A more recent article on long-term opioid therapy for nonterminal pain is available.
Am Fam Physician. 2012;86(3):252-258
Related content: Information and resources on pain management and opioid abuse from the AAFP
Related editorial: Opioid Abuse and Pain Management
Author disclosure: No relevant financial affiliations to disclose.
Opioid prescribing for chronic nonterminal pain has increased in recent years, although evidence for its long-term effectiveness is weak and its potential for harm is significant. Nonmedical use of prescription opioids, diversion, and overdose deaths have also increased sharply, sparking concern about the safety of these medications. Physicians considering initiation or continuation of opioid therapy for a patient with chronic nonterminal pain should first use a structured approach that includes a biopsychosocial evaluation and a treatment plan that encourages patients to set and reach functional goals. There should be a comprehensive evaluation for the cause of pain, assessment for risk of opioid complications (including misuse and addiction), and a detailed treatment history, including a review of medical records and data from the state prescription monitoring program. Opioids should be prescribed on a trial basis, to be continued only if progress toward functional goals is demonstrated. Long-acting morphine is the preferred initial drug, although several alternatives are available. Ongoing monitoring for safety and effectiveness is essential, including regular review of functional progress or maintenance, urine drug testing, and surveillance of data from the state prescription monitoring program. Ineffective, unsafe, or diverted opioid therapy should be promptly tapered or stopped.
Chronic pain affects approximately one-third of U.S. adults, and 5 percent receive opioid treatment1; strong opioids are prescribed in up to 9 percent of all office visits.2 Until the 1990s, physicians generally limited opioid treatment to patients with acute or cancer-related pain. However, studies showed that all types of pain were undertreated; as a result, pain education, advocacy, and policy initiatives led to an increase in opioid prescribing for patients with chronic nonterminal pain.
More expansive prescribing of opioids for chronic nonterminal pain has been accompanied by increasing rates of high-dose, continuous therapy.3–6 Although some patients may benefit from such treatment, others will have significant physiologic and functional compromise. Continuous opioid therapy for as little as two weeks can produce drug tolerance in some patients. For other patients, dosages as low as 30 mg per day of morphine or its equivalent have been shown to lower pain thresholds and create opioid-induced hyperalgesia, in which pain paradoxically worsens as opioid doses are increased.7–9 Many patients experience nausea, vomiting, constipation, depressed mood, and respiratory depression, and tolerance to these toxicities may develop slowly or not at all. A 10-year follow-up study showed that patients who take prescription opioids have a lower quality of life and higher rates of depression and health care utilization.10
Increased opioid prescribing has also resulted in worrying trends of misuse, abuse, and diversion for sale. Between 1997 and 2006, sales of oxycodone (Roxicodone) increased nearly eightfold, and sales of methadone increased ninefold.11 Fifteen percent of 12th graders report that they have taken hydrocodone/acetaminophen (Vicodin) or oxycodone, usually obtained from family members or friends.11 From 2004 to 2008, visits to emergency departments for nonmedical use of opioids tripled.12 The Centers for Disease Control and Prevention reported a nearly sevenfold increase in methadone-related fatalities in 2009.12 Overall, the nonmedical use of controlled prescription medication now exceeds illicit drug use. These statistics have raised serious concerns among state and federal law enforcement, regulatory, and legislative officials, and have sparked efforts to increase prescription oversight.13,14
In this environment, prescribers must ensure that opioid therapy is appropriate, safe, and effective. This review provides a framework for approaching opioid therapy for patients with chronic nonterminal pain, including patient selection; structured trials of opioid therapy; monitoring; and tapering, when indicated. More details and practice tools are available online (http://guidelines.gov/content.aspx?id=25657&search=managing+chronic+pain).15
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Patients with chronic nonterminal pain should receive a comprehensive evaluation, including assessment for potential opioid responsiveness and opioid risk. | C | 4, 6, 18 |
| Chronic nonterminal pain requires treatment of physical and psychological modalities, prescription of nonopioid analgesics, treatment of comorbid mood disorders, and restoration of sleep. | C | 18, 19 |
| Tricyclic antidepressants or selective serotonin-norepinephrine reuptake inhibitors should be included in patients with chronic nonterminal pain with a neuropathic component. | A | 13, 15, 20 |
| Opioid therapy should be avoided in patients with chronic central or visceral pain syndromes such as fibromyalgia, headaches, or abdominal pain. | C | 11, 17 |
| Opioids should be initiated as a trial, to be continued if progress is documented toward functional goals, and if there is no evidence of complications, including misuse or diversion. | C | 6, 15, 18 |
| Opioid dosages exceeding 100 mg of morphine or its equivalent may increase the risk of overdose, and should prompt consideration of tapering or referral to a pain subspecialist. | C | 13, 27 |
Understanding Chronic Pain
Chronic pain is different from acute pain, and must be approached differently. Acute pain is a symptom that plays a functional role in body defenses and resolves with tissue recovery. It is transmitted along intact neural pathways that are effectively modulated by opioids to decrease pain perception.
