
Am Fam Physician. 2018;98(11):661-669
Patient information: See related handout on Crohn's disease, written by the authors of this article.
Author disclosure: No relevant financial affiliations.
Crohn's disease is a chronic inflammatory condition that affects the gastrointestinal tract. It can cause lesions from mouth to anus and may result in extraintestinal complications. The prevalence of Crohn's disease is increasing in adults and children. Genetic predispositions to Crohn's disease have been identified, and specific environmental factors have been associated with its development. Common presenting symptoms include diarrhea, abdominal pain, rectal bleeding, fever, weight loss, and fatigue. Physical examination should identify unstable patients requiring immediate care, include an anorectal examination, and look for extraintestinal complications. Initial laboratory evaluation identifies inflammation and screens for alternative diagnoses. Measurement of fecal calprotectin has value to rule out disease in adults and children. Endoscopy and cross-sectional imaging are used to confirm the diagnosis and determine the extent of disease. Treatment decisions are guided by disease severity and risk of poor outcomes. Patients commonly receive corticosteroids to treat symptom flare-ups. Patients with higher-risk disease are given biologics, with or without immunomodulators, to induce and maintain remission. For children, enteral nutrition is an option for induction therapy. All patients with Crohn's disease should be counseled on smoking avoidance or cessation. Patients with Crohn's disease are at increased risk of cancer, osteoporosis, anemia, nutritional deficiencies, depression, infection, and thrombotic events. Maximizing prevention measures is essential in caring for these patients.
Crohn's disease is a chronic inflammatory condition affecting the gastrointestinal tract that often causes extraintestinal complications. Inflammation may occur at any point from mouth to anus (Table 11). Specific clinical and diagnostic characteristics distinguish Crohn's disease from ulcerative colitis1,2 (Table 21). In the United States, the prevalence is estimated at 58 per 100,000 children and 119 to 241 per 100,000 adults, and is increasing for both groups.3,4 Most cases are diagnosed in the 20s to 40s, but new cases do occur later.5 White race and higher education levels are associated with increased prevalence.4 The estimated annual economic burden to U.S. health care is $6.3 billion.6
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Fecal calprotectin is a useful test for ruling out Crohn's disease in adults. | C | 14 |
| Cross-sectional imaging techniques (i.e., computed tomography, magnetic resonance imaging, and ultrasonography) are the imaging studies of choice for evaluating Crohn's disease. | C | 20, 21 |
| Smoking cessation reduces complications experienced by patients with Crohn's disease, and all patients should be counseled not to smoke and offered cessation assistance. | B | 42 |
| Corticosteroids treat symptom flare-ups and may induce remission of Crohn's disease. | C | 2, 24 |
| Biologics, with or without immunomodulators, induce and maintain remission of Crohn's disease in moderate- to high-risk patients. | C | 2 |
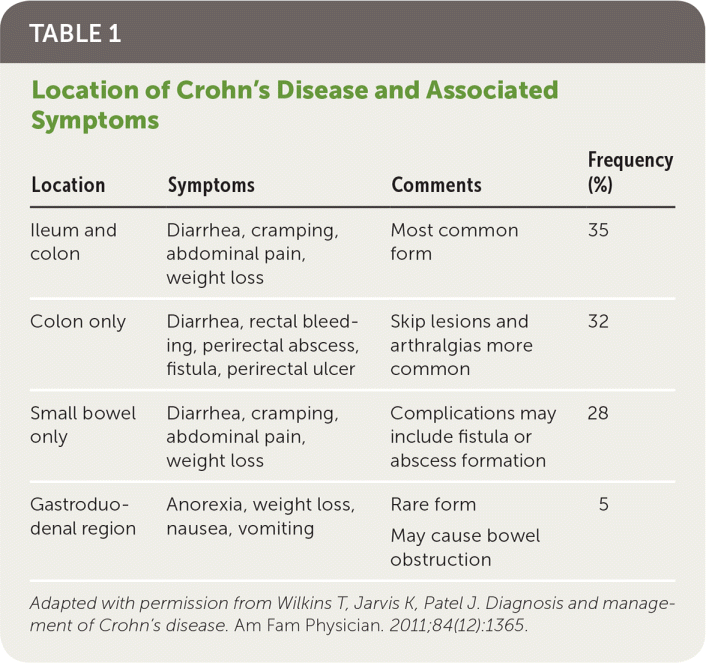
| Location | Symptoms | Comments | Frequency (%) |
|---|---|---|---|
| Ileum and colon | Diarrhea, cramping, abdominal pain, weight loss | Most common form | 35 |
| Colon only | Diarrhea, rectal bleeding, perirectal abscess, fistula, perirectal ulcer | Skip lesions and arthralgias more common | 32 |
| Small bowel only | Diarrhea, cramping, abdominal pain, weight loss | Complications may include fistula or abscess formation | 28 |
| Gastroduodenal region | Anorexia, weight loss, nausea, vomiting | Rare form may cause bowel obstruction | 5 |
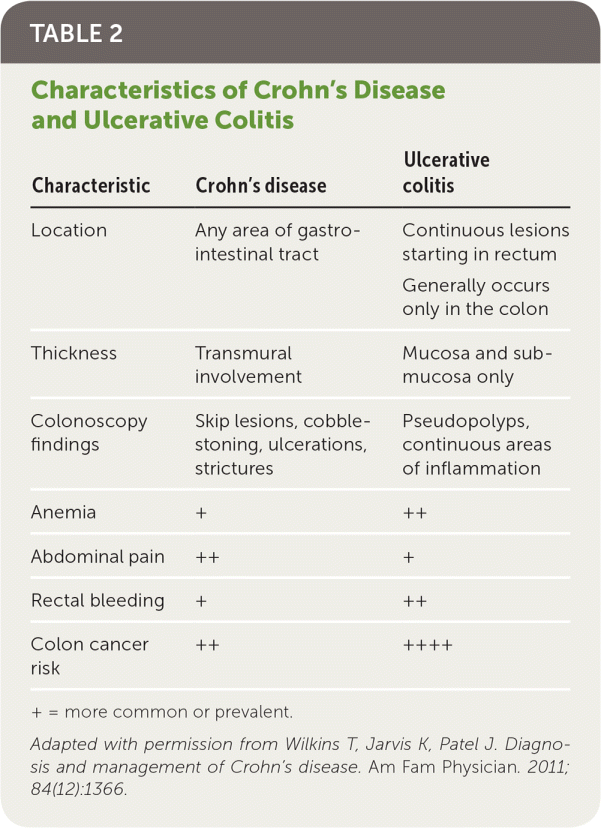
| Characteristic | Crohn's disease | Ulcerative colitis |
|---|---|---|
| Location | Any area of gastrointestinal tract | Continuous lesions starting in rectum |
| Generally occurs only in the colon | ||
| Thickness | Transmural involvement | Mucosa and submucosa only |
| Colonoscopy findings | Skip lesions, cobble-stoning, ulcerations, strictures | Pseudopolyps, continuous areas of inflammation |
| Anemia | + | ++ |
| Abdominal pain | ++ | + |
| Rectal bleeding | + | ++ |
| Colon cancer risk | ++ | ++++ |
Risk Factors
Current data suggest an interplay between genetic susceptibility and environmental factors in the development of Crohn's disease. Genetic loci have been identified that increase risk. For example, homozygosity for the NOD2 gene has shown a 20- to 40-fold increased risk of developing Crohn's disease.5 Environmental factors associated with increased risk include smoking, oral contraceptive use, antibiotic use, regular use of nonsteroidal anti-inflammatory drugs, and urban environment.5,7 Factors associated with decreased risk include exposure to pets and farm animals, bedroom sharing, having more than two siblings, high fiber intake, fruit consumption, and physical activity.5,8 Vaccines have not been associated with the development of Crohn's disease.9
Clinical Findings
HISTORY AND PHYSICAL EXAMINATION
Crohn's disease most often presents insidiously but can present as an acute toxic illness. Common symptoms include diarrhea, abdominal pain, rectal bleeding, fever, weight loss, and fatigue.1,2 A case review of 201 participants compared patients with Crohn's disease and patients without Crohn's disease who had irritable bowel syndrome or were otherwise healthy. The study identified eight red flag findings for Crohn's disease in adults. In order of strength of association, the findings were perianal lesions other than hemorrhoids, a first-degree relative with inflammatory bowel disease, weight loss (5% of usual body weight) in the past three months, abdominal pain for longer than three months, nocturnal diarrhea, fever, no abdominal pain for 30 to 45 minutes after meals, and no rectal urgency.10 A case review of 606 children with chronic abdominal pain identified three red flag findings for children: anemia, hematochezia, and weight loss.11 The history should identify findings specific for Crohn's disease, identify alternative diagnoses (Table 31,2,12), and search for extraintestinal findings (Table 41). Important areas to cover are nocturnal symptoms; urgency findings; food intolerance; travel; medications (including antibiotic exposure); smoking status; family history of inflammatory bowel disease; and eye, joint, or skin symptoms.
