
Am Fam Physician. 2011;84(12):1365-1375
Patient information: See related handout on Crohn's disease, written by the authors of this article.
Author disclosure: No relevant financial affiliations to disclose.
Crohn's disease is a chronic inflammatory condition affecting the gastrointestinal tract at any point from the mouth to the rectum. Patients may experience diarrhea, abdominal pain, fever, weight loss, abdominal masses, and anemia. Extraintestinal manifestations of Crohn's disease include osteoporosis, inflammatory arthropathies, scleritis, nephrolithiasis, cholelithiasis, and erythema nodosum. Acute phase reactants, such as C-reactive protein level and erythrocyte sedimentation rate, are often increased with inflammation and may correlate with disease activity. Levels of vitamin B12, folate, albumin, prealbumin, and vitamin D can help assess nutritional status. Colonoscopy with ileoscopy, capsule endoscopy, computed tomography enterography, and small bowel follow-through are often used to diagnose Crohn's disease. Ultrasonography, computed axial tomography, scintigraphy, and magnetic resonance imaging can assess for extraintestinal manifestations or complications (e.g., abscess, perforation). Mesalamine products are often used for the medical management of mild to moderate colonic Crohn's disease. Antibiotics (e.g., metronidazole, fluoroquinolones) are often used for treatment. Patients with moderate to severe Crohn's disease are treated with corticosteroids, azathioprine, 6-mercaptopurine, or anti–tumor necrosis factor agents (e.g., infliximab, adalimumab). Severe disease may require emergent hospitalization and a multidisciplinary approach with a family physician, gastroenterologist, and surgeon.
Crohn's disease is a chronic inflammatory condition of the gastrointestinal tract characterized by inflammation at any point from the mouth to the rectum (Table 1). The prevalence in the United States is 201 per 100,000 adults.1 Patients with Crohn's disease often present in adolescence, and the median age at diagnosis is 20 to 30 years.2 Crohn's disease is more prevalent in women than men, in developed countries, and in the northern hemisphere.1,2 The annual U.S. economic burden of Crohn's disease is estimated to be $10.9 to 15.5 billion in 2006 U.S. dollars.3
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Ultrasonography, computed axial tomography, scintigraphy, and magnetic resonance imaging are helpful for excluding extramural complications in persons with Crohn's disease. | C | 8, 12 |
| Colonoscopy with ileoscopy and biopsy is a valuable initial test in the diagnosis of ileocolonic Crohn's disease. | C | 8 |
| Esophagogastroduodenoscopy is recommended in patients with Crohn's disease who have upper gastrointestinal symptoms. | C | 8 |
| There is no difference between elemental and nonelemental diets in inducing remission in patients with Crohn's disease. | A | 18 |
| Budesonide (Entocort EC) is effective in inducing, but not maintaining, remission in patients with Crohn's disease. | B | 21, 37 |
| Corticosteroids are more effective than placebo and 5-aminosalicylic acid products in inducing remission in patients with Crohn's disease. | A | 22 |
| Azathioprine (Imuran) and 6-mercaptopurine are effective in inducing remission in patients with active Crohn's disease. | A | 23 |
| Methotrexate is effective in inducing and maintaining remission in patients with Crohn's disease. | B | 25, 33 |
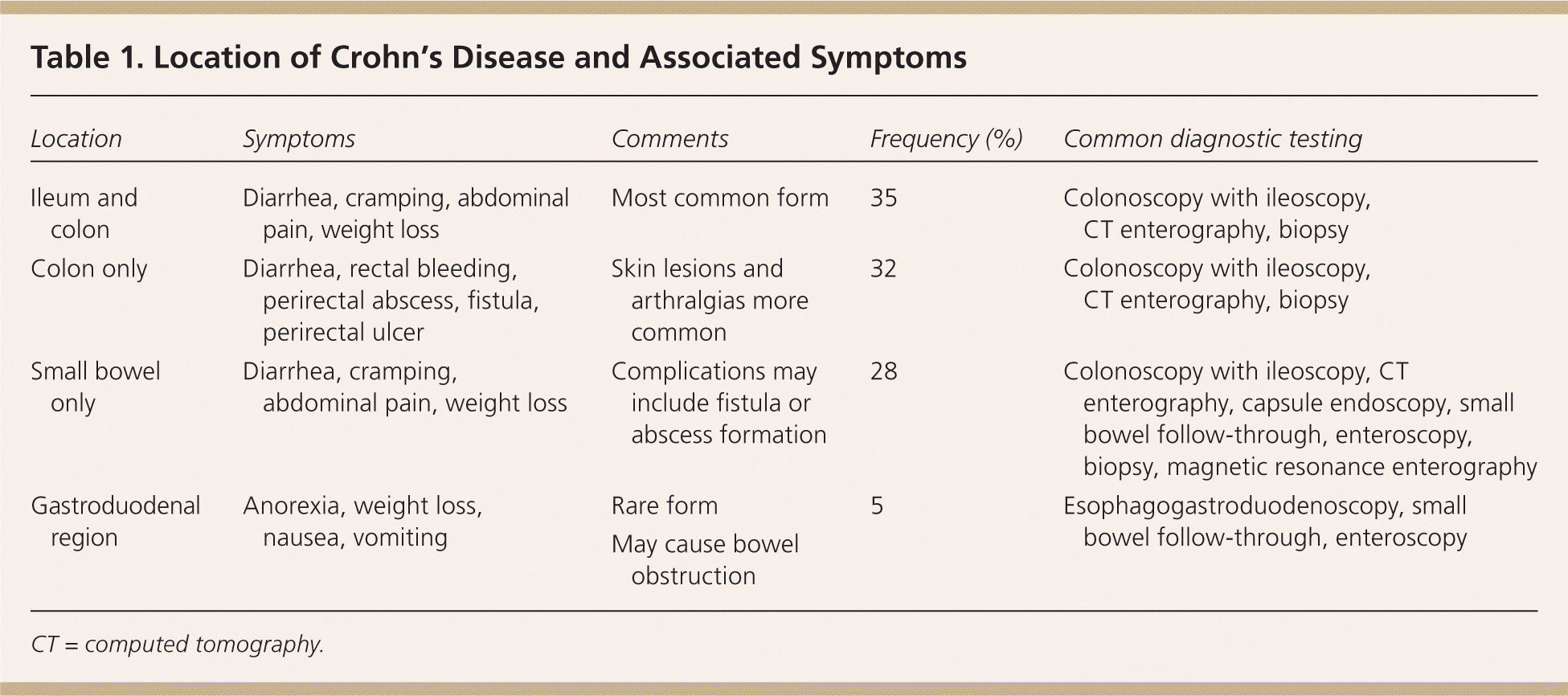
| Location | Symptoms | Comments | Frequency (%) | Common diagnostic testing |
|---|---|---|---|---|
| Ileum and colon | Diarrhea, cramping, abdominal pain, weight loss | Most common form | 35 | Colonoscopy with ileoscopy, CT enterography, biopsy |
| Colon only | Diarrhea, rectal bleeding, perirectal abscess, fistula, perirectal ulcer | Skin lesions and arthralgias more common | 32 | Colonoscopy with ileoscopy, CT enterography, biopsy |
| Small bowel only | Diarrhea, cramping, abdominal pain, weight loss | Complications may include fistula or abscess formation | 28 | Colonoscopy with ileoscopy, CTenterography, capsule endoscopy, small bowel follow-through, enteroscopy, biopsy, magnetic resonance enterography |
| Gastroduodenal region | Anorexia, weight loss, nausea, vomiting | Rare form | 5 | Esophagogastroduodenoscopy, small bowel follow-through, enteroscopy |
| May cause bowel obstruction |
Clinical Features
Inflammatory bowel disease includes two distinct chronic conditions (i.e., Crohn's disease and ulcerative colitis) that have significant clinical and pathologic differences (Table 2).