In contrast, chronic pain has no such functional role, does not resolve with tissue recovery, and can become a primary diagnosis. Chronic pain involves complex central nervous system signaling that can be amplified by stressors such as relationship issues, financial problems, or underlying psychiatric diagnoses. This can result in the perception of worse pain, and an increased likelihood of compromised social functioning.10,16,17 Thus, chronic nonterminal pain is a biopsychosocial condition that requires a comprehensive, multidisciplinary approach to evaluation and management. Continuing typical treatments for acute pain—including opioid therapy—can be ineffective or unsafe in patients with chronic pain.
General Approach to the Patient with Chronic Nonterminal Pain
Effective management of chronic nonterminal pain requires more than prescribing medication.13,15,18 Any potential focal or treatable pain generators should be identified and treated first. Attention should then turn to physical and psychological modalities, prescription of nonopioid and adjuvant analgesic medications, multimodal treatment of mood disorders, and restoration of sleep.18,19 Only after these measures are optimized should a trial of opioid therapy, or continuation of existing opioid therapy, be considered. A comprehensive approach is outlined in Table 1.13,15,18,20

| Identify and treat specific local pain generators, if present |
| Cutaneous stimulators (e.g., transcutaneous electrical nerve stimulation) |
| Local treatment (e.g., physical therapy, manipulation, massage, heat) |
| Minor interventions (e.g., anesthetic or steroid joint injection) |
| Surgery |
| Topical anesthetics (e.g., lidocaine ointment or patches) |
| Promote healthy behaviors |
| Physical activity |
| Weight control |
| Restore sleep |
| Depending on coexisting problems, useful agents may include trazodone, tricyclic antidepressants, gabapentin (Neurontin), pregabalin (Lyrica), mirtazapine (Remeron), melatonin, or quetiapine (Seroquel); use of benzodiazepines or their analogs should be avoided because of tolerance and abuse potential |
| Start adjuvant pain medications (listed in order of recommended treatments) |
| Tricyclic antidepressants are indicated for neuropathic pain; nortriptyline (Pamelor), desipramine (Norpramin), amitriptyline, and doxepin are also useful for localized or generalized pain with coexisting headache, depression, panic disorder, or tobacco addiction |
| Serotonin-norepinephrine reuptake inhibitors are indicated for neuropathic pain; duloxetine (Cymbalta) and milnacipran (Savella) are most effective for localized or generalized pain with depression or anxiety, venlafaxine (Effexor) less so; doses for treatment of pain tend to be higher than those for depression |
| Pregabalin is approved by the U.S. Food and Drug Administration for neuropathic pain and fibromyalgia; gabapentin has similar effectiveness, is available generically, and is more widely used, but it is sedating, and dosing is complex; may be synergistic when combined with tricyclic antidepressants |
| Anticonvulsants; carbamazepine (Tegretol) is a first-line agent for trigeminal neuralgia, but third-line (with oxcarbazepine [Trileptal] and lamotrigine [Lamictal]) for other neuropathic pain |
| Migraine chemoprophylaxis (e.g., beta blockers, calcium channel blockers, tricyclic antidepressants, topiramate [Topamax]) |
| Treat comorbid psychiatric illness |
| Use of pharmacologic and psychotherapeutic measures is essential for improvement of symptoms and function |
| Commonly associated disorders include anxiety, depression, posttraumatic stress disorder in persons who have experienced physical, sexual, or emotional trauma; treatment should include psychotherapy, biofeedback, mindfulness training, posttraumatic stress disorder therapy, relationship counseling, social and financial counseling, and substance abuse counseling |
| Trial of opioid therapy (initiation or continuation) |
| Patients should have no contraindications for opioid therapy |
| Patients with diffuse, centralized pain or headache are less likely to benefit from opioid therapy and may be harmed by opioid toxicities |
| Select patients with specific somatic, peripheral, or neuropathic pain may benefit from low-dose opioid therapy in combination with other treatments |
| Treatment should produce demonstrable functional improvement, not merely a reduction in pain scores, or opioids should be tapered or discontinued |
Opioid Use and Precautions
INITIAL PATIENT SELECTION
Safe and effective opioid therapy requires careful patient selection. In general, chronic somatic or neuropathic pain (e.g., musculoskeletal pain, peripheral neuropathy, postherpetic neuralgia) is at least partially responsive to opioids. However, chronic visceral or central pain syndromes (e.g., abdominal or pelvic pain, fibromyalgia, headaches) are less responsive or nonresponsive.11,17,21,22 Patients with these conditions may have more adverse effects than benefits from chronic opioid therapy.