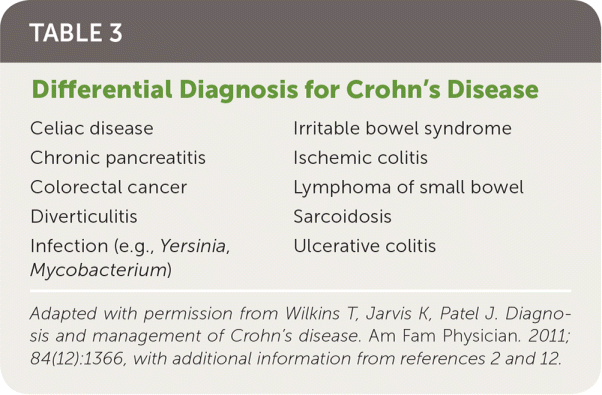
| Celiac disease |
| Chronic pancreatitis |
| Colorectal cancer |
| Diverticulitis |
| Infection (e.g., Yersinia, Mycobacterium) |
| Irritable bowel syndrome |
| Ischemic colitis |
| Lymphoma of small bowel |
| Sarcoidosis |
| Ulcerative colitis |
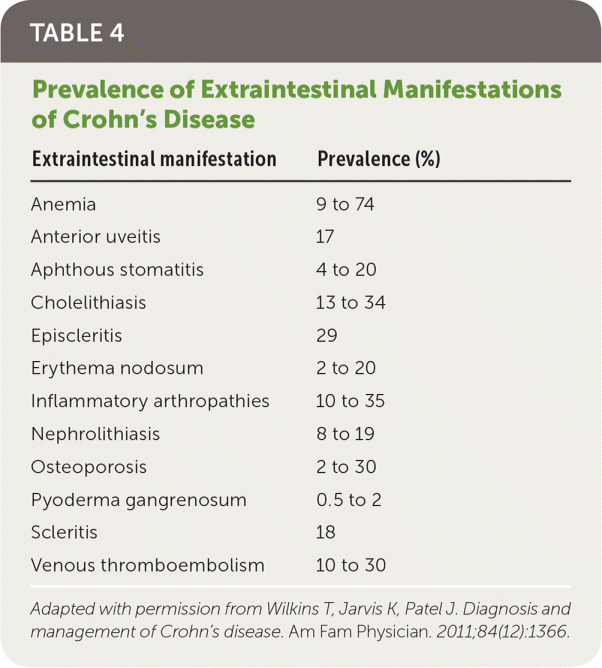
| Extraintestinal manifestation | Prevalence (%) |
|---|---|
| Anemia | 9 to 74 |
| Anterior uveitis | 17 |
| Aphthous stomatitis | 4 to 20 |
| Cholelithiasis | 13 to 34 |
| Episcleritis | 29 |
| Erythema nodosum | 2 to 20 |
| Inflammatory arthropathies | 10 to 35 |
| Nephrolithiasis | 8 to 19 |
| Osteoporosis | 2 to 30 |
| Pyoderma gangrenosum | 0.5 to 2 |
| Scleritis | 18 |
| Venous thromboembolism | 10 to 30 |
The physical examination should first identify unstable patients that need immediate attention. Pulse, blood pressure, temperature, respiratory rate, and body weight should be measured. Abdominal examination findings can include tenderness, distention, and/or masses.1,2 An anorectal examination is required, and a pelvic examination should be considered because abscesses, fissures, or fistulas are common in Crohn's disease.1,2 Perianal findings (e.g., fistulas, abscesses) increase the likelihood of Crohn's disease.10
EXTRAINTESTINAL FINDINGS
The inflammatory effects of Crohn's disease can extend beyond the intestinal lumen, causing abscesses, fissures, and/or fistulas, and can affect organs outside of the intestinal tract. Patients can present with extraintestinal findings before gastrointestinal symptoms are prominent. Areas affected include, but are not limited to, the eyes, hematologic system, joints, and skin (Table 41). History, physical examination, laboratory testing, and imaging are important in identifying these manifestations.1,13
Diagnostic Studies and Monitoring
LABORATORY TESTING
Laboratory testing has multiple purposes for the evaluation of Crohn's disease, including diagnosis, monitoring of disease activity, and tracking adverse effects and effectiveness of medications. Fecal calprotectin is a reasonable test to rule out Crohn's disease for adults (sensitivity of 83% to 100%; specificity of 60% to 100%) and children (sensitivity of 95% to 100%; specificity of 44% to 93%) with equivocal symptoms, and may spare them from more invasive testing.2,14 When the diagnosis of Crohn's disease is considered, a complete blood count; a complete metabolic panel; pregnancy test; C-reactive protein level; erythrocyte sedimentation rate; and stool studies for Clostridium difficile, ova and parasites, and culture may be useful. Results can provide information to support the diagnosis, identify the severity of disease, or determine alternative diagnoses.1,2 Measurement of C-reactive protein, fecal calprotectin, and stool lactoferrin can help assess disease activity and potentially limit the need for endoscopy in disease management decisions.15
Anemia is common, so hemoglobin and hematocrit should be monitored periodically. Deficiencies of folate, iron, and 25-hydroxyvitamin D are also common; thus, screening is prudent. Patients with extensive bowel resection have increased risk of vitamin B12 deficiency and should be screened.16 Tuberculosis screening should be considered before using biologic agents. A complete blood count and renal and hepatic function testing should be done periodically when methotrexate, thiopurines, and/or biologic agents are used for treatment.16 Therapeutic drug monitoring can guide therapy.17
ENDOSCOPY AND IMAGING
Endoscopy and imaging are essential tools for diagnosing and monitoring Crohn's disease. Endoscopic procedures allow direct visualization of and access to the bowel lumen. Direct visualization allows for identification of characteristic lesions, monitoring the success or failure of therapy, and screening for colorectal cancer. Endoscopic procedures (except capsule endoscopy) also allow for biopsy and therapeutic interventions (Table 52,13,18,19).