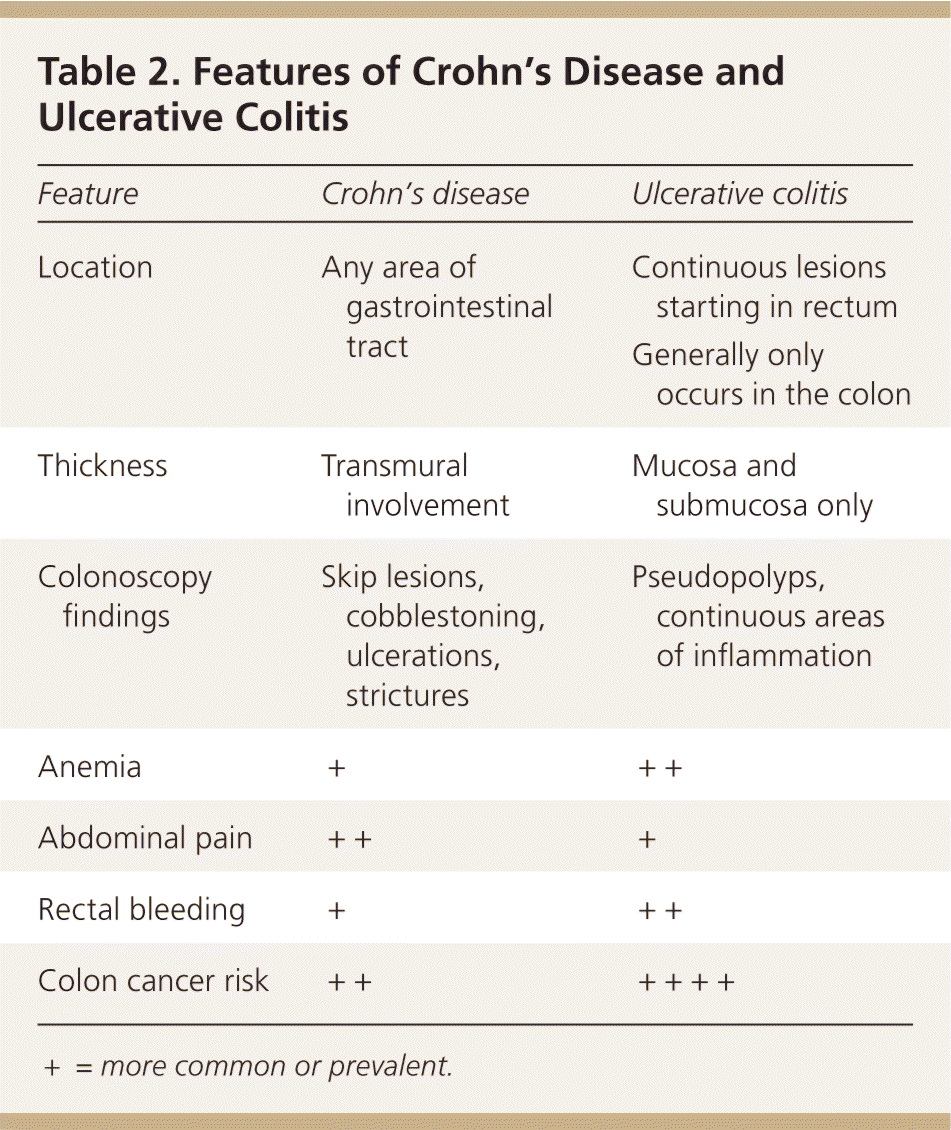
| Feature | Crohn's disease | Ulcerative colitis |
|---|---|---|
| Location | Any area of gastrointestinal tract | Continuous lesions starting in rectum |
| Generally only occurs in the colon | ||
| Thickness | Transmural involvement | Mucosa and submucosa only |
| Colonoscopy findings | Skip lesions, cobblestoning, ulcerations, strictures | Pseudopolyps, continuous areas of inflammation |
| Anemia | + | ++ |
| Abdominal pain | ++ | + |
| Rectal bleeding | + | ++ |
| Colon cancer risk | ++ | ++++ |
HISTORY AND PHYSICAL EXAMINATION
Common symptoms of Crohn's disease include abdominal pain, diarrhea, fatigue, fever, gastrointestinal bleeding, and weight loss. The history should address the onset, severity, and pattern of symptoms, especially frequency and consistency of bowel movements. History targeting risk factors and possible alternative diagnoses includes recent travel, exposure to antibiotics, food intolerance, medications, smoking, and family history of inflammatory bowel disease8 (Table 39 ). Specific questions addressing extraintestinal manifestations include eye and joint problems and symptoms of anemia (Table 4).10 Questions about the impact of symptoms should include time missed from school or work.
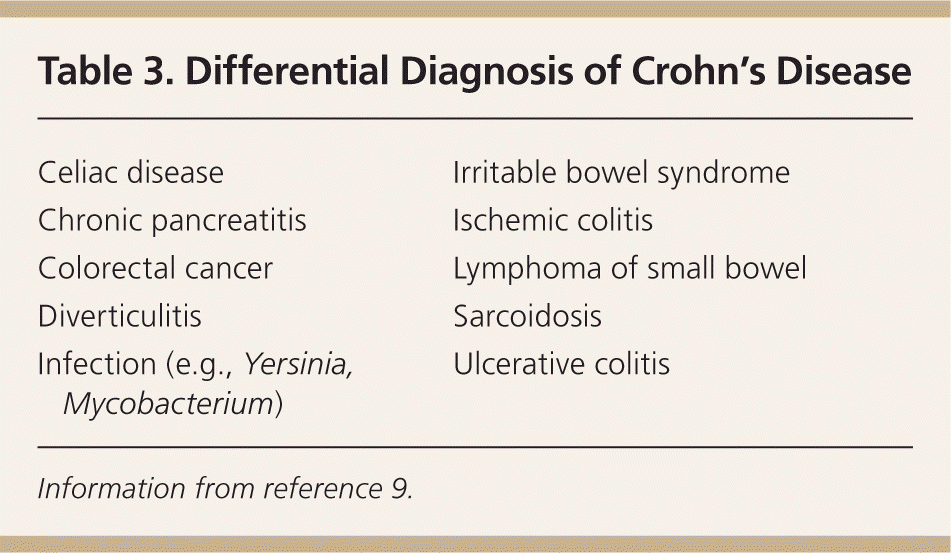
| Celiac disease |
| Chronic pancreatitis |
| Colorectal cancer |
| Diverticulitis |
| Infection (e.g., Yersinia, Mycobacterium) |
| Irritable bowel syndrome |
| Ischemic colitis |
| Lymphoma of small bowel |
| Sarcoidosis |
| Ulcerative colitis |
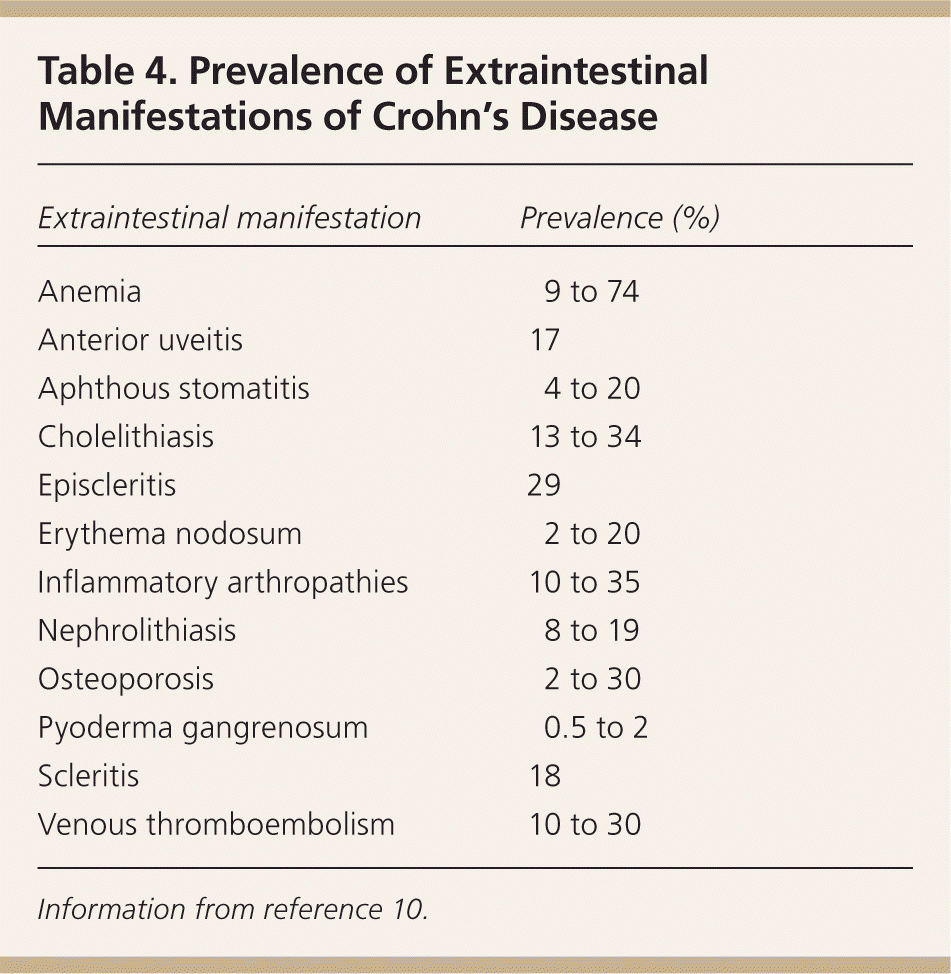
| Extraintestinal manifestation | Prevalence (%) |
|---|---|
| Anemia | 9 to 74 |
| Anterior uveitis | 17 |
| Aphthous stomatitis | 4 to 20 |
| Cholelithiasis | 13 to 34 |
| Episcleritis | 29 |
| Erythema nodosum | 2 to 20 |
| Inflammatory arthropathies | 10 to 35 |
| Nephrolithiasis | 8 to 19 |
| Osteoporosis | 2 to 30 |
| Pyoderma gangrenosum | 0.5 to 2 |
| Scleritis | 18 |
| Venous thromboembolism | 10 to 30 |
During the physical evaluation, heart rate, blood pressure, temperature, and body weight should be measured.8 Abdominal examination may reveal tenderness, distention, or masses.8 An anorectal examination should be performed because one-third of patients have a perirectal abscess, fissure, or fistula at some time during the illness.11
EXTRAINTESTINAL MANIFESTATIONS
Extraintestinal manifestations of Crohn's disease are common and include anemia, cholelithiasis, erythema nodosum, inflammatory arthropathies, nephrolithiasis, osteoporosis, uveitis, scleritis, and venous thromboembolism (Table 4).10 Ultrasonography, computed axial tomography, scintigraphy, and magnetic resonance imaging are helpful for excluding extramural complications.8,12 The diagnostic accuracy of these tests is provided in Table 5.12
