
Am Fam Physician. 2012;86(6):521-526
This is part I of a two-part article on TIA. Part II, “Risk Factor Modification and Treatment,” appears in this issue of AFP.
Patient information: See related handout on TIA, written by the authors of this article.
For a commentary on the AHA/ASA guidelines on TIA, which are featured in this article, see the AFP Journal Club critique in the June 15, 2012, issue at https://www.aafp.org/afp/2012/0615/p1179.html.
Author disclosure: No relevant financial affiliations to disclose.
Transient ischemic attack is defined as transient neurologic symptoms without evidence of acute infarction. It is a common and important risk factor for future stroke, but is greatly underreported. Common symptoms are sudden and transient, and include unilateral paresis, speech disturbance, and monocular blindness. Correct and early diagnosis of transient ischemic attack versus mimicking conditions is important because early interventions can significantly reduce risk of future stroke. Nonspecific symptoms and gradual onset are more likely with mimics than with true transient ischemic attacks. Transient ischemic attacks are more likely with sudden onset, focal neurologic deficit, or speech disturbance. Urgent evaluation is necessary in patients with symptoms of transient ischemic attack and includes neuroimaging, cervicocephalic vasculature imaging, cardiac evaluation, blood pressure assessment, and routine laboratory testing. The ABCD2 (age, blood pressure, clinical presentation, diabetes mellitus, duration of symptoms) score should be determined during the initial evaluation and can help assess the immediate risk of repeat ischemia and stroke. Patients with higher ABCD2 scores should be treated as inpatients, whereas those with lower scores are at lower risk of future stroke and can be treated as outpatients.
Over the past 10 years, transient ischemic attack (TIA) has been redefined multiple times to reflect the transient nature of not only the symptoms, but also cerebral ischemia. The classic definition for TIA of a sudden, focal neurologic deficit for less than 24 hours was established in the 1960s and was the accepted definition for 40 years.1,2 In 2002, the TIA Working Group redefined TIA as brief neurologic dysfunction with symptoms typically lasting less than one hour, without evidence of acute infarction.1 This definition was well received; however, it has been shown that no time cutoff