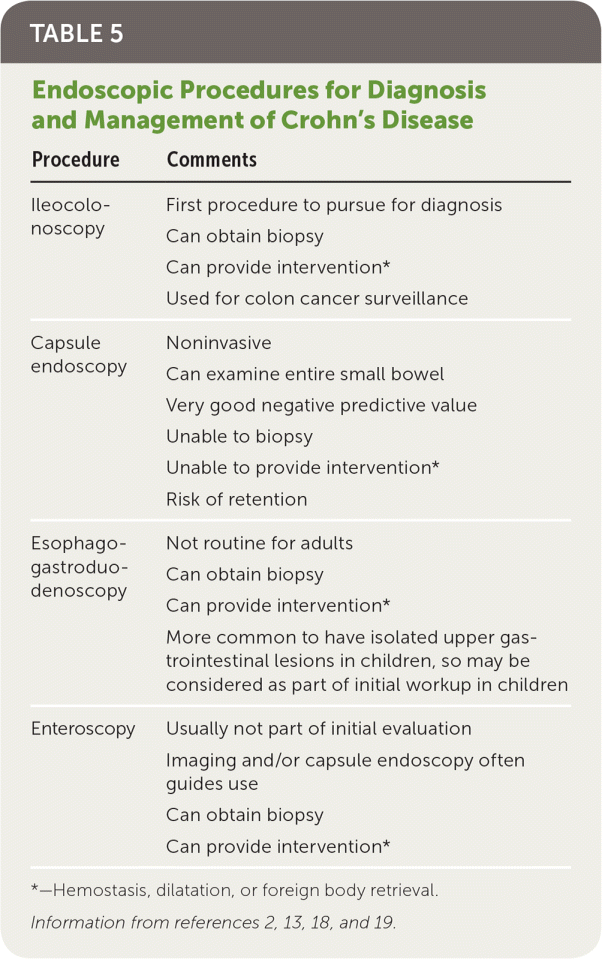
| Procedure | Comments |
|---|---|
| Ileocolonoscopy | First procedure to pursue for diagnosis* |
| Can obtain biopsy | |
| Can provide intervention | |
| Used for colon cancer surveillance | |
| Capsule endoscopy | Noninvasive |
| Can examine entire small bowel | |
| Very good negative predictive value | |
| Unable to biopsy | |
| Unable to provide intervention* | |
| Risk of retention | |
| Esophagogastroduodenoscopy | Not routine for adults |
| Can obtain biopsy | |
| Can provide intervention* | |
| More common to have isolated upper gastrointestinal lesions in children, so may be considered as part of initial workup in children | |
| Enteroscopy | Usually not part of initial evaluation |
| Imaging and/or capsule endoscopy often guides use | |
| Can obtain biopsy | |
| Can provide intervention* |
Cross-sectional imaging techniques, including computed tomography (CT), magnetic resonance imaging, and ultrasonography, have come to the forefront in the management of Crohn's disease. These techniques are all useful and provide similar accuracy for making the initial diagnosis, monitoring disease activity, and identifying complications (e.g., fistulas, abscesses).20,21 They complement endoscopy because they can identify extraluminal pathology and examine the gastrointestinal tract not accessible to endoscopic procedures. If the patient can tolerate the contrast load, CT and magnetic resonance enterography are preferred to standard CT and magnetic resonance imaging protocols. CT studies provide the most consistent results but have the downside of radiation exposure. Magnetic resonance studies have no radiation exposure, but are expensive, may have limited availability, and are more difficult for patients to tolerate. Ultrasonography is readily available and has no radiation exposure, but it is highly operator dependent and can be limited by body habitus. Choosing which modality to pursue depends on the patient's age, pregnancy status, current clinical condition, local expertise, and availability13,20,21 (Table 613,21,22).
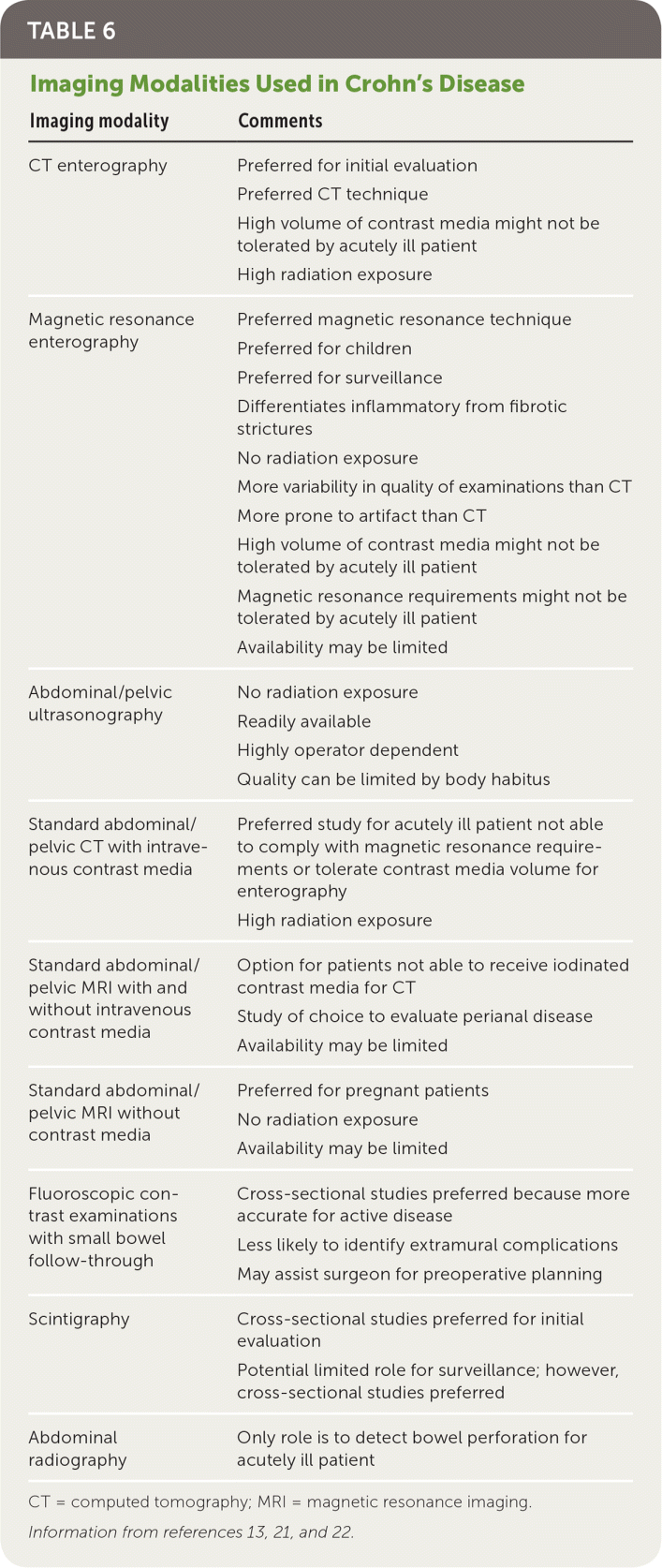
| Imaging modality | Comments |
|---|---|
| CT enterography | Preferred for initial evaluation |
| Preferred CT technique | |
| High volume of contrast media might not be tolerated by acutely ill patient | |
| High radiation exposure | |
| Magnetic resonance enterography | Preferred magnetic resonance technique |
| Preferred for children | |
| Preferred for surveillance | |
| Differentiates inflammatory from fibrotic strictures | |
| No radiation exposure | |
| More variability in quality of examinations than CT | |
| More prone to artifact than CT | |
| High volume of contrast media might not be tolerated by acutely ill patient | |
| Magnetic resonance requirements might not be tolerated by acutely ill patient | |
| Availability may be limited | |
| Abdominal/pelvic ultrasonography | No radiation exposure |
| Readily available | |
| Highly operator dependent | |
| Quality can be limited by body habitus | |
| Standard abdominal/pelvic CT with intravenous contrast media | Preferred study for acutely ill patient not able to comply with magnetic resonance requirements or tolerate contrast media volume for enterography |
| High radiation exposure | |
| Standard abdominal/pelvic MRI with and without intravenous contrast media | Option for patients not able to receive iodinated contrast media for CT |
| Study of choice to evaluate perianal disease | |