| Test | Sensitivity (%) | Specificity (%) | Positive likelihood ratio†‡ | Negative likelihood ratio†‡ | Positive predictive value (%)† | Negative predictive value (%)† |
|---|---|---|---|---|---|---|
| Computed axial tomography | 84.3 | 95.1 | 3.8 | 0.03 | 79.0 | 96.5 |
| Magnetic resonance imaging | 93.0 | 92.8 | 2.8 | 0.02 | 73.9 | 98.3 |
| Scintigraphy | 87.8 | 84.5 | 1.2 | 0.03 | 55.4 | 96.9 |
| Ultrasonography | 89.7 | 95.6 | 4.4 | 0.02 | 81.6 | 97.5 |
Diagnostic Studies
LABORATORY TESTING
Laboratory tests are useful for diagnosing Crohn's disease, assessing disease activity, identifying complications, and monitoring response to therapy. Initial testing often includes white blood cell count; platelet count; measurement of hemoglobin, hematocrit, blood urea nitrogen, creatinine, liver enzymes, and C-reactive protein; and erythrocyte sedimentation rate. Stool culture and testing for Clostridium difficile toxin should be considered.8 Presence of antibodies to Escherichia coli outer membrane porin and Saccharomyces cerevisiae is suggestive of Crohn's disease, whereas perinuclear antineutrophil cytoplasmic antibody is more suggestive of ulcerative colitis.13
Subsequent testing may include measurement of iron, ferritin, total iron-binding capacity, vitamin B12, folate, albumin, prealbumin, calcium, and vitamin D to monitor common complications. Fecal lactoferrin and calprotectin are surrogate markers for bowel inflammation and may help distinguish between inflammatory conditions and irritable bowel syndrome.14,15 An elevated fecal calprotectin level reliably indicates relapse in patients with Crohn's disease (sensitivity of 80 percent; specificity of 90.7 percent; positive likelihood ratio = 1.9; negative likelihood ratio = 0.04).14 Table 6 lists laboratory tests to assess disease activity and complications in patients with Crohn's disease.8,14,15
| Category | Test | Initial testing | Subsequent testing | Comments |
|---|---|---|---|---|
| General | White blood cell count | ✓ | ✓ | Elevated with inflammation or infection, or secondary to glucocorticoid use |
| Decreased with 6-mercaptopurine and azathioprine (Imuran) use | ||||
| Hemoglobin and hematocrit level | ✓ | ✓ | Anemia | |
| Acute phase reactants | Platelet count | ✓ | ✓ | Increased with inflammation or decreased with treatment (e.g., azathioprine) |
| C-reactive protein level and erythrocyte sedimentation rate | ✓ | ✓ | If elevated, may correlate with disease activity | |
| Stool studies | Stool for culture, ova and parasites, and Clostridium difficile toxin | ✓ | ✓ | To rule out major infectious cause of diarrhea |