can reliably predict if cerebral ischemia is reversible.3 This led to the 2009 revision by the American Heart Association/American Stroke Association (AHA/ASA), which now defines TIA as a transient episode of neurologic dysfunction caused by focal cerebral, spinal cord, or retinal ischemia, without acute infarction.2 A lack of evidence of infarction on magnetic resonance imaging (MRI) in patients who have symptoms consistent with cerebral ischemia distinguishes TIA from minor stroke. This article, part I of a two-part series, focuses on the diagnosis of TIA. Part II discusses treatment after TIA.4
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| All patients presenting with symptoms of possible TIA should undergo urgent evaluation including neuroimaging, cervicocephalic vasculature imaging, and electrocardiography. | C | 2 |
| Echocardiography, prolonged cardiac monitoring, and routine blood tests are reasonable in the evaluation of TIA. | C | 2 |
| Patients with TIA who present within 72 hours of symptom resolution should be hospitalized if they have an ABCD2 (age, blood pressure, clinical presentation, diabetes mellitus, duration of symptoms) score of 3 or greater, have evidence of focal ischemia, or are unable to complete outpatient workup within 48 hours. | C | 2, 29–31 |
Epidemiology
Using imaging results instead of a time cutoff to diagnose TIA will impact interpretation of future and past epidemiologic data on incidence and prevalence of TIA. One study evaluated MRI in patients with TIA based on the classic definition and found that 33 percent had evidence of cerebral infarction.5 Under the new definition, those with evidence of infarction should be redefined as having a stroke, leading to a lower overall incidence of TIA.5 However, TIA is likely underreported. In a telephone survey, 2.3 percent of individuals reported that they were told by their physician that they had a TIA, but an additional 3.2 percent of patients reported having symptoms consistent with TIA but never sought medical attention.6 The overall incidence of TIA is estimated to be 200,000 to 500,000 cases per year.2 TIA is a major risk factor for future ischemic stroke, with the greatest risk occurring in the period immediately after TIA.7–9 The odds ratio for ischemic stroke following TIA is 30.4 during the first 30 days, 18.9 at one to three months, 3.16 at four to six months, and 1.87 after five years.7
TIA Mimics
