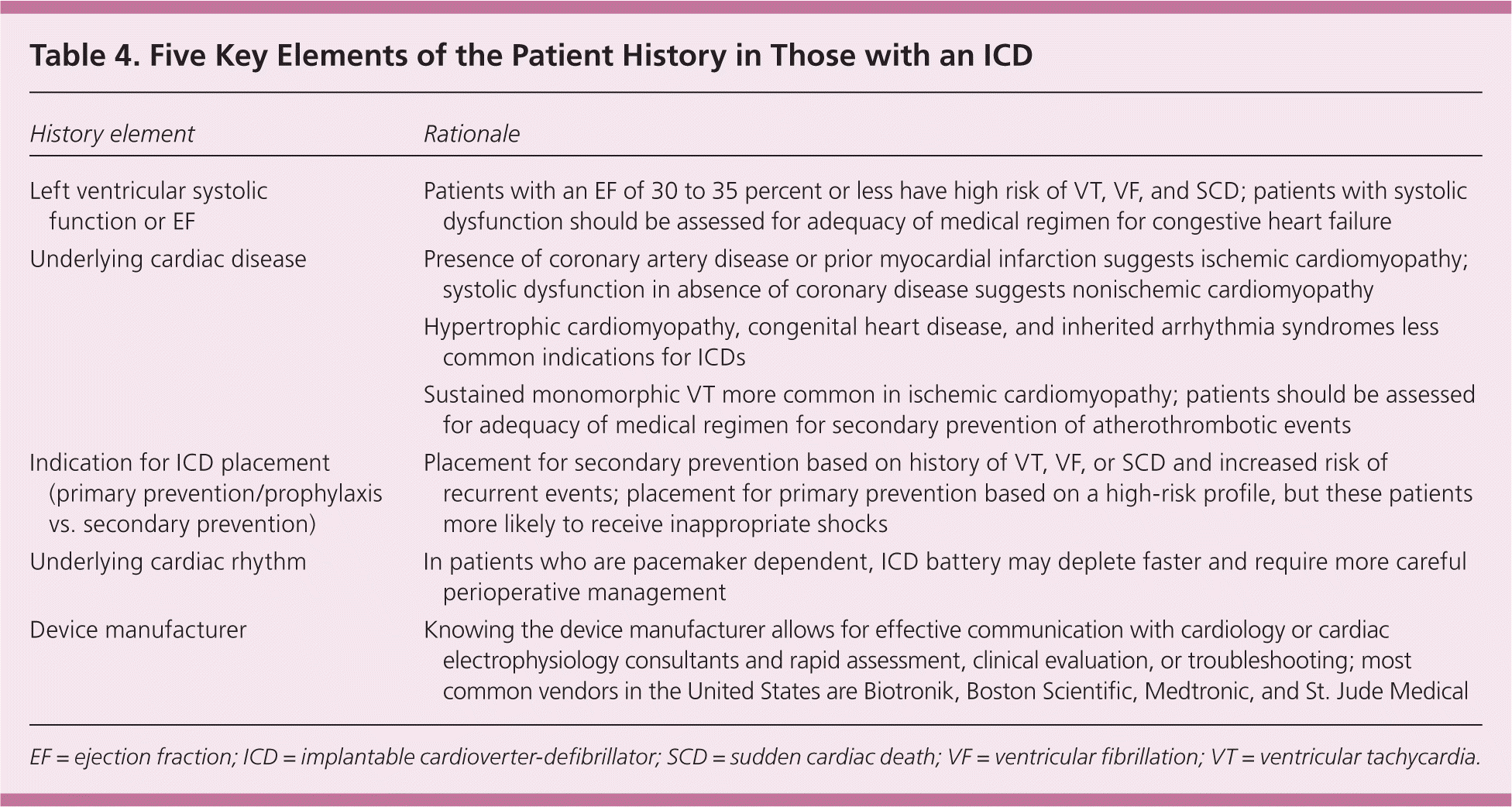
| History element | Rationale |
|---|---|
| Left ventricular systolic function or EF | Patients with an EF of 30 to 35 percent or less have high risk of VT, VF, and SCD; patients with systolic dysfunction should be assessed for adequacy of medical regimen for congestive heart failure |
| Underlying cardiac disease | Presence of coronary artery disease or prior myocardial infarction suggests ischemic cardiomyopathy; systolic dysfunction in absence of coronary disease suggests nonischemic cardiomyopathy |
| Hypertrophic cardiomyopathy, congenital heart disease, and inherited arrhythmia syndromes less common indications for ICDs | |
| Sustained monomorphic VT more common in ischemic cardiomyopathy; patients should be assessed for adequacy of medical regimen for secondary prevention of atherothrombotic events | |
| Indication for ICD placement (primary prevention/prophylaxis vs. secondary prevention) | Placement for secondary prevention based on history of VT, VF, or SCD and increased risk of recurrent events; placement for primary prevention based on a high-risk profile, but these patients more likely to receive inappropriate shocks |
| Underlying cardiac rhythm | In patients who are pacemaker dependent, ICD battery may deplete faster and require more careful perioperative management |
| Device manufacturer | Knowing the device manufacturer allows for effective communication with cardiology or cardiac electrophysiology consultants and rapid assessment, clinical evaluation, or troubleshooting; most common vendors in the United States are Biotronik, Boston Scientific, Medtronic, and St. Jude Medical |
The manufacturer of the ICD generator should be documented in case urgent consultation is required. Most patients know the manufacturer of their ICD and carry a device identification card. New patients should be asked the date and result of their last ICD check; follow-up device interrogations should be scheduled at three- or six-month intervals. The patient should be asked about any history of ICD shocks, palpitations, heart failure exacerbations, and syncope, and whether these were addressed at the previous device interrogation. Battery life is typically five to seven years, although there may be substantial variation.