| Nutritional status | Iron, ferritin, vitamin B12, and folate levels; total iron-binding capacity | ✓ | Decreased absorption or increased iron loss leading to anemia | |
| Albumin and prealbumin levels | ✓ | Decreased with poor nutritional status and with protein-losing enteropathy | ||
| Vitamin D and calcium levels | ✓ | Decreased secondary to malabsorption, small bowel resection, or corticosteroid impairment of vitamin D metabolism | ||
| Measure when initiating corticosteroid therapy | ||||
| Complications | Liver function testing | ✓ | ✓ | Performed to rule out sclerosing cholangitis, screen for adverse effects of therapies |
| Blood urea nitrogen and creatinine levels | ✓ | ✓ | Monitor renal function | |
| Diagnosis | Fecal lactoferrin and calprotectin levels | ✓ | Surrogate marker for bowel inflammation | |
| May distinguish between flare-up of Crohn's disease and symptoms of irritable bowel syndrome | ||||
| Antibodies to Escherichia coli outer membrane porin and Saccharomyces cerevisiae; perinuclear antineutrophil cytoplasmic antibody | ✓ | Distinguish between Crohn's disease and ulcerative colitis |
ENDOSCOPY AND RELATED INVESTIGATIONS
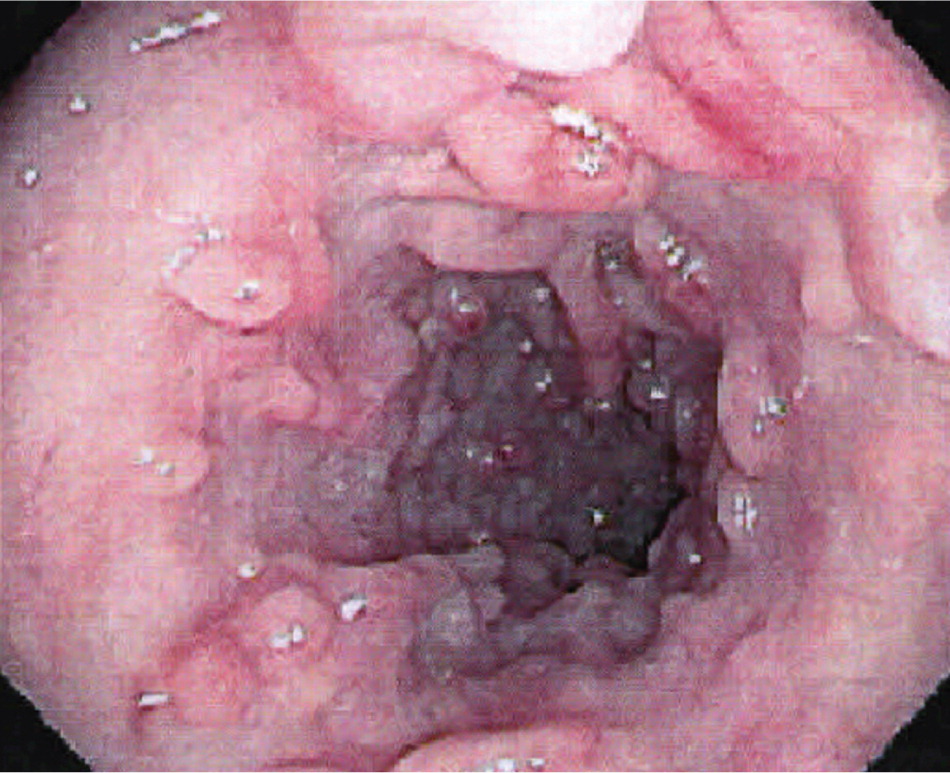
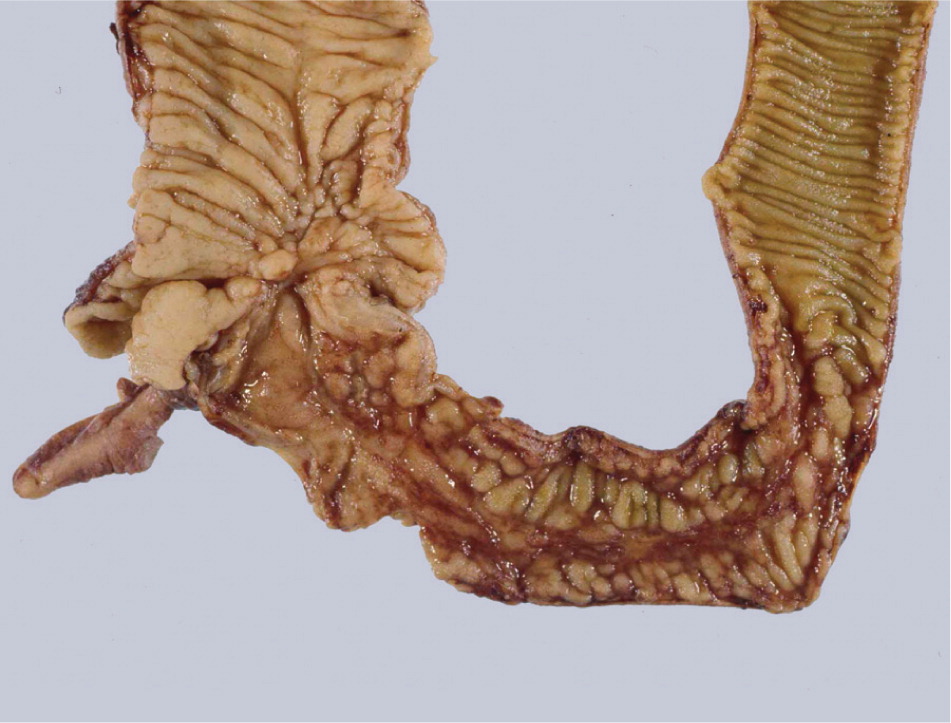
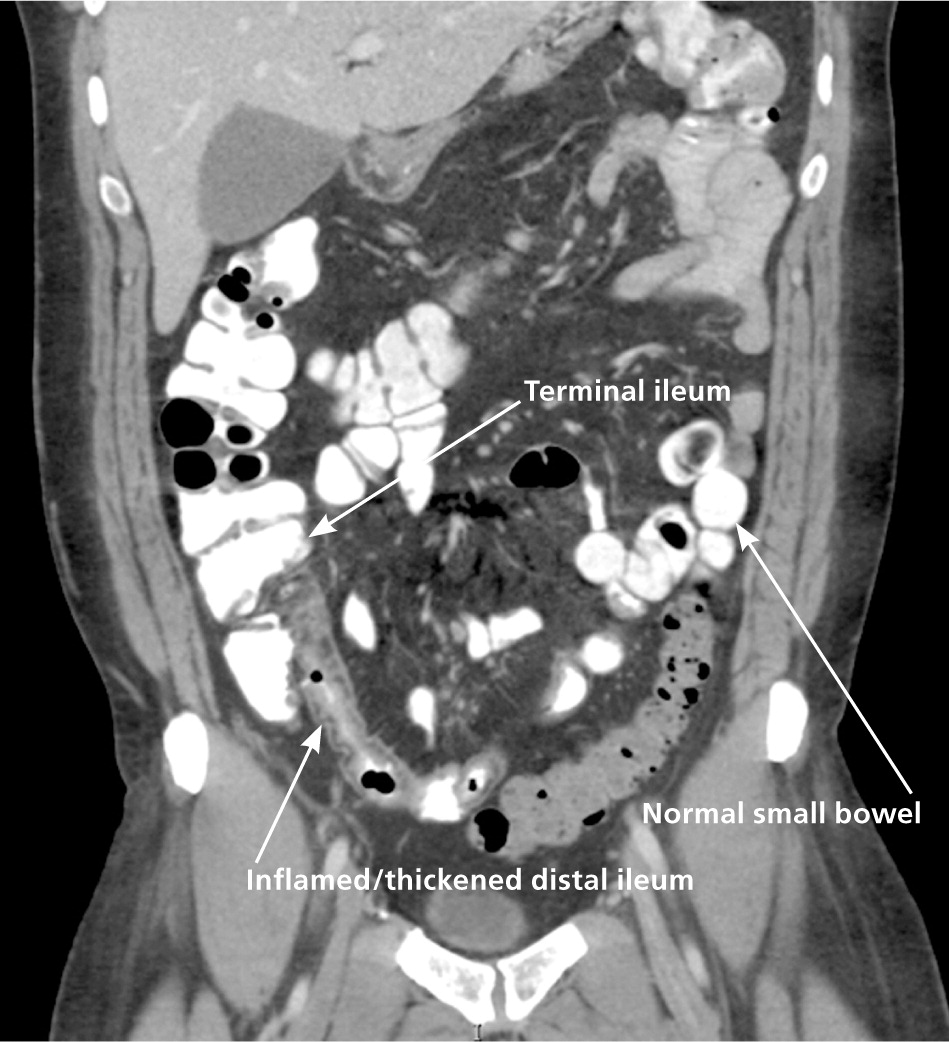
Colonoscopy with ileoscopy and biopsy is valuable in the diagnosis of Crohn's disease at the junction of the ileum and colon8 (Figure 1). Characteristic endoscopic findings include skip lesions, cobblestoning (Figure 2), ulcerations, and strictures. Histology may show neutrophilic inflammation, noncaseating granulomas, Paneth cell metaplasia, and intestinal villi blunting. Other diagnostic tests useful in the diagnosis of small bowel Crohn's disease include capsule endoscopy, computed tomography enterography (Figure 3), magnetic resonance enterography, and small bowel follow-through (Tables 716 and 8). Capsule endoscopy should be avoided in patients with small bowel strictures because capsule retention may occur. Esophagogastroduodenoscopy is recommended in patients with upper gastrointestinal symptoms; asymptomatic patients with iron deficiency anemia; and patients with active Crohn's disease who have a normal colonoscopy.8
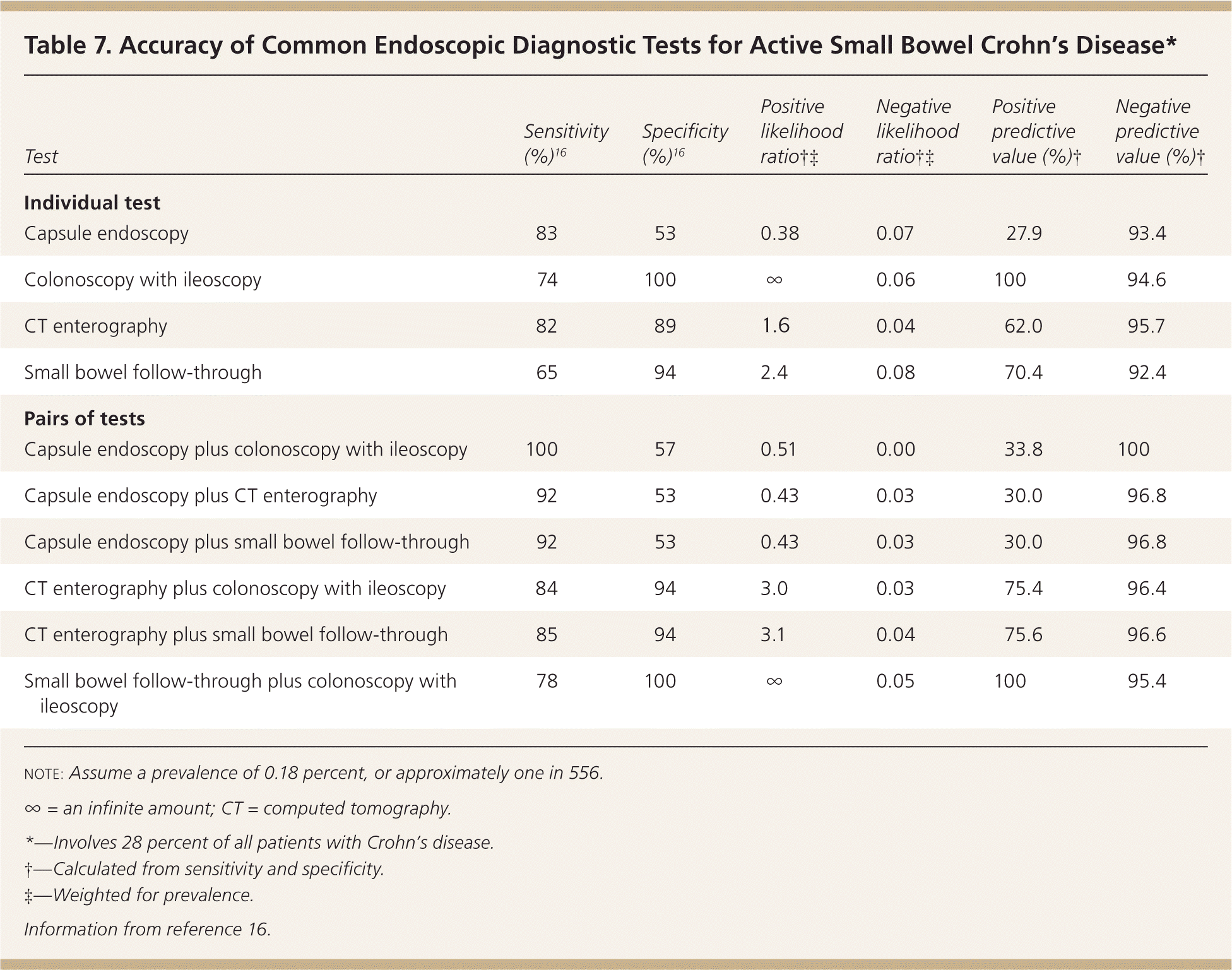
| Test | Sensitivity (%)16 | Specificity (%)16 | Positive likelihood ratio†‡ | Negative likelihood ratio†‡ | Positive predictive value (%)† | Negative predictive value (%)† |
|---|---|---|---|---|---|---|
| Individual test | ||||||
| Capsule endoscopy | 83 | 53 | 0.38 | 0.07 | 27.9 | 93.4 |
| Colonoscopy with ileoscopy | 74 | 100 | ∞ | 0.06 | 100 | 94.6 |