The patient's risk of opioid misuse or abuse must be assessed. Several validated tools are available, including the Opioid Risk Tool (Table 2).23 Risks should be acknowledged openly and managed, whenever possible, before prescribing opioids. Although no specific cutoffs have been established, patients with scores of 8 or more on the Opioid Risk Tool should be prescribed opioids only after all other treatment modalities have been exhausted, under close supervision, and ideally in consultation with a pain or addiction subspecialist. Opioids should not be prescribed to patients who are actively using illicit substances, as detected during assessment or on pretreatment urine testing.
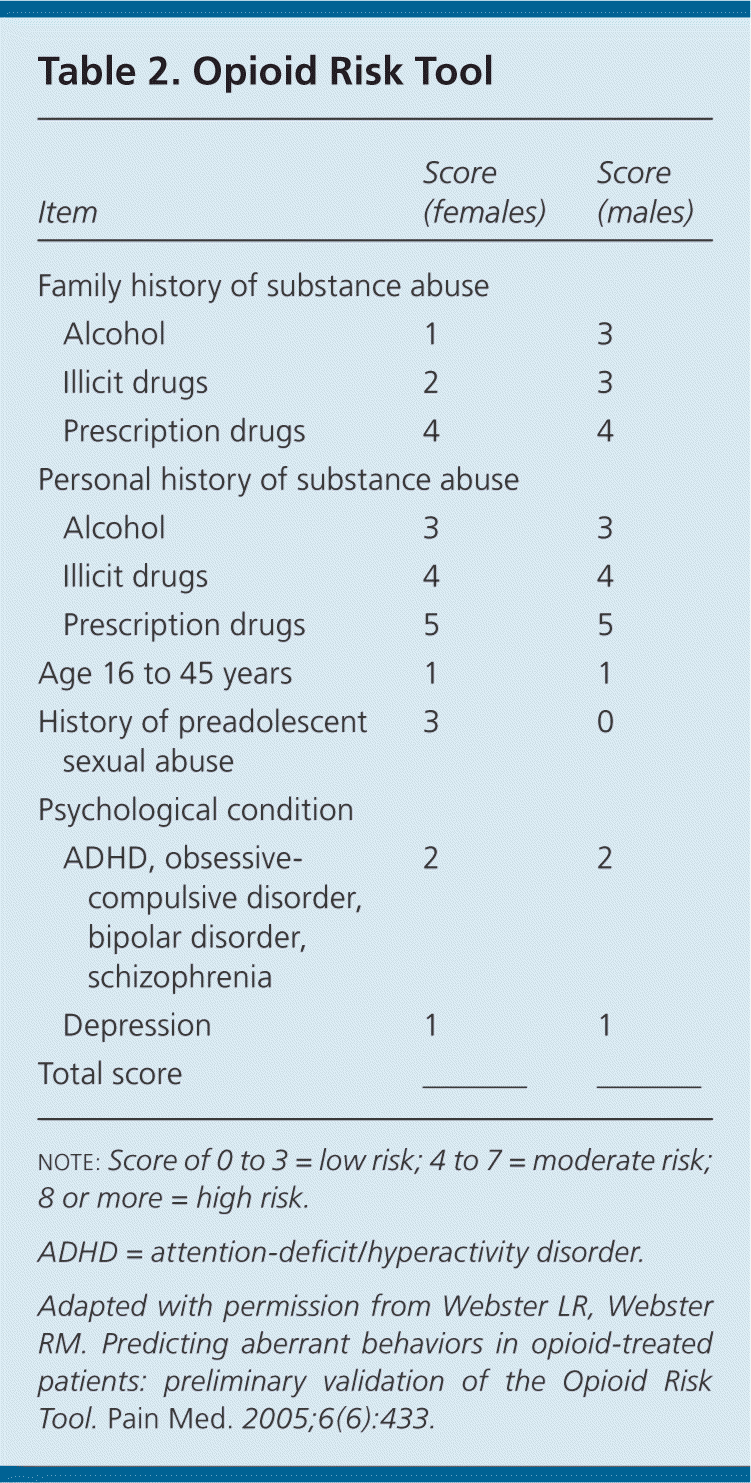
| Item | Score (females) | Score (males) | |
|---|---|---|---|
| Family history of substance abuse | |||
| Alcohol | 1 | 3 | |
| Illicit drugs | 2 | 3 | |
| Prescription drugs | 4 | 4 | |
| Personal history of substance abuse | |||
| Alcohol | 3 | 3 | |
| Illicit drugs | 4 | 4 | |
| Prescription drugs | 5 | 5 | |
| Age 16 to 45 years | 1 | 1 | |
| History of preadolescent sexual abuse | 3 | 0 | |
| Psychological condition | |||
| ADHD, obsessive-compulsive disorder, bipolar disorder, schizophrenia | 2 | 2 | |
| Depression | 1 | 1 | |
| Total score | –––– | –––– | |
After careful screening, opioid therapy may be initiated on a trial basis. Guidance for all phases of opioid prescribing—decision, initiation, maintenance, and tapering—is given in Table 3.13,15,18,19,24 These steps should be followed in all patients as part of a universal precautions approach. Controlled medications should not be prescribed on a first visit because of pressure from the patient or a sense of duty to treat.
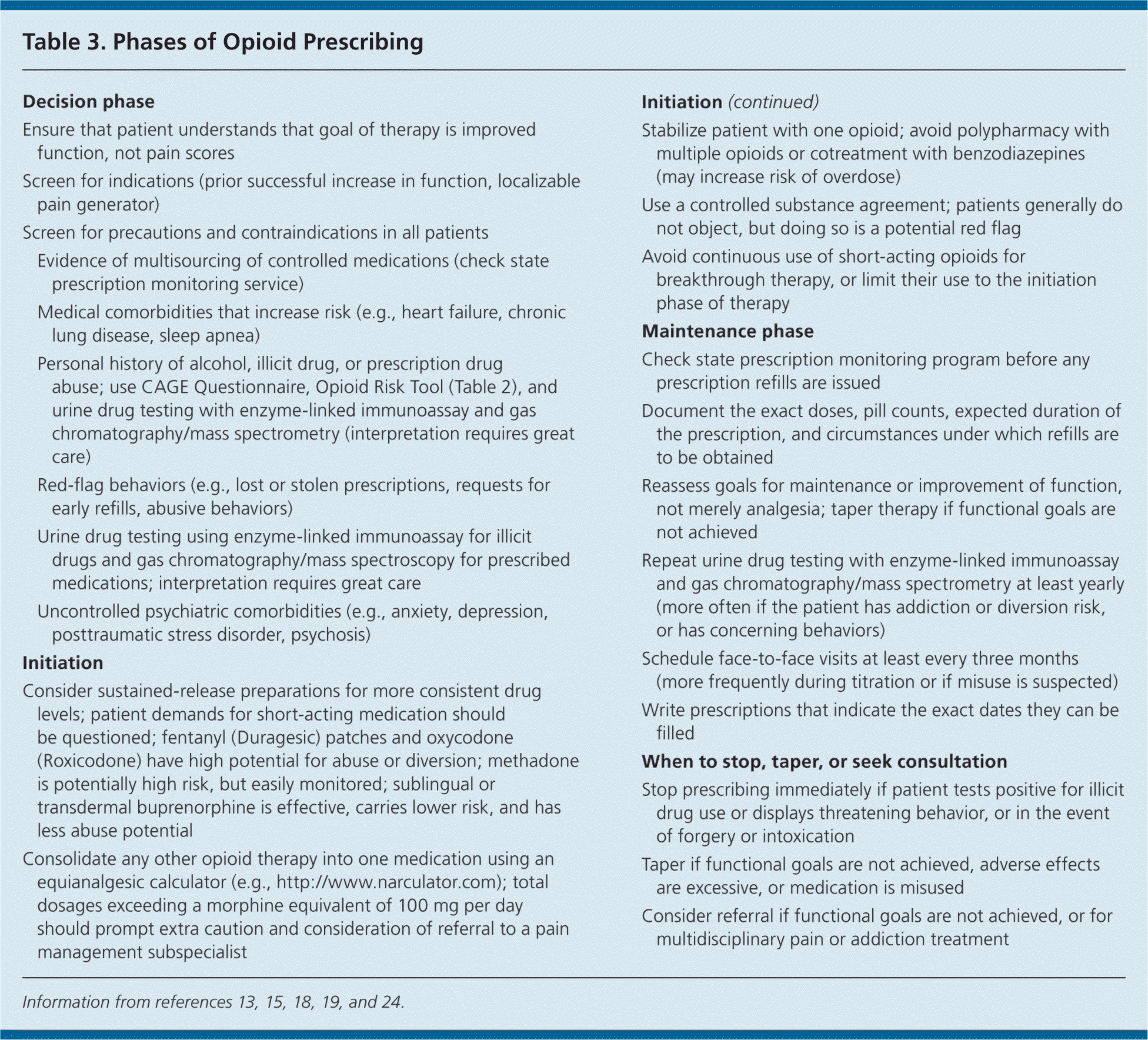
| Decision phase | |
| Ensure that patient understands that goal of therapy is improved function, not pain scores | |