One of the greatest challenges family physicians face when evaluating possible TIA is distinguishing true TIA and ischemic events from TIA mimics. Physician accuracy in determining this distinction in the outpatient primary care setting historically has been poor, and a study showed that even stroke-trained neurologists have a fair amount of disagreement when diagnosing TIA.10,11
Correct and early diagnosis of TIA versus mimics is critical because early interventions (e.g., antiplatelet agents, statin therapy, blood pressure–lowering therapy, anticoagulation when appropriate) can lead to an 80 percent reduction in risk of recurrent ischemic events.12 The most common TIA mimics are seizures, migraines, metabolic disturbances, and syncope.10,13,14 Mimics are more likely with gradual onset of symptoms and with nonspecific symptoms (Table 113 ), such as memory loss or headache.13–15
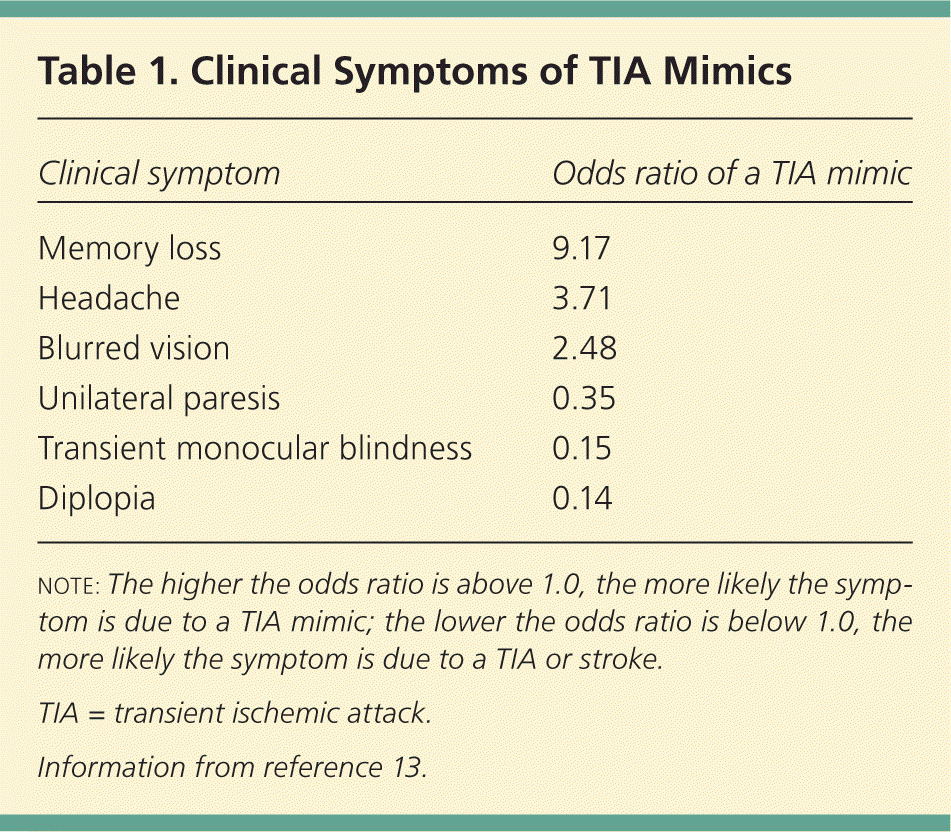
| Clinical symptom | Odds ratio of a TIA mimic |
|---|---|
| Memory loss | 9.17 |
| Headache | 3.71 |
| Blurred vision | 2.48 |
| Unilateral paresis | 0.35 |
| Transient monocular blindness | 0.15 |
| Diplopia | 0.14 |
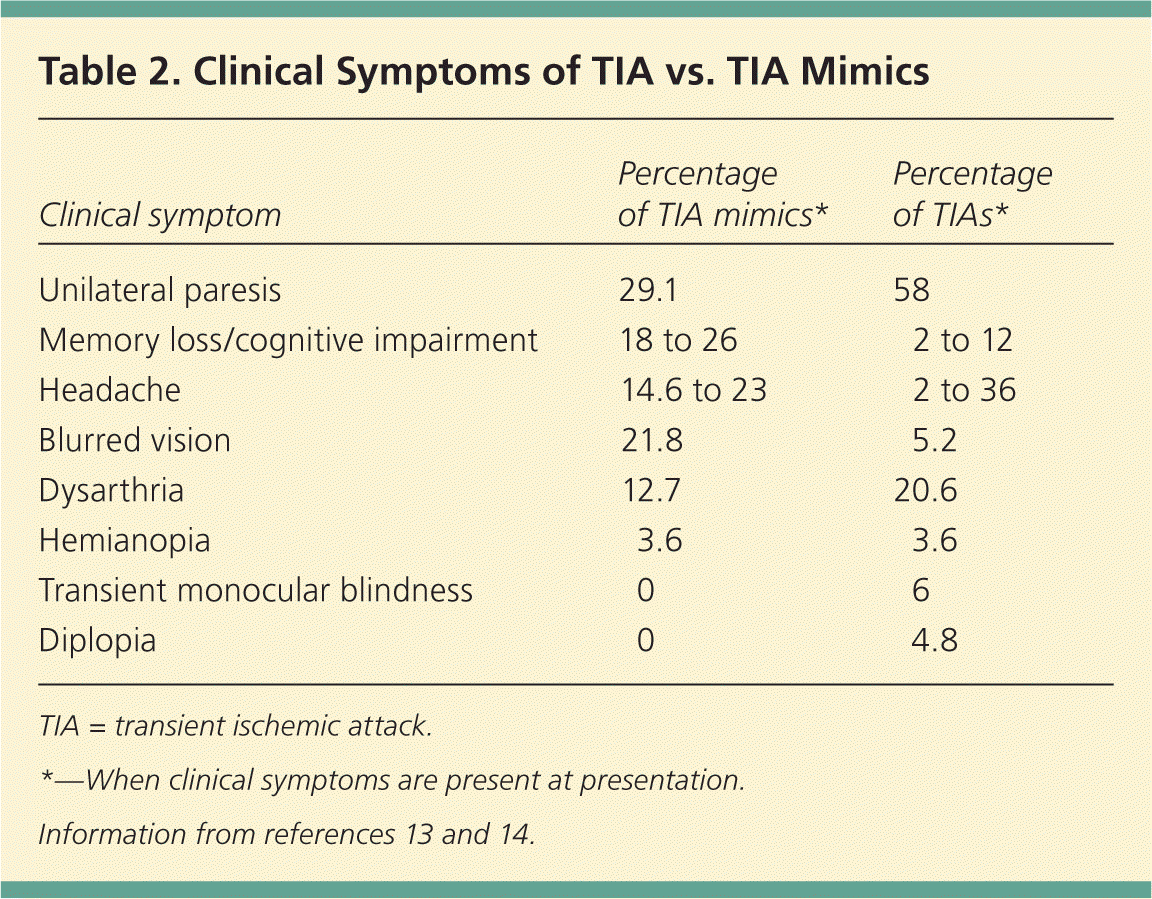
TIA is more likely with sudden onset, unilateral paresis, speech disturbance, or transient monocular blindness.13,14,16 Table 2 shows which symptoms are more likely with mimics versus true TIA.13,14 A complete history and physical examination are necessary to correctly diagnose TIA in the outpatient setting.
Presentation
HISTORY
At initial presentation, a comprehensive history should include identification of symptoms consistent with a focal neurologic deficit, and the timing of symptom onset and resolution. This is crucial because symptoms often resolve by the time of presentation. Attention should also be given to the presence or absence of nonspecific symptoms common in TIA mimics. Witnesses of the event can also be helpful in describing symptoms not perceived by the patient. The history should elicit risk factors associated with ischemic disease, such as cigarette smoking, obesity, diabetes mellitus, dyslipidemia, and hypertension, as well as personal or family history of hypercoagulability disorders, stroke, or TIA. Symptoms of TIA occur suddenly and include a neurologic deficit or loss of function.17 It is imperative to ask about recurrent symptoms of TIA because recent, recurrent TIA (crescendo TIA) requires urgent evaluation.