| CT enterography | 82 | 89 | 1.6 | 0.04 | 62.0 | 95.7 |
| Small bowel follow-through | 65 | 94 | 2.4 | 0.08 | 70.4 | 92.4 |
| Pairs of tests | ||||||
| Capsule endoscopy plus colonoscopy with ileoscopy | 100 | 57 | 0.51 | 0.00 | 33.8 | 100 |
| Capsule endoscopy plus CT enterography | 92 | 53 | 0.43 | 0.03 | 30.0 | 96.8 |
| Capsule endoscopy plus small bowel follow-through | 92 | 53 | 0.43 | 0.03 | 30.0 | 96.8 |
| CT enterography plus colonoscopy with ileoscopy | 84 | 94 | 3.0 | 0.03 | 75.4 | 96.4 |
| CT enterography plus small bowel follow-through | 85 | 94 | 3.1 | 0.04 | 75.6 | 96.6 |
| Small bowel follow-through plus colonoscopy with ileoscopy | 78 | 100 | ∞ | 0.05 | 100 | 95.4 |
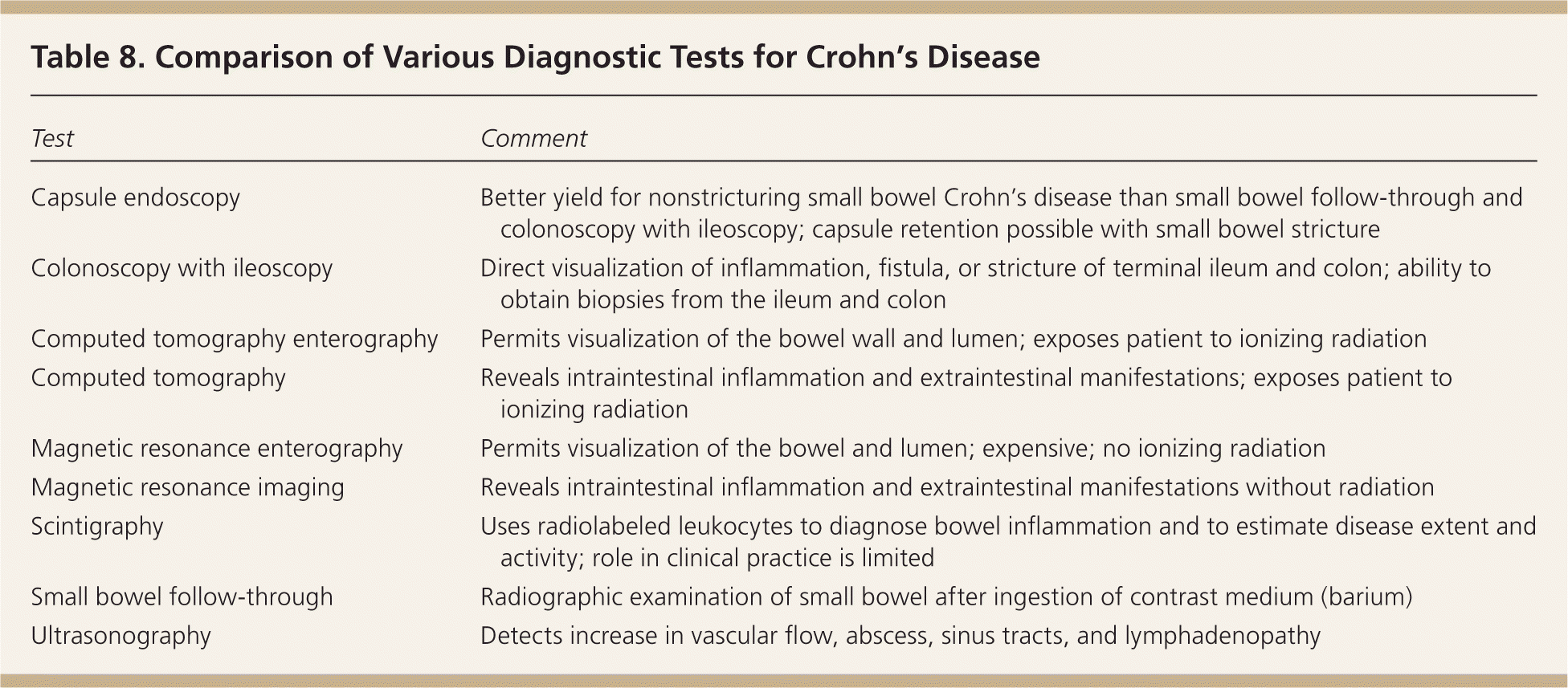
| Test | Comment |
|---|---|
| Capsule endoscopy | Better yield for nonstricturing small bowel Crohn's disease than small bowel follow-through and colonoscopy with ileoscopy; capsule retention possible with small bowel stricture |
| Colonoscopy with ileoscopy | Direct visualization of inflammation, fistula, or stricture of terminal ileum and colon; ability to obtain biopsies from the ileum and colon |
| Computed tomography enterography | Permits visualization of the bowel wall and lumen; exposes patient to ionizing radiation |
| Computed tomography | Reveals intraintestinal inflammation and extraintestinal manifestations; exposes patient to ionizing radiation |
| Magnetic resonance enterography | Permits visualization of the bowel and lumen; expensive; no ionizing radiation |
| Magnetic resonance imaging | Reveals intraintestinal inflammation and extraintestinal manifestations without radiation |
| Scintigraphy | Uses radiolabeled leukocytes to diagnose bowel inflammation and to estimate disease extent and activity; role in clinical practice is limited |
| Small bowel follow-through | Radiographic examination of small bowel after ingestion of contrast medium (barium) |
| Ultrasonography | Detects increase in vascular flow, abscess, sinus tracts, and lymphadenopathy |
Active Treatment
Therapeutic recommendations are determined by disease location, activity, and severity, and by disease-associated complications. The goals of therapy are control of symptoms, induction of clinical remission, and maintenance of remission with minimal adverse effects.17 Two principal strategies are currently used for Crohn's disease management. A traditional “step-up” approach begins with corticosteroids or mesalamine products and advances to immunomodulators or anti–tumor necrosis factor (TNF) agents based on severity of disease (Table 9). A “top-down” approach begins with anti-TNF agents. The optimal treatment strategy remains controversial.