| Availability may be limited | |
| Standard abdominal/pelvic MRI without contrast media | Preferred for pregnant patients |
| No radiation exposure | |
| Availability may be limited | |
| Fluoroscopic contrast examinations with small bowel follow-through | Cross-sectional studies preferred because more accurate for active disease |
| Less likely to identify extramural complications | |
| May assist surgeon for preoperative planning | |
| Scintigraphy | Cross-sectional studies preferred for initial evaluation |
| Potential limited role for surveillance; however, cross-sectional studies preferred | |
| Abdominal radiography | Only role is to detect bowel perforation for acutely ill patient |
Diagnostic Approach
The diagnosis of Crohn's disease results from clinical findings coupled with endoscopic, histologic, radiologic, and/or biochemical testing. History, physical examination, and basic laboratory findings drive the decision to pursue the diagnosis. If the patient has a toxic presentation, standard CT should be the first test. If the patient does not have a fulminant presentation, ileocolonoscopy with biopsy should be the first test, and esophagogastroduodenoscopy should be considered for children. Cross-sectional imaging should follow so that the full extent of disease seen by endoscopy can be determined or to identify disease not visualized by endoscopy. Identifying the complete extent of disease is important for developing a treatment plan. When ileocolonoscopy and cross-sectional imaging are negative and concern for Crohn's disease is still high, capsule endoscopy would be the next step. If this study is negative, it is moderately certain that the disease is not present1,2,13,20,21 (Figure 11,2,13,14,18,19,21).
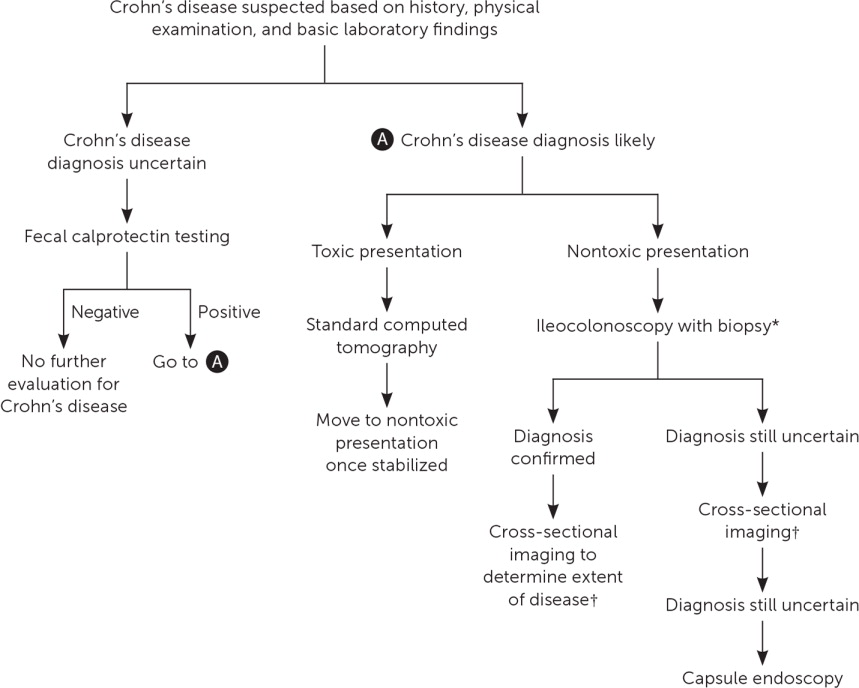
Management
Management has two aims. First is treating the inflammatory process and its associated complications (e.g., abscesses, fistulas, strictures, intestinal obstructions) with the goal of achieving and maintaining remission. Second is minimizing the negative health impacts from Crohn's disease itself and the therapies used to treat it.13,16,22,23
MEDICAL TREATMENT
Treatment decisions are guided by age, comorbidities, symptoms, inflammation status, disease location and extent, and overall risk of more severe and complicated disease (Table 72,24). More severe disease and the presence of risk factors that predict poor prognosis justify the use of high-risk medications.2,24
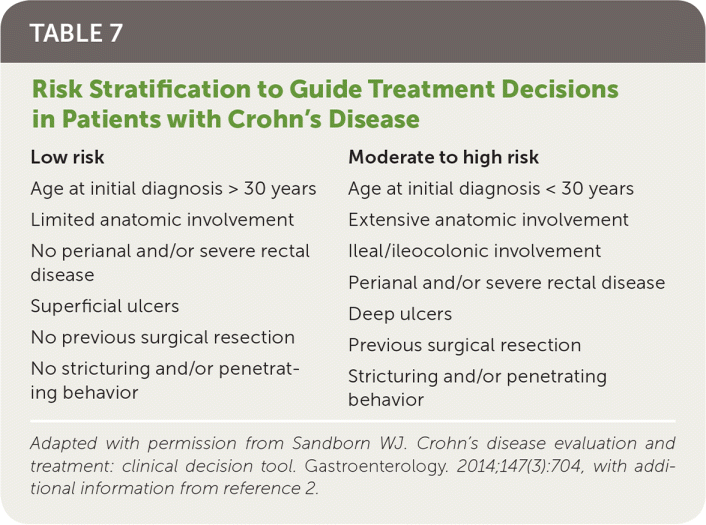
| Low risk | Moderate to high risk |
|---|---|
| Age at initial diagnosis > 30 years | Age at initial diagnosis < 30 years |
| Limited anatomic involvement | Extensive anatomic involvement |
| No perianal and/or severe rectal disease | Ileal/ileocolonic involvement |
| Perianal and/or severe rectal disease | |
| Superficial ulcers | Deep ulcers |
| No previous surgical resection | Previous surgical resection |
| No stricturing and/or penetrating behavior | Stricturing and/or penetrating behavior |
Significant advancements have been made in treatment. Whereas 5-aminosalicylates were once commonly used and are still prescribed for symptom management in mild to moderate disease, mucosal healing has not been demonstrated.2 Antibiotics, also widely used, should be limited to treating complications such as abscesses and fistulas.2,25,26
Medication management includes corticosteroids, immunomodulators, and biologics (eTable A). Each plays an important role in inducing or maintaining remission.