Mimics are more common in patients with a history of cognitive disorders, seizures, postural hypotension, and vertigo.10,14 Symptoms that generally are not suggestive of TIA include generalized weakness, dizziness, confusion, loss of consciousness, tinnitus, dysphagia, scotoma, headache, eye pain, and chest pain.17,18 It is important to note that the presence of common mimic symptoms does not exclude TIA from the diagnosis; however, mimics should be considered in the absence of concurrent focal deficits. Table 3 presents the differential diagnosis of TIA.
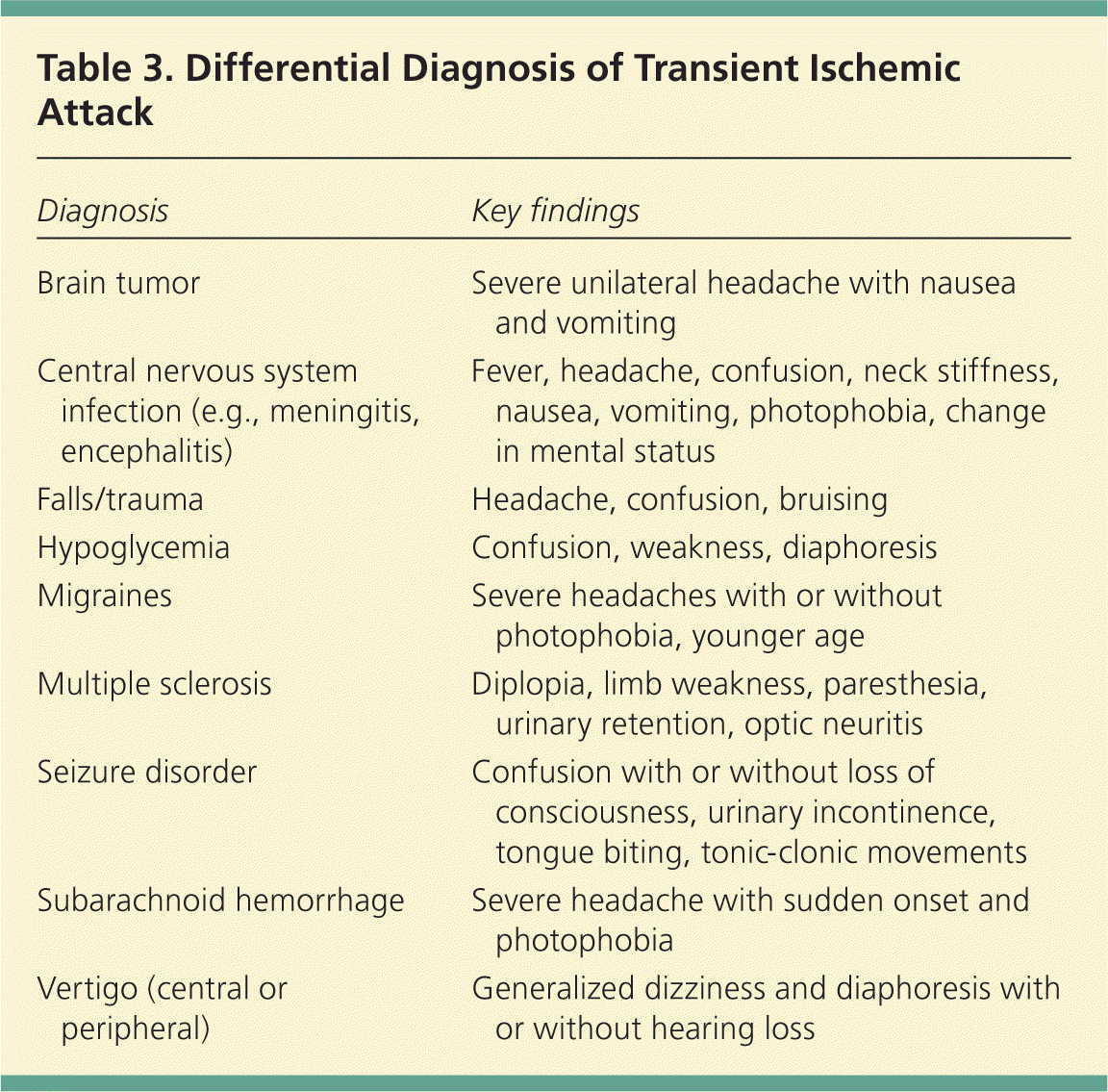
| Diagnosis | Key findings |
|---|---|
| Brain tumor | Severe unilateral headache with nausea and vomiting |
| Central nervous system infection (e.g., meningitis, encephalitis) | Fever, headache, confusion, neck stiffness, nausea, vomiting, photophobia, change in mental status |
| Falls/trauma | Headache, confusion, bruising |
| Hypoglycemia | Confusion, weakness, diaphoresis |
| Migraines | Severe headaches with or without photophobia, younger age |
| Multiple sclerosis | Diplopia, limb weakness, paresthesia, urinary retention, optic neuritis |
| Seizure disorder | Confusion with or without loss of consciousness, urinary incontinence, tongue biting, tonic-clonic movements |
| Subarachnoid hemorrhage | Severe headache with sudden onset and photophobia |
| Vertigo (central or peripheral) | Generalized dizziness and diaphoresis with or without hearing loss |
PHYSICAL EXAMINATION
A clinical presentation that demonstrates motor weakness and speech deficits is highly suggestive of TIA, and also may be associated with a higher risk of having an early stroke after TIA.19 The physical examination should include measurement of vital signs, a cardiovascular examination, and a comprehensive neurologic examination. Blood pressure is commonly elevated with cerebral ischemia and should be assessed, along with an evaluation for carotid bruits or cardiac arrhythmias.