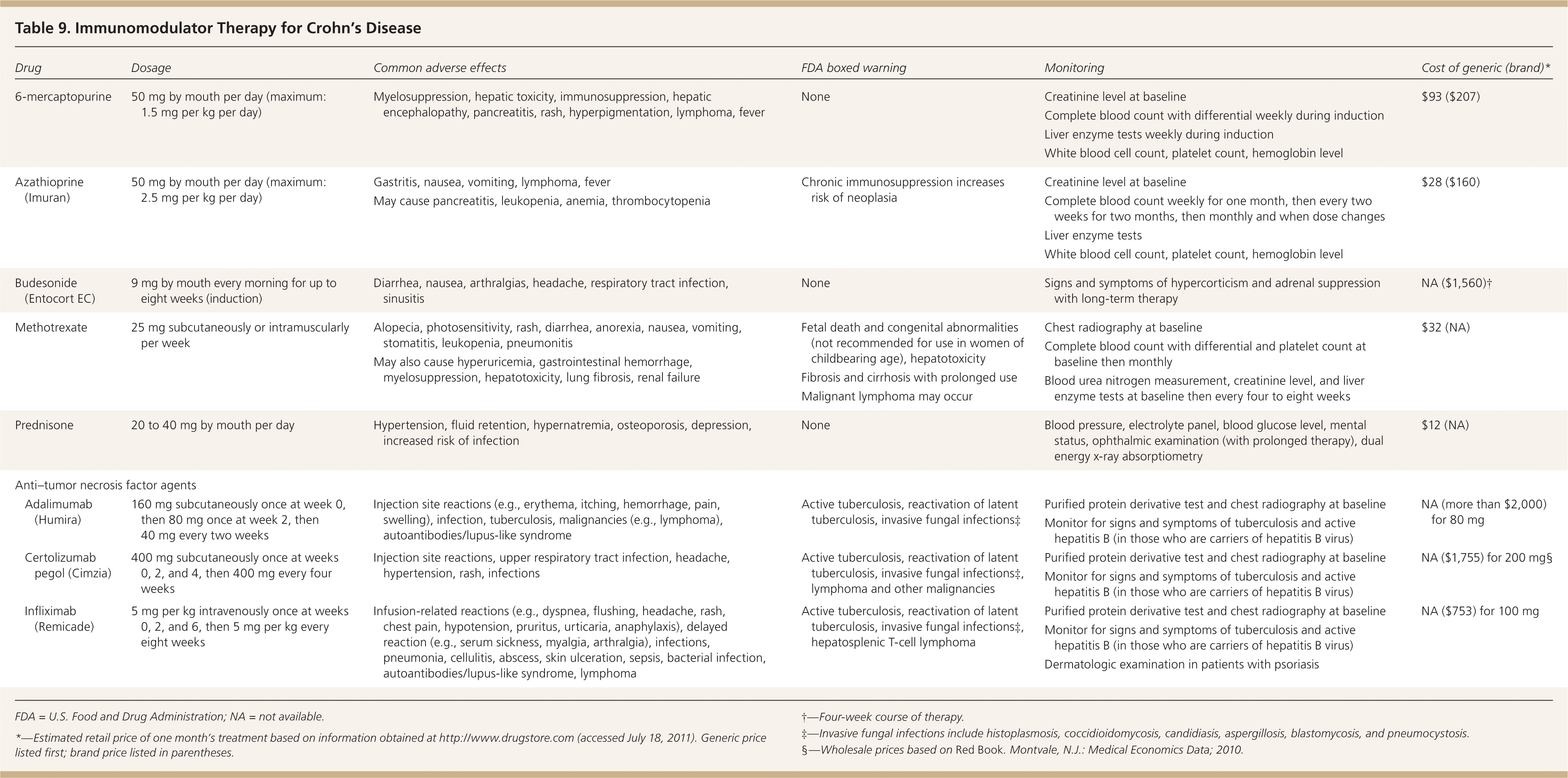
| Drug | Dosage | Common adverse effects | FDA boxed warning | Monitoring | Cost of generic (brand)* | |
|---|---|---|---|---|---|---|
| 6-mercaptopurine | 50 mg by mouth per day (maximum: 1.5 mg per kg per day) |
| None |
| $93 ($207) | |
| Azathioprine (Imuran) | 50 mg by mouth per day (maximum: 2.5 mg per kg per day) |
| Chronic immunosuppression increases risk of neoplasia |
| $28 ($160) | |
| Budesonide (Entocort EC) | 9 mg by mouth every morning for up to eight weeks (induction) |
| None |
| NA ($1,560)† | |
| Methotrexate | 25 mg subcutaneously or intramuscularly per week |
| Fetal death and congenital abnormalities (not recommended for use in women of childbearing age), hepatotoxicity |
| $32 (NA) | |
| Fibrosis and cirrhosis with prolonged use | ||||||
| Malignant lymphoma may occur | ||||||
| Prednisone | 20 to 40 mg by mouth per day |
| None |
| $12 (NA) | |
| Anti–tumor necrosis factor agents | ||||||
| Adalimumab (Humira) | 160 mg subcutaneously once at week 0, then 80 mg once at week 2, then 40 mg every two weeks |
| Active tuberculosis, reactivation of latent tuberculosis, invasive fungal infections‡ |
| NA (more than $2,000) for 80 mg | |
| Certolizumab pegol (Cimzia) | 400 mg subcutaneously once at weeks 0, 2, and 4, then 400 mg every four weeks |
| Active tuberculosis, reactivation of latent tuberculosis, invasive fungal infections‡, lymphoma and other malignancies |
| NA ($1,755) for 200 mg§ | |
| Infliximab (Remicade) | 5 mg per kg intravenously once at weeks 0, 2, and 6, then 5 mg per kg every eight weeks |
| Active tuberculosis, reactivation of latent tuberculosis, invasive fungal infections‡, hepatosplenic T-cell lymphoma |
| NA ($753) for 100 mg | |
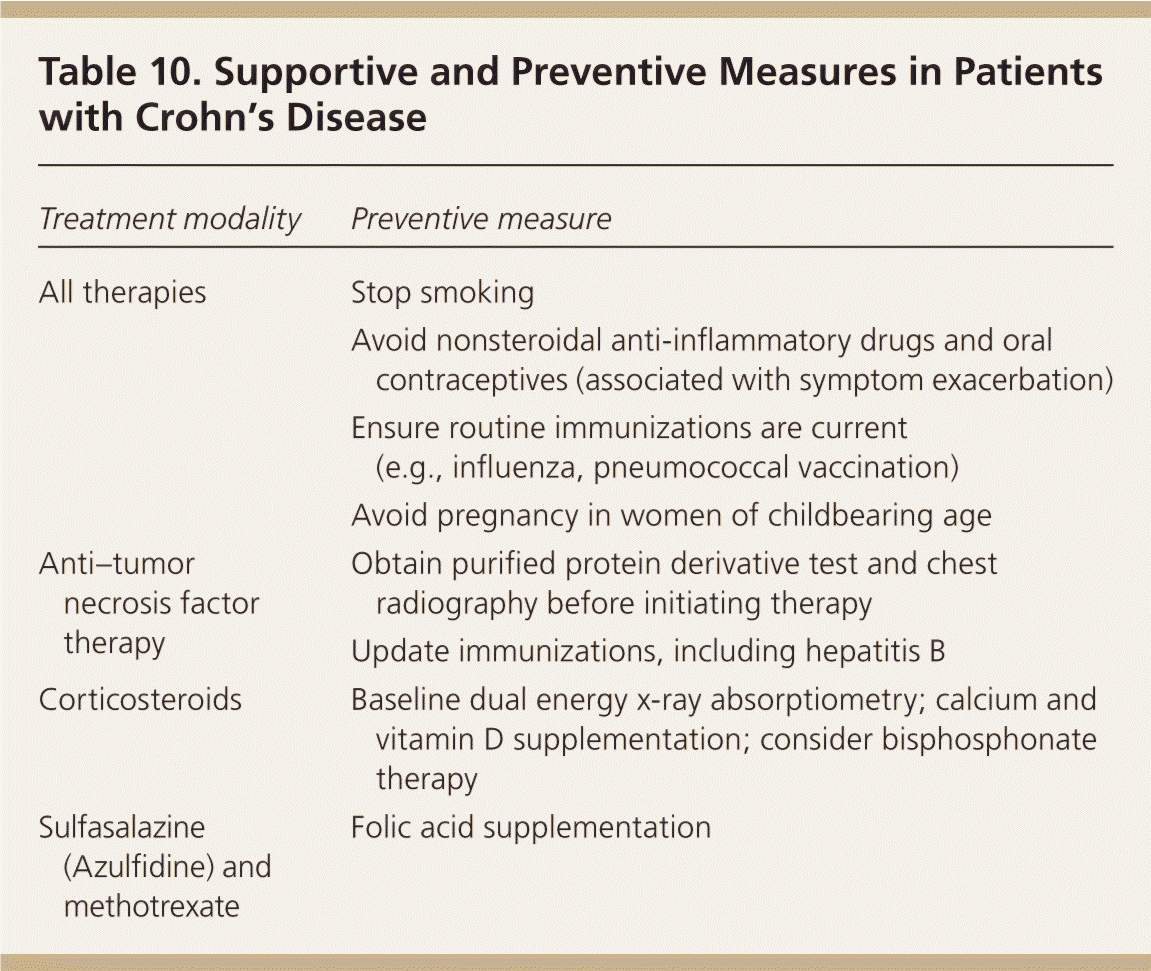
| Treatment modality | Preventive measure |
|---|---|
| All therapies | Stop smoking |
| Avoid nonsteroidal anti-inflammatory drugs and oral contraceptives (associated with symptom exacerbation) | |
| Ensure routine immunizations are current (e.g., influenza, pneumococcal vaccination) | |
| Avoid pregnancy in women of childbearing age | |
| Anti–tumor necrosis factor therapy | Obtain purified protein derivative test and chest radiography before initiating therapy |
| Update immunizations, including hepatitis B | |
| Corticosteroids | Baseline dual energy x-ray absorptiometry; calcium and vitamin D supplementation; consider bisphosphonate therapy |
| Sulfasalazine (Azulfidine) and methotrexate | Folic acid supplementation |
MILD DISEASE ACTIVITY
Patients with mild disease activity and no systemic symptoms are ambulatory and able to tolerate oral diet and medications.8