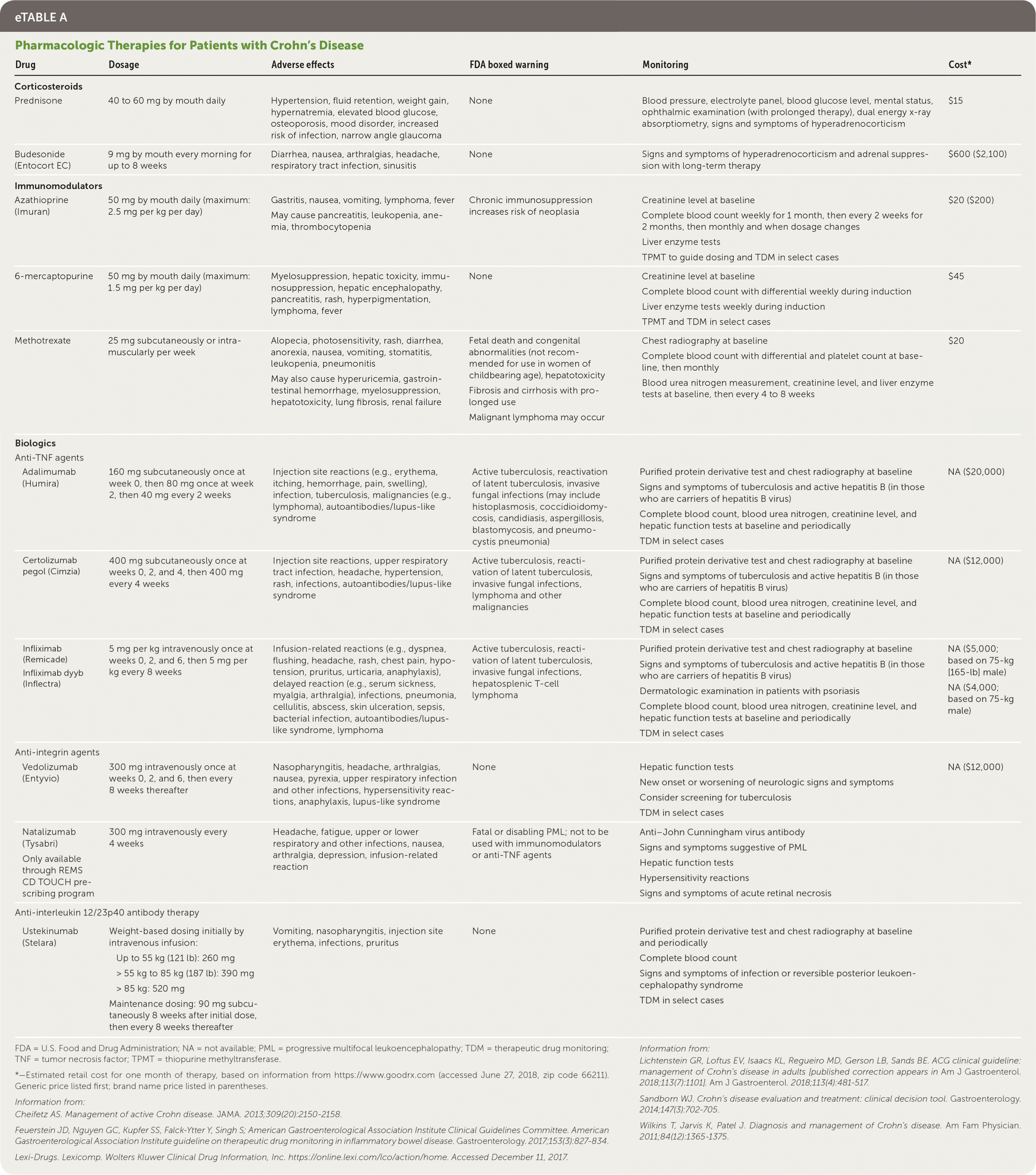
| Drug | Dosage | Adverse effects | FDA boxed warning | Monitoring | Cost* |
|---|---|---|---|---|---|
| Corticosteroids | |||||
| Prednisone | 40 to 60 mg by mouth daily | Hypertension, fluid retention, weight gain, hypernatremia, elevated blood glucose, osteoporosis, mood disorder, increased risk of infection, narrow angle glaucoma | None | Blood pressure, electrolyte panel, blood glucose level, mental status, ophthalmic examination (with prolonged therapy), dual energy x-ray absorptiometry, signs and symptoms of hyperadrenocorticism | $15 |
| Budesonide (Entocort EC) | 9 mg by mouth every morning for up to 8 weeks | Diarrhea, nausea, arthralgias, headache, respiratory tract infection, sinusitis | None | Signs and symptoms of hyperadrenocorticism and adrenal suppression with long-term therapy | $600 ($2,100) |
| Immunomodulators | |||||
| Azathioprine (Imuran) | 50 mg by mouth daily (maximum: 2.5 mg per kg per day) | Gastritis, nausea, vomiting, lymphoma, fever May cause pancreatitis, leukopenia, anemia, thrombocytopenia | Chronic immunosuppression increases risk of neoplasia | Creatinine level at baseline Complete blood count weekly for 1 month, then every 2 weeks for 2 months, then monthly and when dosage changes Liver enzyme tests TPMT to guide dosing and TDM in select cases | $20 ($200) |
| 6-mercaptopurine | 50 mg by mouth daily (maximum: 1.5 mg per kg per day) | Myelosuppression, hepatic toxicity, immunosuppression, hepatic encephalopathy, pancreatitis, rash, hyperpigmentation, lymphoma, fever | None | Creatinine level at baseline Complete blood count with differential weekly during induction Liver enzyme tests weekly during induction TPMT and TDM in select cases | $45 |
| Methotrexate | 25 mg subcutaneously or intramuscularly per week | Alopecia, photosensitivity, rash, diarrhea, anorexia, nausea, vomiting, stomatitis, leukopenia, pneumonitis May also cause hyperuricemia, gastrointestinal hemorrhage, myelosuppression, hepatotoxicity, lung fibrosis, renal failure | Fetal death and congenital abnormalities (not recommended for use in women of childbearing age), hepatotoxicity Fibrosis and cirrhosis with prolonged use Malignant lymphoma may occur | Chest radiography at baseline Complete blood count with differential and platelet count at baseline, then monthly Blood urea nitrogen measurement, creatinine level, and liver enzyme tests at baseline, then every 4 to 8 weeks | $20 |
| Biologics | |||||
| Anti-TNF agents | |||||
| Adalimumab (Humira) | 160 mg subcutaneously once at week 0, then 80 mg once at week 2, then 40 mg every 2 weeks | Injection site reactions (e.g., erythema, itching, hemorrhage, pain, swelling), infection, tuberculosis, malignancies (e.g., lymphoma), autoantibodies/lupus-like syndrome | Active tuberculosis, reactivation of latent tuberculosis, invasive fungal infections (may include histoplasmosis, coccidioidomycosis, candidiasis, aspergillosis, blastomycosis, and pneumocystis pneumonia) | Purified protein derivative test and chest radiography at baseline Signs and symptoms of tuberculosis and active hepatitis B (in those who are carriers of hepatitis B virus) Complete blood count, blood urea nitrogen, creatinine level, and hepatic function tests at baseline and periodically TDM in select cases | NA ($20,000) |
| Certolizumab pegol (Cimzia) | 400 mg subcutaneously once at weeks 0, 2, and 4, then 400 mg every 4 weeks | Injection site reactions, upper respiratory tract infection, headache, hypertension, rash, infections, autoantibodies/lupus-like syndrome | Active tuberculosis, reactivation of latent tuberculosis, invasive fungal infections, lymphoma and other malignancies | Purified protein derivative test and chest radiography at baseline Signs and symptoms of tuberculosis and active hepatitis B (in those who are carriers of hepatitis B virus) Complete blood count, blood urea nitrogen, creatinine level, and hepatic function tests at baseline and periodically TDM in select cases | NA ($12,000) |
| Infliximab (Remicade) Infliximab dyyb (Inflectra) | 5 mg per kg intravenously once at weeks 0, 2, and 6, then 5 mg per kg every 8 weeks | Infusion-related reactions (e.g., dyspnea, flushing, headache, rash, chest pain, hypotension, pruritus, urticaria, anaphylaxis), delayed reaction (e.g., serum sickness, myalgia, arthralgia), infections, pneumonia, cellulitis, abscess, skin ulceration, sepsis, bacterial infection, autoantibodies/lupus-like syndrome, lymphoma | Active tuberculosis, reactivation of latent tuberculosis, invasive fungal infections, hepatosplenic T-cell lymphoma | Purified protein derivative test and chest radiography at baseline Signs and symptoms of tuberculosis and active hepatitis B (in those who are carriers of hepatitis B virus) Dermatologic examination in patients with psoriasis Complete blood count, blood urea nitrogen, creatinine level, and hepatic function tests at baseline and periodically TDM in select cases | NA ($5,000; based on 75-kg [165-lb] male) NA ($4,000; based on 75-kg male) |
| Anti-integrin agents | |||||
| Vedolizumab (Entyvio) | 300 mg intravenously once at weeks 0, 2, and 6, then every 8 weeks thereafter | Nasopharyngitis, headache, arthralgias, nausea, pyrexia, upper respiratory infection and other infections, hypersensitivity reactions, anaphylaxis, lupus-like syndrome | None | Hepatic function tests New onset or worsening of neurologic signs and symptoms Consider screening for tuberculosis TDM in select cases | NA ($12,000) |
| Natalizumab (Tysabri) Only available through REMS CD TOUCH prescribing program | 300 mg intravenously every 4 weeks | Headache, fatigue, upper or lower respiratory and other infections, nausea, arthralgia, depression, infusion-related reaction | Fatal or disabling PML; not to be used with immunomodulators or anti-TNF agents | Anti–John Cunningham virus antibody Signs and symptoms suggestive of PML Hepatic function tests Hypersensitivity reactions Signs and symptoms of acute retinal necrosis | |
| Anti-interleukin 12/23p40 antibody therapy | |||||
| Ustekinumab (Stelara) | Weight-based dosing initially by intravenous infusion Up to 55 kg (121 lb): 260 mg > 55 kg to 85 kg (187 lb): 390 mg > 85 kg: 520 mg Maintenance dosing: 90 mg subcutaneously 8 weeks after initial dose, then every 8 weeks thereafter | Vomiting, nasopharyngitis, injection site erythema, infections, pruritus | None | Purified protein derivative test and chest radiography at baseline and periodically Complete blood count Signs and symptoms of infection or reversible posterior leukoencephalopathy syndrome TDM in select cases | |
CORTICOSTEROIDS