Careful attention should be given to focal neurologic deficits and their represented neurovascular distribution. Cranial nerve, somatic motor strength, somatic sensory, speech and language, and cerebellar system testing should be performed. The most common findings for TIA in the cranial nerve examination are diplopia, hemianopia, monocular blindness, disconjugate gaze, facial drooping, lateral tongue movement, dysphagia, and vestibular dysfunction.17,18 Cerebellar system testing includes ocular movement and finger-to-nose and heel-to-shin movement, which may reveal nystagmus, past-pointing, dystaxia, or ataxia. Motor testing suggestive of TIA may reveal spasticity, clonus, rigidity, or unilateral weakness in the upper or lower extremities, face, and tongue.
Unilateral weakness and speech disturbance are the most common presenting symptoms in patients with TIA, and these symptoms are more likely to be associated with acute cerebral infarction on MRI.20,21 In an analysis of persons with TIA, 31 to 54 percent presented with focal weakness, 25 to 42 percent presented with speech changes, 16 to 32 percent had symptoms lasting one hour or less, and 37 to 72 percent had symptoms lasting more than one hour.3
Evaluation
The diagnostic evaluation of suspected TIA should be initiated as soon as possible to stratify risk of recurrent events. According to AHA/ASA guidelines, the goals of the diagnostic evaluation are to evaluate the vasculature for the mechanism and origin of the patient's symptoms and to exclude nonischemic etiologies.2 Symptomatic patients should be considered as having an active stroke and evaluated urgently in an emergency department.