Mesalamine Products. Sulfasalazine (Azulfidine) and 5-aminosalicylic acid (5-ASA) are often used in the medical management of mild to moderate colonic Crohn's disease (Table 11). Sulfasalazine can cause nausea, headache, fever, rash, male infertility, and rarely agranulocytosis, which usually occurs within the first two months of therapy. 5-ASA is believed to have anti-inflammatory and immunosuppressive properties. 5-ASA products are well tolerated and are preferred to sulfasalazine because they have fewer adverse effects. Headache, nausea, diarrhea, and abdominal pain may occur with 5-ASA. Pancreatitis or pneumonitis may occur with sulfasalazine and mesalamine.
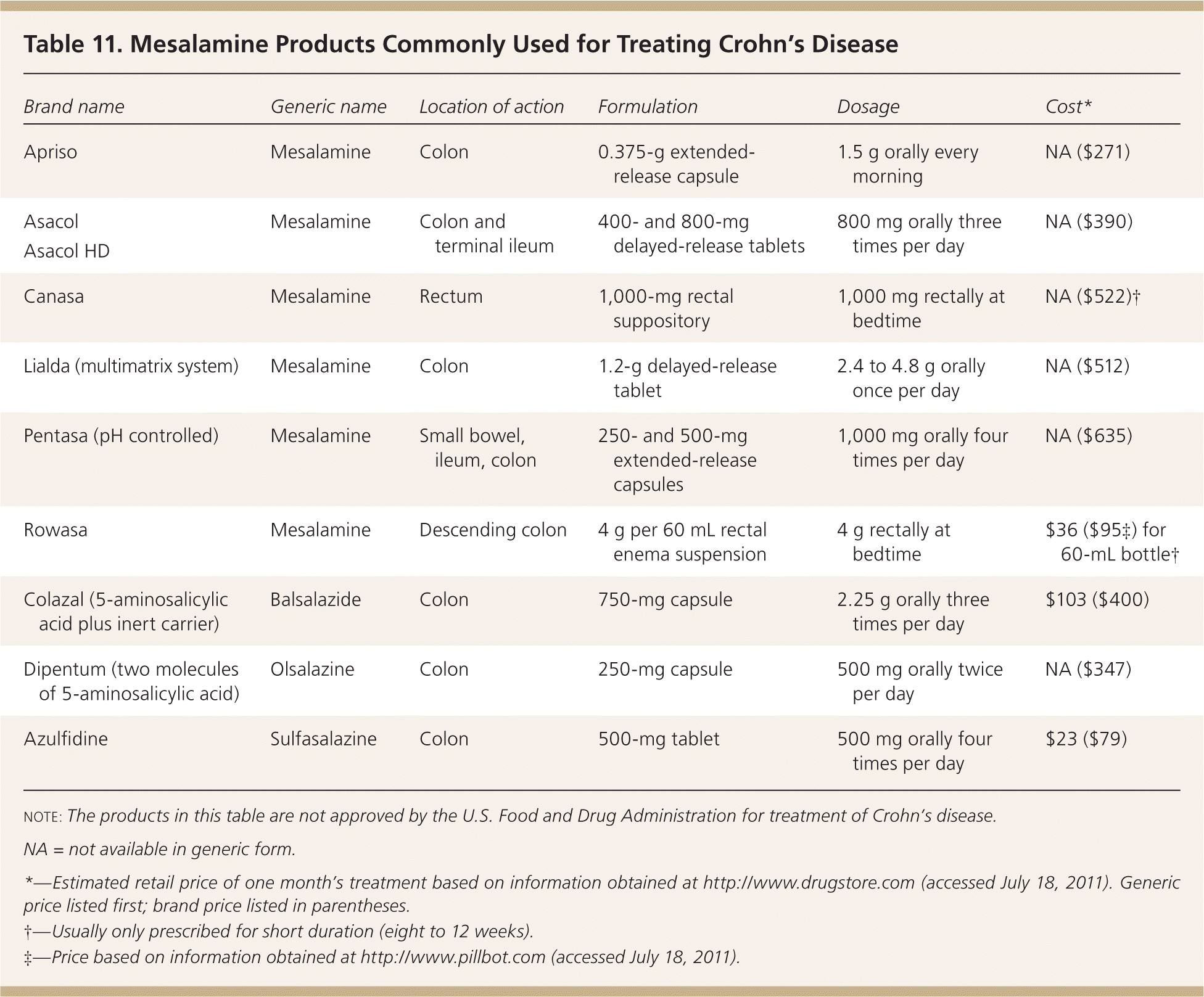
| Brand name | Generic name | Location of action | Formulation | Dosage | Cost* |
|---|---|---|---|---|---|
| Apriso | Mesalamine | Colon | 0.375-g extended-release capsule | 1.5 g orally every morning | NA ($271) |
| Asacol Asacol HD | Mesalamine | Colon and terminal ileum | 400- and 800-mg delayed-release tablets | 800 mg orally three times per day | NA ($390) |
| Canasa | Mesalamine | Rectum | 1,000-mg rectal suppository | 1,000 mg rectally at bedtime | NA ($522)† |
| Lialda (multimatrix system) | Mesalamine | Colon | 1.2-g delayed-release tablet | 2.4 to 4.8 g orally once per day | NA ($512) |
| Pentasa (pH controlled) | Mesalamine | Small bowel, ileum, colon | 250- and 500-mg extended-release capsules | 1,000 mg orally four times per day | NA ($635) |
| Rowasa | Mesalamine | Descending colon | 4 g per 60 mL rectal enema suspension | 4 g rectally at bedtime | $36 ($95A) for 60-mL bottle† |
| Colazal (5-aminosalicylic acid plus inert carrier) | Balsalazide | Colon | 750-mg capsule | 2.25 g orally three times per day | $103 ($400) |
| Dipentum (two molecules of 5-aminosalicylic acid) | Olsalazine | Colon | 250-mg capsule | 500 mg orally twice per day | NA ($347) |
| Azulfidine | Sulfasalazine | Colon | 500-mg tablet | 500 mg orally four times per day | $23 ($79) |
Budesonide. Budesonide (Entocort EC) is an oral, controlled-release glucocorticoid that is useful for treating Crohn's disease at the junction of the ileum and colon or ascending colon. A Cochrane review found that budesonide was more effective than placebo (relative risk [RR] = 1.96; 95% CI, 1.2 to 3.2) or mesalamine (RR = 1.63; 95% CI, 1.2 to 2.2) for induction of remission in patients with Crohn's disease.21
MODERATE DISEASE ACTIVITY
Outpatients with moderate disease activity are defined by failed treatment for mild disease or by fever, weight loss, abdominal pain, nausea or vomiting without obstruction, or anemia.8 Many of these patients are treated by gastroenterologists.