Corticosteroids are often used for symptom management.2,24 Tapering courses of prednisone, with initial doses of 40 to 60 mg based on disease severity, are recommended. Depending on response and how quickly remission is achieved, first tapering by 5 mg per week until the patient reaches a dose of 20 mg, and then tapering by 2.5 to 5 mg weekly until discontinuation is an appropriate strategy.2 When disease is diffuse or located in the left colon, prednisone is preferred; however, formulations of controlled ileal-release budesonide (Entocort EC) are an option for disease affecting the ileum and/or proximal colon and may be preferred because of their unique delivery specifically to that area.2 Budesonide undergoes significant first-pass metabolism in the liver, resulting in improved tolerability.26 Because corticosteroids do not maintain remission, adverse effects are common, and because perforating complications are higher in patients who take corticosteroids, they are most often used to treat symptom flare-ups while patients transition to more effective therapies.
IMMUNOMODULATORS
Thiopurines and methotrexate are the immunomodulators used in Crohn's disease. Azathioprine (Imuran) and 6-mercaptopurine are no more effective than placebo in inducing remission, and therefore, have limited use as monotherapy.2 Immunomodulators have a relatively slow onset of action, but they are often used adjunctively for their steroid-sparing effects. In moderate- to high-risk patients, azathioprine combined with anti–tumor necrosis factor (TNF) agents, such as infliximab (Remicade), demonstrated improved effectiveness over either agent used alone.2 This combination decreases corticosteroid exposure, resulting in fewer adverse effects, and reduces the rate of immunogenicity against anti-TNF agents.2,27 Based on more limited evidence, methotrexate may also be used for induction or maintenance of remission; however, time to clinical response should be considered.2,24,28,29
BIOLOGICS
Several monoclonal antibodies have been approved for the treatment of Crohn's disease: anti-TNF agents, anti-integrin agents, and anti-interleukin-12/23p40 antibody therapy. Whichever agent induces remission should be continued for maintenance. All monoclonal antibodies increase the risk of certain cancers and infections, including reactivation of tuberculosis.1,30,31 Despite these risks, a recent meta-analysis indicates that patients are more likely to continue treatment with biologics than immunomodulators because of improved effectiveness and tolerability.32
Anti-TNF agents, such as certolizumab pegol (Cimzia), adalimumab (Humira), and infliximab, induce and maintain remission in moderate- to high-risk patients, or in patients with inadequate response to corticosteroids and immunomodulators. Onset of action and clinical benefit are often seen within two weeks of therapy initiation. The overall effectiveness of anti-TNF agents is greatest when given within two years of disease onset.2
Natalizumab (Tysabri) and vedolizumab (Entyvio) are anti-integrin agents that target leukocyte trafficking. Natalizumab is associated with progressive multifocal leukoencephalopathy and should only be used in patients who are not seropositive for anti–John Cunningham virus antibody; progressive multifocal leukoencephalopathy has not been observed in patients treated with vedolizumab. Vedolizumab is preferred because of its specificity to leukocyte trafficking in the gut, and has demonstrated effectiveness in achieving clinical response, remission, and corticosteroid-free remission.2
Anti-interleukin-12/23p40 antibody therapy (ustekinumab [Stelara]) is an emerging treatment option for patients when standard therapies have been ineffective.2 The U.S. Food and Drug Administration approved the use of ustekinumab for Crohn's disease in September 2016. Although ustekinumab has been shown to be better than placebo for achieving remission and decreasing symptoms of Crohn's disease, no head-to-head studies have demonstrated the effectiveness of ustekinumab over other therapies, and clinical experience is limited.2,33,34
OTHER THERAPIES
Enteral nutrition is first-line therapy to induce remission in children.23,35 Small studies also show benefit for enteral nutrition for maintenance therapy in adults, so it may be considered when medications are contraindicated or refused.13,36 Current evidence does not support the use of probiotics or omega-3 fatty acids.13
Perianal and Fistulizing Disease
These complex situations are associated with worse outcomes37 and are best managed by a multidisciplinary approach involving subspecialty care from gastroenterologists and surgeons. Perianal disease is best evaluated by contrast-enhanced magnetic resonance imaging. Endoscopic anorectal ultrasonography and clinical examination under anesthesia are also useful diagnostic procedures.21,22
Perianal fistulization is classified as simple or complex. Simple disease is managed with surgical drainage of abscesses and antibiotic therapy (commonly metronidazole [Flagyl] with or without ciprofloxacin). Complex disease first requires surgical drainage of abscesses followed by therapy with an anti-TNF agent (i.e., infliximab or adalimumab). The addition of ciprofloxacin or thiopurines can enhance the effect of anti-TNF agents. Evidence for the management of other fistulas (e.g., enterocutaneous, enteroenteric, enterovesical, enterogynecologic) is limited. When treatment is necessary, they usually require surgical intervention. Anti-TNF agents, thiopurines, and tacrolimus (Prograf; enterocutaneous fistulas) may also provide benefit.2,22
Surgical Treatment
The need for surgery is common, with up to 57% of patients requiring at least one surgery.38 Surgery is often needed to treat fistulas, abscesses, and perianal disease. Other indications include medically resistant disease, perforation, obstruction, strictures, uncontrolled bleeding, dysplasia, and malignancy.1,22,26 Early resection may be an option for patients with disease confined to the ileocecal region who wish to minimize adverse effects of medical therapy.23,39 If resection is required for colonic disease, segmental, as opposed to total, is preferable.22 Strictureplasty and endoscopic dilatation are alternatives to resection for the treatment of strictures. Strictureplasty is not recommended in the colon.22,23
Preventive Measures
Crohn's disease itself and treatments for the disease are associated with medical comorbidities. Preventive measures can mitigate these complications. Patients with Crohn's disease are at increased risk of cancer, osteoporosis, anemia, nutritional deficiencies, depression, infection, and thrombotic events16,41 (Table 816,41–51 and eTable B).