| Screen for indications (prior successful increase in function, localizable pain generator) | |
| Screen for precautions and contraindications in all patients | |
| Evidence of multisourcing of controlled medications (check state prescription monitoring service) | |
| Medical comorbidities that increase risk (e.g., heart failure, chronic lung disease, sleep apnea) | |
| Personal history of alcohol, illicit drug, or prescription drug abuse; use CAGE Questionnaire, Opioid Risk Tool (Table 2), and urine drug testing with enzyme-linked immunoassay and gas chromatography/mass spectrometry (interpretation requires great care) | |
| Red-flag behaviors (e.g., lost or stolen prescriptions, requests for early refills, abusive behaviors) | |
| Urine drug testing using enzyme-linked immunoassay for illicit drugs and gas chromatography/mass spectroscopy for prescribed medications; interpretation requires great care | |
| Uncontrolled psychiatric comorbidities (e.g., anxiety, depression, posttraumatic stress disorder, psychosis) | |
| Initiation | |
| Consider sustained-release preparations for more consistent drug levels; patient demands for short-acting medication should be questioned; fentanyl (Duragesic) patches and oxycodone (Roxicodone) have high potential for abuse or diversion; methadone is potentially high risk, but easily monitored; sublingual or transdermal buprenorphine is effective, carries lower risk, and has less abuse potential | |
| Consolidate any other opioid therapy into one medication using an equianalgesic calculator (e.g., http://www.narculator.com); total dosages exceeding a morphine equivalent of 100 mg per day should prompt extra caution and consideration of referral to a pain management subspecialist | |
| Stabilize patient with one opioid; avoid polypharmacy with multiple opioids or cotreatment with benzodiazepines (may increase risk of overdose) | |
| Use a controlled substance agreement; patients generally do not object, but doing so is a potential red flag | |