Corticosteroid Therapy. Patients with moderate to severe Crohn's disease are treated with prednisone until improvement of symptoms. Corticosteroids are more effective than placebo (RR = 1.99; 95% CI, 1.51 to 2.64; P < .00001) and 5-ASA products (RR =1.65; 95% CI, 1.33 to 2.03; P < .00001) at inducing remission in patients with Crohn's disease.22 If symptoms are not controlled with adequate doses of prednisone, urgent gastroenterology consultation is warranted. No standards have been established for corticosteroid tapering; however, reduction by 5 to 10 mg per week to 20 mg and then by 2.5 to 5 mg per week until discontinuation is reasonable.
Azathioprine and 6-Mercaptopurine. Azathioprine (Imuran) and 6-mercaptopurine can effectively induce remission in patients with active Crohn's disease within three to six months of achieving the maximal dose (OR = 2.5; 95% CI, 1.6 to 3.9; number needed to treat [NNT] = 5).23 These agents are primarily used for long-term maintenance of remission and are typically combined with corticosteroids or occasionally with anti-TNF preparations. Routine monitoring of white blood cell count, platelet count, and hemoglobin and creatinine levels is recommended.24 Adverse effects of azathioprine and 6-mercaptopurine include leukopenia, thrombocytopenia, bone marrow suppression, immunosuppression, pancreatitis, hypersensitivity reaction, lymphoma, nausea, vomiting, elevated liver enzymes, and fever.
Methotrexate. Methotrexate is an alternative therapy for patients intolerant of azathioprine or 6-mercaptopurine. It effectively induces remission and enables withdrawal from corticosteroids in patients with refractory Crohn's disease (NNT = 5).25 Potential adverse effects include bone marrow suppression, leukopenia, nausea, vomiting, hepatic fibrosis, and pneumonitis. Chest radiography, complete blood count, and liver enzyme tests are recommended before initiating treatment.24 Risk factors for hepatotoxicity include obesity, diabetes mellitus, chronic alcohol use, abnormal liver chemistries, and a cumulative dose of methotrexate exceeding 1.5 g.26
Anti-TNF Agents. Three TNF antagonist (anti-TNF) therapies (infliximab [Remicade], adalimumab [Humira], and certolizumab pegol [Cimzia]) are approved by the U.S. Food and Drug Administration for moderate to severe Crohn's disease. Anti-TNF therapy may be considered in patients with moderate to severe active Crohn's disease that does not respond to corticosteroids or immunosuppressive therapy. It is also used for patients in whom corticosteroids are contraindicated or not desired. Relative or absolute contraindications to anti-TNF therapy include sepsis, tuberculosis, optic neuritis, infusion reaction, and cancer. A negative purified protein derivative test and chest radiography before treatment with anti-TNF agents are important because this therapy is associated with reactivation of tuberculosis.27 Anti-TNF therapy has been shown to effectively induce and maintain remission in patients with moderate to severe Crohn's disease.24,28,29
SEVERE DISEASE ACTIVITY
Patients with severe disease activity have persistent symptoms despite therapy, or they present with fever, vomiting, evidence of intestinal obstruction, involuntary guarding or rebound tenderness, cachexia, or evidence of abscess. These patients require emergent hospitalization and gastroenterology consultation.8 Evaluation often includes abdominal imaging and laboratory tests, including a complete blood count, complete metabolic panel, blood cultures, urinalysis, urine culture, stool culture, and C. difficile stool antigen test. Computed tomography or magnetic resonance enterography may differentiate inflammatory from fibrotic strictures. Urgent surgical evaluation is recommended for patients with symptoms of intestinal obstruction or abdominal mass. An abscess requires percutaneous or open surgical drainage. Fluid resuscitation, parenteral corticosteroids, and broad-spectrum antibiotics should be administered, and nutritional support should be provided using elemental feeding or parenteral hyperalimentation.30 Use of anti-TNF agents is controversial in the treatment of severe Crohn's disease. Failure to respond or worsening symptoms may require surgical intervention.
PERIANAL AND FISTULIZING DISEASE
Suppurative conditions (abscess) are treated with drainage and should be jointly managed by gastroenterologists and surgeons. Chronic fistulae and perianal fissures are treated with antibiotics (metronidazole alone or in combination with ciprofloxacin), immunosuppressives, or anti-TNF agents.31 A placebo-controlled trial suggested benefits with infliximab in the closure of cutaneous Crohn's disease fistulae that had not responded to previous therapy with antibiotics, corticosteroids, or immunomodulators.11 No data are available from controlled trials concerning treatment by internal fistulae closure (enteroenteric, enterocolic, enterovesicular, and enterovaginal) with alternative immunomodulatory agents. Surgery may be considered.
Maintenance Therapy
Azathioprine is effective for maintenance of remission in patients with Crohn's disease (OR = 2.1; 95% CI, 1.4 to 3.5; NNT = 7).32 In a randomized controlled trial with 24 weeks of follow-up, 65 percent of patients maintained remission with methotrexate.33 Increasing evidence supports that “top-down” therapy beginning with infliximab and azathioprine may offer corticosteroid-sparing benefits for corticosteroid-naive patients.34 Evidence demonstrates that low-dose conventional corticosteroids and 5-ASA preparations are ineffective in maintaining remission in patients with Crohn's disease, and high-dose corticosteroids have not been evaluated as maintenance therapy.35,36 No published studies have evaluated antibiotics in the maintenance of remission. Budesonide is no more effective than placebo for maintenance of remission in patients with Crohn's disease at 12 months (RR = 1.13; 95% CI, 0.94 to 1.35; P = .19).37
Surgical Therapy
The most common indications for surgery include refractory disease, intractable hemorrhage, perforation, obstruction, abscess, dysplasia, cancer, and unresponsive fulminant disease. Patients with active luminal Crohn's disease that fails to improve within seven to 10 days of intensive inpatient medical management should be considered for surgery. The most common surgical procedures for Crohn's disease include surgical resection, stricturoplasty, and drainage of abscess. In a recent review of six population-based studies involving 25,870 patients with an average follow-up of 11.1 years, surgery was required in one-third of patients after corticosteroids were initiated, and the risk of postoperative recurrence over 10 years was 44 to 55 percent.38
In this study, one-half of all patients required surgery within 10 years of the diagnosis of Crohn's disease, and only 10 percent of patients had a prolonged clinical remission.38 Limited segmental resection is superior to subtotal colectomy in terms of fewer symptoms (P = .039), fewer loose stools (P = .002), and better anorectal function (P = .027).39 Postoperative infections are not associated with azathioprine, 6-mercaptopurine, or infliximab, but are associated with corticosteroid therapy.40 Stricturoplasty has been recommended in selected patients with small bowel disease to avoid impaired nutrient absorption, bile salt diarrhea, steatorrhea, bacterial overgrowth, and short bowel syndrome, but is not recommended for colonic disease.