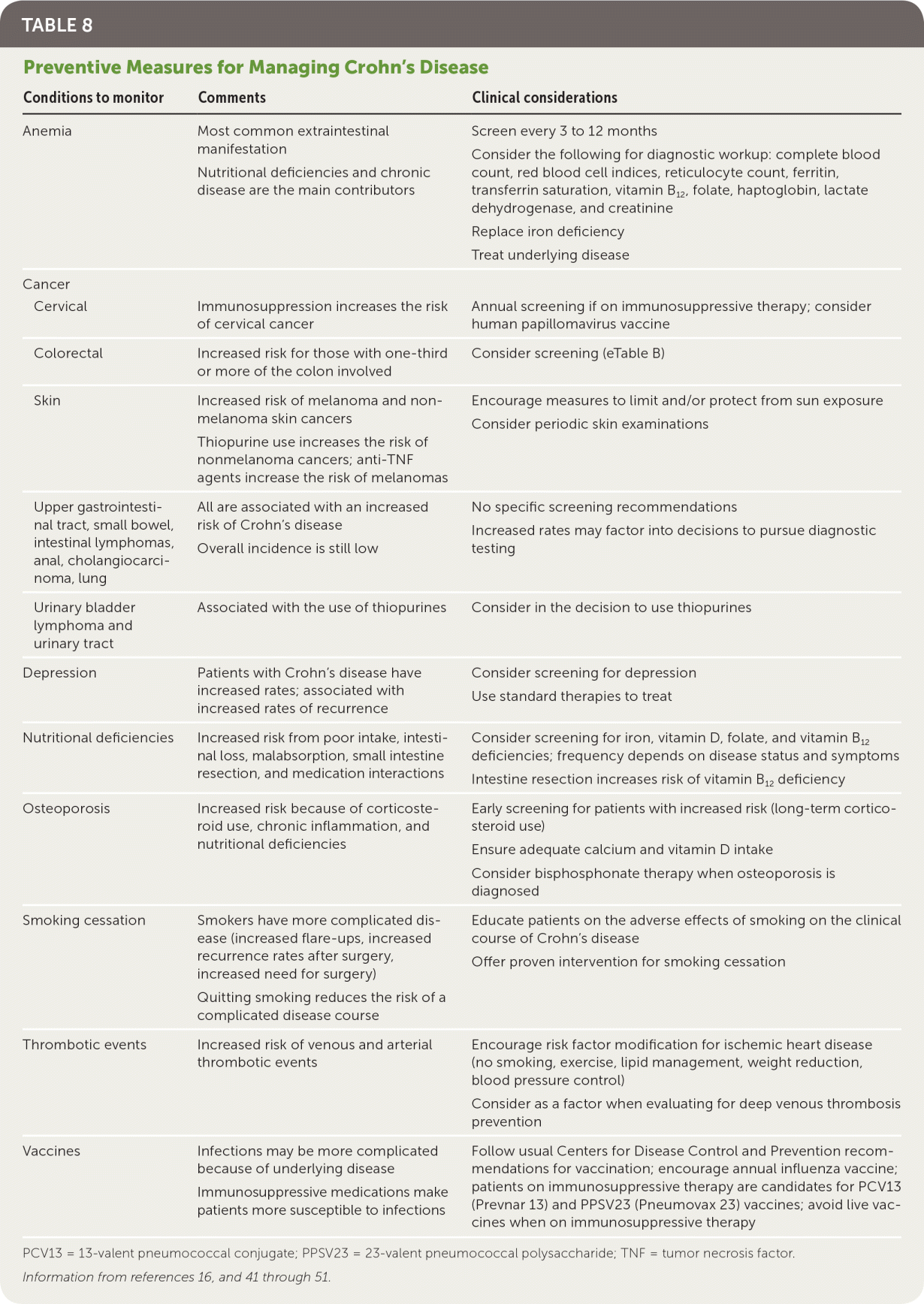
| Conditions to monitor | Comments | Clinical considerations |
|---|---|---|
| Anemia | Most common extraintestinal manifestation Nutritional deficiencies and chronic disease are the main contributors | Screen every 3 to 12 months Consider the following for diagnostic workup: complete blood count, red blood cell indices, reticulocyte count, ferritin, transferrin saturation, vitamin B12, folate, haptoglobin, lactate dehydrogenase, and creatinine Replace iron deficiency Treat underlying disease |
| Cancer Cervical | Immunosuppression increases the risk of cervical cancer | Annual screening if on immunosuppressive therapy; consider human papillomavirus vaccine |
| Colorectal | Increased risk for those with one-third or more of the colon involved | Consider screening (eTable B) |
| Skin | Increased risk of melanoma and nonmelanoma skin cancers Thiopurine use increases the risk of nonmelanoma cancers; anti-TNF agents increase the risk of melanomas | Encourage measures to limit and/or protect from sun exposure Consider periodic skin examinations |
| Upper gastrointestinal tract, small bowel, intestinal lymphomas, anal, cholangiocarcinoma, lung | All are associated with an increased risk of Crohn's disease Overall incidence is still low | No specific screening recommendations Increased rates may factor into decisions to pursue diagnostic testing |
| Urinary bladder lymphoma and urinary tract | Associated with the use of thiopurines | Consider in the decision to use thiopurines |
| Depression | Patients with Crohn's disease have increased rates; associated with increased rates of recurrence | Consider screening for depression Use standard therapies to treat |
| Nutritional deficiencies | Increased risk from poor intake, intestinal loss, malabsorption, small intestine resection, and medication interactions | Consider screening for iron, vitamin D, folate, and vitamin B12 deficiencies; frequency depends on disease status and symptoms Intestine resection increases risk of vitamin B12 deficiency |
| Osteoporosis | Increased risk because of corticosteroid use, chronic inflammation, and nutritional deficiencies | Early screening for patients with increased risk (long-term corticosteroid use) Ensure adequate calcium and vitamin D intake Consider bisphosphonate therapy when osteoporosis is diagnosed |
| Smoking cessation | Smokers have more complicated disease (increased flare-ups, increased recurrence rates after surgery, increased need for surgery) Quitting smoking reduces the risk of a complicated disease course | Educate patients on the adverse effects of smoking on the clinical course of Crohn's disease Offer proven intervention for smoking cessation |
| Thrombotic events | Increased risk of venous and arterial thrombotic events | Encourage risk factor modification for ischemic heart disease (no smoking, exercise, lipid management, weight reduction, blood pressure control) Consider as a factor when evaluating for deep venous thrombosis prevention |