| Avoid continuous use of short-acting opioids for breakthrough therapy, or limit their use to the initiation phase of therapy | |
| Maintenance phase | |
| Check state prescription monitoring program before any prescription refills are issued | |
| Document the exact doses, pill counts, expected duration of the prescription, and circumstances under which refills are to be obtained | |
| Reassess goals for maintenance or improvement of function, not merely analgesia; taper therapy if functional goals are not achieved | |
| Repeat urine drug testing with enzyme-linked immunoassay and gas chromatography/mass spectrometry at least yearly (more often if the patient has addiction or diversion risk, or has concerning behaviors) | |
| Schedule face-to-face visits at least every three months (more frequently during titration or if misuse is suspected) | |
| Write prescriptions that indicate the exact dates they can be filled | |
| When to stop, taper, or seek consultation | |
| Stop prescribing immediately if patient tests positive for illicit drug use or displays threatening behavior, or in the event of forgery or intoxication | |
| Taper if functional goals are not achieved, adverse effects are excessive, or medication is misused | |
| Consider referral if functional goals are not achieved, or for multidisciplinary pain or addiction treatment | |
If an initial evaluation raises questions about whether opioid therapy is appropriate, the physician should openly acknowledge these concerns with the patient and commit to treat the pain comprehensively through other means. If opioids are not appropriate in a patient who is already taking them, therapy should not be continued because of concerns about withdrawal. Although it is uncomfortable, opioid withdrawal is generally not life-threatening, and the patient can be referred to an addiction subspecialist for management.