IMAGING
The AHA/ASA recommends neuroimaging within 24 hours of symptom onset. Diffusion-weighted MRI is the preferred modality because it is more sensitive than computed tomography (CT).2 However, CT is more commonly used than MRI because of its availability and ability to quickly identify intracerebral hemorrhage.22 If a patient receives an emergent CT, a follow-up MRI should be performed when available because of its superiority in identifying cerebral infarction.2
The presence of infarction on MRI can have important prognostic implications. A study of classically defined TIA showed that patients with infarction on MRI had an in-hospital stroke rate of 19.4 percent, compared with 1.3 percent in those without evidence of infarction.23 Using the new definition, many patients with classically defined TIA would be redefined as having a minor stroke if there is evidence of acute infarction on MRI. A recent study used the new definition of TIA to evaluate patients whose symptoms resolved within 24 hours. For those with evidence of infarction on MRI (now defined as minor stroke), 7.1 percent had a stroke within the next seven days, compared with just 0.4 percent of patients without evidence of infarction.24
In patients with TIA, the cervicocephalic vasculature should be assessed for treatable atherosclerotic lesions using carotid ultrasonography/transcranial Doppler ultrasonography, magnetic resonance angiography, or CT angiography.2 A reasonable approach is to perform carotid imaging within one week of symptom onset in patients who are candidates for carotid endarterectomy.25 A meta-analysis showed that magnetic resonance angiography had 92.2 percent sensitivity and 75.7 percent specificity for the diagnosis of carotid stenosis, compared with 87.5 percent sensitivity and 75.7 percent specificity with carotid ultrasonography.26 Another study demonstrated 81 percent sensitivity and 96 percent specificity with CT angiography, compared with 92 percent sensitivity and 98 percent specificity with magnetic resonance angiography.27
CARDIAC ASSESSMENT
Electrocardiography should be performed during the initial evaluation. Transthoracic or transesophageal echocardiography can be used to look for a cardioembolic source and to determine the presence of patent foramen ovale, valvular disease, cardiac thrombus, and atherosclerosis.2 Prolonged cardiac monitoring with telemetry in the inpatient setting or Holter monitor in the outpatient setting is reasonable, primarily to evaluate for paroxysmal atrial fibrillation.
LABORATORY TESTING
In the initial evaluation of TIA symptoms, blood glucose and serum electrolyte levels should be measured to help rule out hypoglycemia or an electrolyte imbalance as the cause of change in mental status. Complete blood count and coagulation studies can help determine the likelihood of hemorrhage and thrombotic disorders.2,28 For younger patients and when there is clinical suspicion of central nervous system infection, drug intoxication, or clotting disorders, additional workup to assess the potential contribution of these disorders should include rapid plasma reagin testing, cerebrospinal fluid examination, urine drug screening, and full hypercoagulability workup.2,28 A fasting lipid panel should be performed to determine cardiovascular risk and for baseline cholesterol levels, to determine the appropriate starting dose of statin therapy needed to achieve target low-density lipoprotein levels.2