| Vaccines | Infections may be more complicated because of underlying disease Immunosuppressive medications make patients more susceptible to infections | Follow usual Centers for Disease Control and Prevention recommendations for vaccination; encourage annual influenza vaccine; patients on immunosuppressive therapy are candidates for PCV13 (Prevnar 13) and PPSV23 (Pneumovax 23) vaccines; avoid live vaccines when on immunosuppressive therapy |
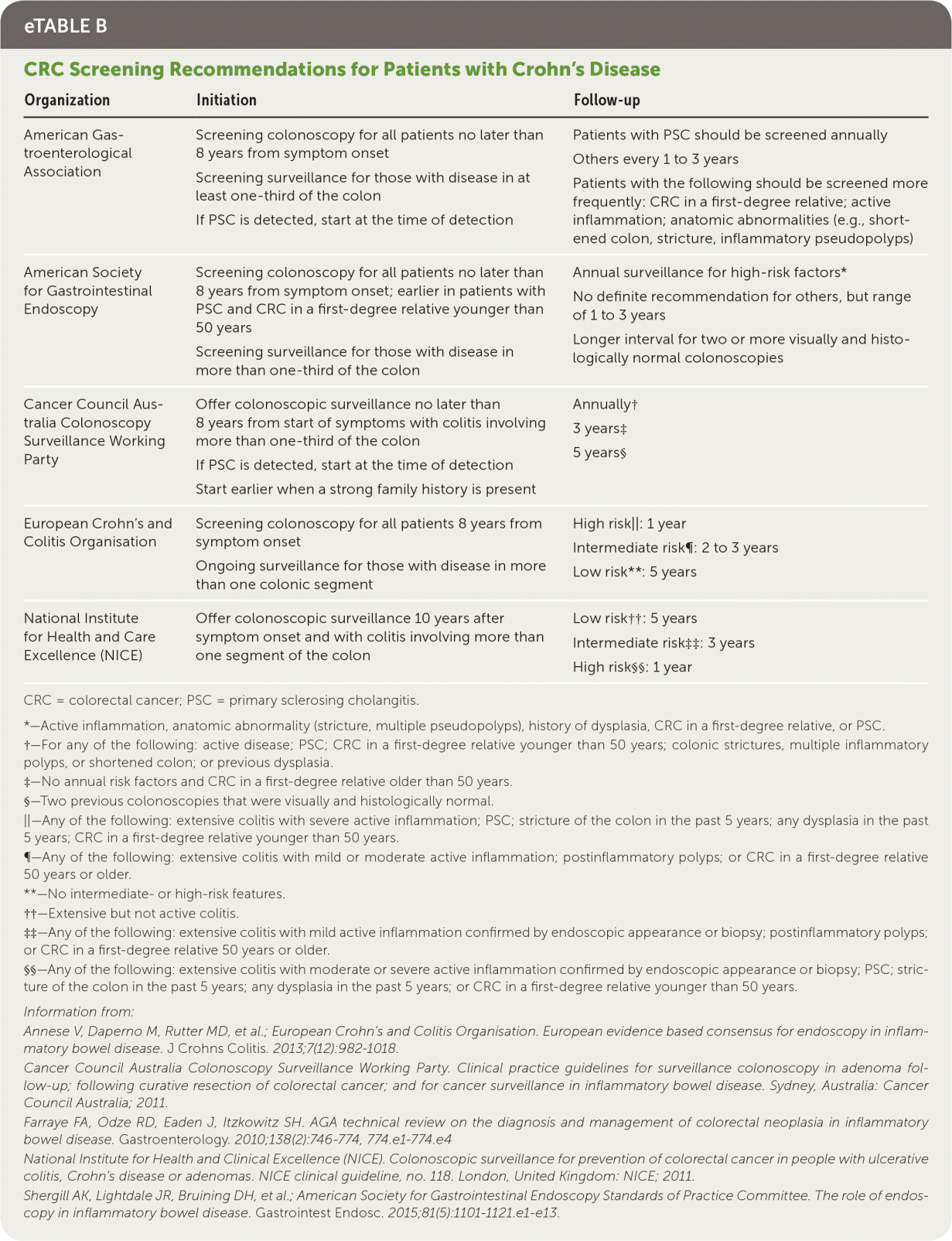
| Organization | Initiation | Follow-up |
|---|---|---|
| American Gastroenterological Association | Screening colonoscopy for all patients no later than 8 years from symptom onset Screening surveillance for those with disease in at least one-third of the colon If PSC is detected, start at the time of detection | Patients with PSC should be screened annually Others every 1 to 3 years Patients with the following should be screened more frequently: CRC in a first-degree relative; active inflammation; anatomic abnormalities (e.g., shortened colon, stricture, inflammatory pseudopolyps) |
| American Society for Gastrointestinal Endoscopy | Screening colonoscopy for all patients no later than 8 years from symptom onset; earlier in patients with PSC and CRC in a first-degree relative younger than 50 years Screening surveillance for those with disease in more than one-third of the colon | Annual surveillance for high-risk factors* No definite recommendation for others, but range of 1 to 3 years Longer interval for two or more visually and histologically normal colonoscopies |
| Cancer Council Australia Colonoscopy Surveillance Working Party | Offer colonoscopic surveillance no later than 8 years from start of symptoms with colitis involving more than one-third of the colon If PSC is detected, start at the time of detection Start earlier when a strong family history is present | Annually† 3 years‡ 5 years§ |
| European Crohn's and Colitis Organisation | Screening colonoscopy for all patients 8 years from symptom onset Ongoing surveillance for those with disease in more than one colonic segment | High risk||: 1 year Intermediate risk¶: 2 to 3 years Low risk**: 5 years |
| National Institute for Health and Care Excellence (NICE) | Offer colonoscopic surveillance 10 years after symptom onset and with colitis involving more than one segment of the colon | Low risk††: 5 years Intermediate risk‡‡: 3 years High risk§§: 1 year |
This article updates previous articles on this topic by Botoman, et al.52 ; Feller, et al.53 ; Knutson, et al.54 ; and Wilkins, et al.1
Data Sources: We searched the National Guideline Clearinghouse using the term Crohn disease; PubMed was searched using the terms Crohn disease and cost United States, prevalence of Crohn disease, and Crohn disease AND prevalence AND United States, and using the Clinical Queries function with the term Crohn's Disease. Also searched were the Cochrane database and Bandolier database using the term Crohn's, and the Institute for Clinical Systems Improvement database for the term Crohn disease. Essential Evidence was also searched. Search dates: November 1, 2016, through July 2, 2018.