Evaluation of an ICD Shock
Patients who experience an ICD shock may seek urgent or emergency department care, or first contact their primary care physician. Because patients are usually conscious when shocked, they often can recall the number of shocks, which can cause intense pain (similar to a blow to the chest) and chest wall soreness. In patients with a single shock, the physician should determine whether the patient has had a change in clinical status. The patient should be asked about chest pain, progression of heart failure symptoms, concurrent illnesses, and medication nonadherence. A focused clinical evaluation for ischemia and heart failure should be performed.
Table 5 lists causes of ICD shocks. Consultation with a cardiac electrophysiologist or cardiologist and interrogation of the ICD can help determine if the shocks were appropriate. A shock is defined as appropriate when a tachyarrhythmia is correctly classified as VT or VF by the device and treated. A shock is inappropriate if it was delivered for any other reason. Because ICDs detect tachyarrhythmias primarily by ventricular rate, which is nonspecific for VT or VF, inappropriate shocks can occur. The most common cause of an inappropriate shock is a supraventricular tachycardia, such as rapid atrial fibrillation in which the ventricular rate falls into the VT or VF detection zone. With the development of more sophisticated arrhythmia discrimination algorithms, the frequency of shocks for supraventricular tachycardia has decreased, but they continue to occur in 15 percent of patients.42–44 Causes related to device programming and integrity must also be excluded.

| Appropriate shocks | |
| Monomorphic ventricular tachycardia | |
| Ventricular fibrillation | |
| Polymorphic ventricular tachycardia (torsades de pointes) | |
| Inappropriate shocks | |
| Supraventricular tachycardia meeting ventricular rate detection criteria | |
| Atrial fibrillation | |
| Atrial flutter or atrial tachycardia | |
| Atrioventricular nodal reentrant tachycardia | |
| Atrioventricular reentrant tachycardia | |
| Junctional tachycardia | |
| Sinus tachycardia | |
| Multiple premature ventricular complexes | |
| Device oversensing with no system defect or failure | |
| Oversensing of T wave or double counting of ventricular electrogram | |
| Oversensing of diaphragmatic myopotentials | |
| External electromagnetic interference | |
| Device oversensing with system defect or failure | |
| ICD lead fracture | |
| ICD lead insulation break | |
| Electrical chatter from lead dislodgement or loose header set screw | |
Unlike a single shock, repeated ICD discharges require prompt evaluation and more urgent consultation.1,20 Patients should be promptly triaged to emergency care with electrocardiographic monitoring. The patient may have an ongoing arrhythmia (with failure to terminate) or recurrent or incessant ventricular arrhythmias (i.e., an electrical storm). Lead fracture, in which the conductor of the pace/sense electrode breaks, can cause electrical noise in the system that is inappropriately interpreted and treated as VF.45 Older leads may be more vulnerable to fracture, although premature failure and high fracture rates recently prompted the recall of one popular ICD lead.46–48
Recurrent shocks should be treated by addressing the underlying cause. Ischemia, heart failure, antiarrhythmic toxicity, and electrolyte derangement should be excluded. Recurrent monomorphic VT may be treated with antiarrhythmic drugs or catheter ablation. Single or recurrent shocks are associated with anxiety, posttraumatic stress, and depression.49 Physicians should screen for these symptoms and consider referral for psychological evaluation and counseling. Finally, optimal pharmacologic therapy and risk factor modification in patients with coronary disease, systolic dysfunction, and heart failure improve quality of life and survival independent of the placement of an ICD. Therefore, ICD placement should be regarded as an adjunct to rather than a substitute for medical therapy for the underlying cardiovascular condition.
Perioperative Considerations
In patients with ICDs, the goals during a surgical procedure are to maintain a stable and normal cardiac rhythm and to avoid inappropriate ICD shocks from electromagnetic interference (e.g., electrocautery, lithotripsy, electroconvulsive therapy).50 There are no definitive guidelines or clinical studies that define optimal perioperative management.50 Therefore, the physician should inform the anesthesiologist and surgeon of the presence of the device and provide key points of the patient history (Table 4). The cardiac electrophysiologist should be informed in advance of the procedure.
To prevent ICD lead damage or dislodgement, elective cannulation of the internal jugular or subclavian veins for central venous access should be avoided or performed with fluoroscopic guidance. Peripherally inserted central catheters should be placed contralateral to the device leads. For large surgeries with thoracic or abdominal manipulation, the device should be interrogated postoperatively to ensure that leads were not dislodged or damaged.
Magnetic resonance imaging (MRI) is generally contraindicated because of possible heating of and damage to the ICD components from electromagnetic flux, although it may be feasible with certain MRI protocols51 and certain MRI-compatible systems. For emergency surgery or when there is insufficient time for consultation, the placement of a donut-shaped magnet over the ICD generator pocket will suspend tachyarrhythmia detection without affecting bradycardia pacing. If VT or VF occurs intraoperatively, removal of the magnet will allow the ICD to resume detection and therapy, usually within 10 seconds, as long as the device does not have a feature that permanently inactivates the ICD with magnet placement.52 Magnet use should be performed in coordination with an electrophysiologist or cardiologist.
Regardless of approach, external defibrillation pads should be in place and attached to an external defibrillator during the surgical procedure. ICDs should not be regarded as a substitute for cardiac telemetry. According to Advanced Cardiac Life Support guidelines, arrhythmia management should not be delayed or suspended while waiting for an ICD to respond.52