Risk Stratification and Hospitalization Criteria
The ABCD2 (age, blood pressure, clinical presentation, diabetes mellitus, duration of symptoms) score (Table 419 ) is a modified version of the original ABCD score, which was developed to determine stroke risk following TIA.19,29 The ABCD2 score has been shown to be highly predictive of the severity of stroke; higher scores correlate with higher disability and length of hospitalization.30 Additionally, a population-based study of TIA demonstrated that the ABCD2 score is highly predictive of a stroke occurring within 24 hours.29 In this study, 76 percent of patients with a recurrence had an ABCD2 score of 5 or greater.31
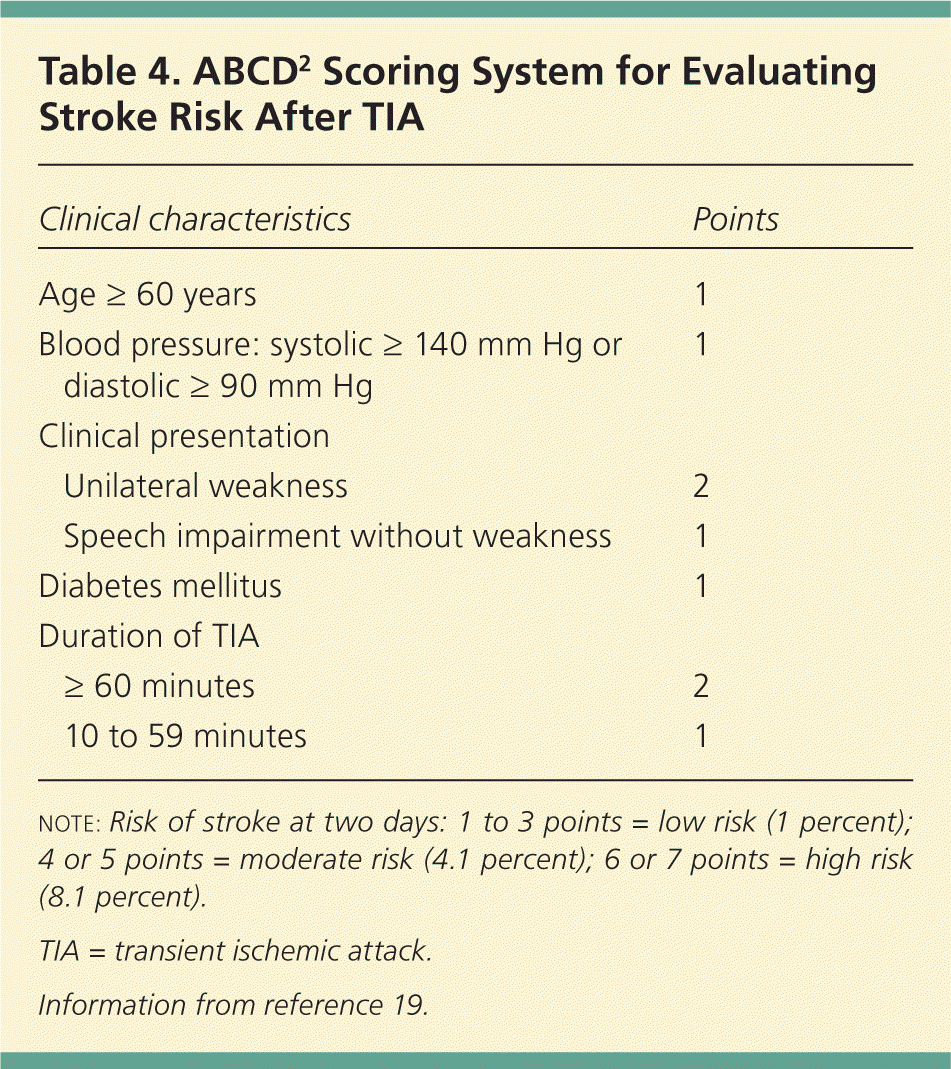
| Clinical characteristics | Points | |
|---|---|---|
| Age ≥ 60 years | 1 | |
| Blood pressure: systolic ≥ 140 mm Hg or diastolic ≥ 90 mm Hg | 1 | |
| Clinical presentation | ||
| Unilateral weakness | 2 | |
| Speech impairment without weakness | 1 | |
| Diabetes mellitus | 1 | |
| Duration of TIA | ||
| ≥ 60 minutes | 2 | |
| 10 to 59 minutes | 1 | |
In a recent study, an emergency department used the ABCD2 score in a novel triage protocol. Patients with an ABCD2 score of 0 to 3 were discharged from the emergency department with an appointment for outpatient MRI and magnetic resonance angiography and an appointment with an outpatient neurology-based TIA clinic within two business days. Those with a score of 4 or 5 received cervical and intracranial vessel imaging in the emergency department. If a symptomatic lesion was identified, they were admitted. If no lesion was identified, they were discharged with the follow-up appointments. All patients with an ABCD2 score greater than 5 were admitted. This approach led to lower rates of admission and lower-than-expected rates of recurrent stroke, which are consistent with expedited specialized outpatient management.32
This is a practical approach that can be instituted at most facilities. However, if urgent imaging is not available through the emergency department or if urgent outpatient neurology follow-up is not available, it is reasonable to admit for observation any patient with an ABCD2 score of 3 or greater who presents within 72 hours of symptom resolution, who has evidence of focal ischemia, or who cannot complete outpatient workup within 48 hours.2,29–31 Anyone with active signs or symptoms or any intracranial lesion on imaging should be considered as having a stroke and managed accordingly.
Data Sources: We searched Medline via Ovid and PubMed, Essential Evidence Plus, the National Guideline Clearinghouse, and the Cochrane database. Search terms included TIA, transient ischemic attack, TIA mimics, ABCD2, and cerebral ischemia. Search dates: January 2011 to February 2012.