VISIT CHECKLISTS
Visit checklists can be used to evaluate whether initiation or continuation of opioid therapy is appropriate. Items should include evidence or absence of progress toward pain relief and functional goals, evidence of red-flag behaviors (e.g., lost prescriptions, frequent early requests for refills), and the presence of uncontrolled psychiatric illness or medical comorbidities. Such checklists, if used routinely, may be especially useful in group practices where multiple physicians may care for the same patients.
URINE TESTING
All patients should undergo urine drug testing before opioid therapy is initiated, and then at least yearly unless patient behavior suggests the need for more frequent testing. An enzyme-linked immunoassay for drugs of abuse (e.g., amphetamine, marijuana, cocaine) and gas chromatography/mass spectrometry to detect prescribed agents should be ordered. The opioid portion of most enzyme-linked immunoassays reliably detects only morphine or codeine, and misses hydrocodone, oxycodone, hydromorphone (Dilaudid), oxymorphone, fentanyl (Duragesic), methadone, and buprenorphine. These drugs are detected by gas chromatography/ mass spectrometry, especially if the laboratory is asked to check for these or other specific drugs in patients known to be prescribed one or more of them. Both tests may produce false-positive or false-negative results, so care must be taken to avoid misinterpretation of patient adherence. Concise guides to test interpretation are available.13,15,25
PRESCRIPTION MONITORING
Many states have established prescription monitoring programs (http://www.painpolicy.wisc.edu/domestic/pmp.htm). Online access is available in most states and can identify a patient's controlled prescriptions for the preceding year by drug name, strength, quantity, date of prescription, date prescription was filled, prescriber, and pharmacy used. Office staff can routinely obtain this information before visits by patients receiving opioid therapy.
WRITTEN AGREEMENTS
Many sample pain and controlled medication agreements are available (e.g., http://www.aapainmanage.org/literature/Articles/OpioidAgreements.pdf). These agreements outline appropriate intervals for follow-up, refill policies, participation in any indicated multimodal management plan (e.g., physical therapy, psychological treatment), use of only one prescriber and one pharmacy for all controlled medications, and prohibition of illicit substance use or prescription diversion. As with urine testing and prescription monitoring program reporting, written agreements should be part of an ongoing treatment plan for all patients receiving chronic opioid therapy, thereby avoiding reliance on physician judgment, suspicion, or bias.
Selecting an Initial Opioid
Morphine should be the first choice for chronic potent opioid therapy. Reliable, inexpensive, long-acting oral morphine preparations are available for patients receiving chronic therapy. If the patient must later be switched to another medication, morphine doses are the units on which opioid equianalgesic calculations are based. Common adverse effects include constipation, nausea, pruritus, and drowsiness, all of which are more common than morphine allergy. Morphine should be used with caution (especially its long-acting preparations) in patients with renal failure.
Oxycodone is an alternative for patients with morphine intolerance or allergy. However, it may have a higher risk of abuse and should be used with caution in patients with higher risk scores. Long-acting oxycodone is not recommended for patients with chronic pain because it is not truly long-acting, is expensive, and has a high street value. Transdermal fentanyl may be a better alternative, although it is expensive and can produce tolerance relatively quickly. Fentanyl is lipophilic, and absorption is affected in patients with little subcutaneous fat and in those prone to edema at application sites.
Methadone can be effective for many patients and may produce less tolerance than other opioids. It is inexpensive, long-acting, and has a combination of opioid and N-methyl-d-aspartate receptor activity that may make it a good choice for patients with mixed somatic and neuropathic pain. However, physicians who prescribe methadone must be familiar with its unique pharmacokinetics: it has a very long elimination half-life, and its morphine-equivalent equianalgesic conversion ratio increases as dosages increase. Starting dosages in opioid-naive patients are 2.5 to 5 mg two or three times per day. Dosages should be titrated slowly and no more than once per week. Methadone can prolong the QT interval, especially in patients who are taking other QT-prolonging medications. Serum drug levels can be used for monitoring. Methadone does not interfere with urine testing for other opioids.
Buprenorphine is a partial opioid agonist that is less likely to produce tolerance.26 It is effective for treatment of pain, has lower abuse potential, and is easily monitored. However, it is expensive, and its use requires special prescriber training (except for the transdermal patch).
Breakthrough Dosing
Short-acting opioids (e.g., morphine, oxycodone, hydromorphone) may be used during initiation of long-acting opioids, but should not be used continuously as primary or breakthrough treatment. Breakthrough dosing has not been shown to improve outcomes and can increase the risk of overdose, misuse, and further opioid tolerance.24 Because of the high street value of many short-acting opioids, requests for breakthrough doses should be resisted; if they are prescribed, only small quantities should be given (e.g., 10 doses per month).
Multiple Opioids and High-Dose Therapy
The use of multiple opioids should be avoided whenever possible; equianalgesic conversions can be used to consolidate therapies (a tool is available at http://www.narculator.com). Total opioid doses exceeding 100 mg of morphine or its equivalent are associated with an increased risk of overdose and should prompt consideration of tapering or referral to a pain subspecialist.13,27 The need for such referral should be framed as an issue of medication safety and effectiveness, not suspicion or judgment of patients or their behavior.
Tapering or Discontinuing Therapy
Opioid therapy should be reassessed for safety and effectiveness in all patients at least quarterly. Issues of patient safety or illegal activity should prompt discontinuation (Table 4).

| Immediate discontinuation |
| Belligerent or threatening behavior toward physician or staff |
| Confirmation of diversion, multisourcing, or prescription forgery |
| Confirmation of illicit drug use, including marijuana |
| Overdose |
| Rapid tapering |
| Frequent requests for early refills, despite adequate titration of long-acting opioids |
| Major adverse effects or intoxication (e.g., somnolence, slurred speech, altered sensorium) |
| Opioid-induced hyperalgesia |
| Other nonadherence to pain medication agreement |
| Gradual tapering |
| Functional goals not met |
| Morphine equivalent dosage greater than 100 mg per day, without clear benefit to function or pain |
| Persistent adverse effects despite opioid rotation (e.g., pruritus, nausea, refractory constipation) |
Before tapering, all opioid therapy should be consolidated into a single long-acting medication using equianalgesic calculations. Dose reduction should then proceed at a rate that avoids opioid withdrawal symptoms, loss of patient confidence, or pain escalation,28 and should continue until symptoms or function is improved. Gradual tapering can be accomplished by reducing the original total opioid dose by 10 percent every one to four weeks until 20 percent of the original total dose remains, then reducing by 5 percent of the original dose until it is discontinued. Rapid tapering can be accomplished by reducing the original dose by 25 percent every three to seven days; this will avoid severe withdrawal symptoms.
Despite tapering, some patients may have withdrawal symptoms when opioids are completely discontinued. These symptoms should be managed supportively; traditional withdrawal management medications such as clonidine (Catapres), tramadol (Ultram), and muscle relaxants are generally ineffective, although temporary use of nonbenzodiazepine sleep aids can be helpful. Follow-up visits should be scheduled frequently for ongoing multimodal pain management and encouragement that function will improve over weeks to months.
Data Sources: Searches of the human, English literature were conducted in Medline, Cochrane reports, and the National Guideline Clearinghouse for clinical trials and guidelines published from January 1, 2000, through April 25, 2011, using the major keywords of chronic pain, non-malignant, non-cancer, and non-terminal. Terms used for specific topic searches included pain assessment, psychiatric comorbidities, addiction risk, opioid abuse, emergency department visits, pain mechanisms, opioid-induced hyperalgesia, opioid tolerance, opioid taper, long-term outcomes, and buprenorphine. Search date: January 2011 through April 2011.