Am Fam Physician. 2014;89(12):938-941
Author disclosure: No relevant financial affiliations.
In a recently issued report, the Centers for Disease Control and Prevention (CDC) estimated the national burden of illnesses and deaths caused by the most common and most worrisome antibiotic-resistant pathogens.1 The report focused on 16 antimicrobial-resistant bacterial pathogens, as well as Candida infections, which together account for more than 2 million illnesses and at least 23,000 deaths every year in the United States.1 The report also included information on Clostridium difficile infections, which, like antibiotic resistance, are driven by antibiotic use. C. difficile causes more than 250,000 clinical infections annually and is associated with more than 14,000 deaths every year in the United States.1
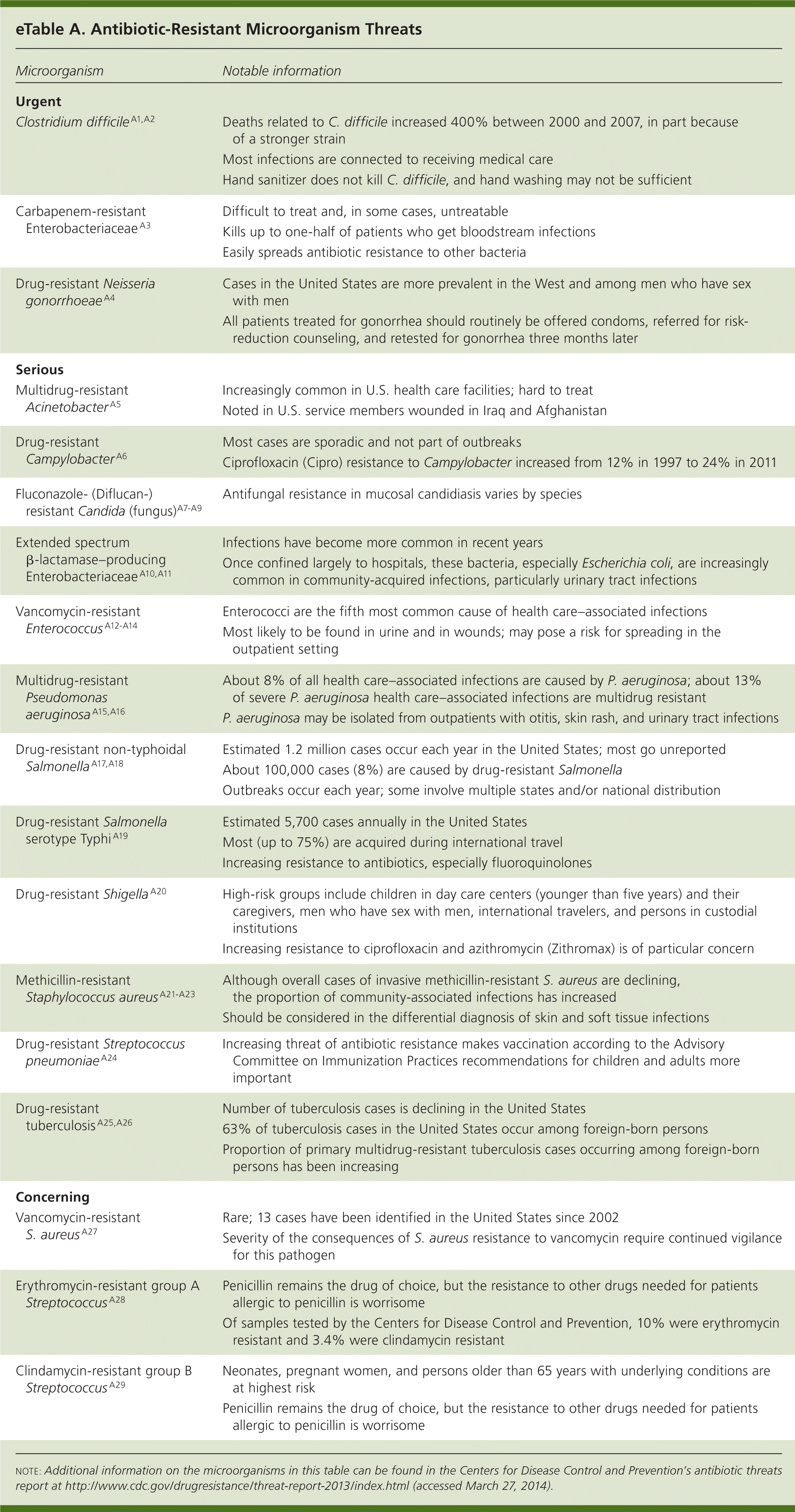
In this report, the CDC categorized 18 pathogens (eTable A) into three groups (urgent, serious, and concerning) based on seven criteria: clinical impact, economic impact, incidence, 10-year projection of incidence, transmissibility, availability of effective antibiotics, and barriers to prevention.1 Three types of bacteria were included in the urgent category: carbapenem-resistant Enterobacteriaceae, drug-resistant Neisseria gonorrhoeae, and C. difficile. In the past, drug-resistant strains of Enterobacteriaceae and N. gonorrhoeae have shown a propensity to spread rapidly in the United States and around the world. Some strains of carbapenem-resistant Enterobacteriaceae are currently untreatable with available antibiotics, and the cephalosporin agents to which some gonococci are now showing emerging resistance are the last available drugs to effectively treat this infection. Thus, further spread of these strains constitutes a public health crisis. C. difficile infections already cause significant morbidity and mortality, and a recently emerging epidemic strain, BI/NAP1/027, appears to be more virulent.
| Microorganism | Notable information |
|---|---|
| Urgent | |
| Clostridium difficileA1 ,A2 | Deaths related to C. difficile increased 400% between 2000 and 2007, in part because of a stronger strain |
| Most infections are connected to receiving medical care | |
| Hand sanitizer does not kill C. difficile, and hand washing may not be sufficient | |
| Carbapenem-resistant Enterobacteriaceae A3 | Difficult to treat and, in some cases, untreatable |
| Kills up to one-half of patients who get bloodstream infections | |
| Easily spreads antibiotic resistance to other bacteria | |
| Drug-resistant Neisseria gonorrhoeaeA4 | Cases in the United States are more prevalent in the West and among men who have sex with men |
| All patients treated for gonorrhea should routinely be offered condoms, referred for risk-reduction counseling, and retested for gonorrhea three months later | |
| Serious | |
| Multidrug-resistant AcinetobacterA5 | Increasingly common in U.S. health care facilities; hard to treat |
| Noted in U.S. service members wounded in Iraq and Afghanistan | |
| Drug-resistant CampylobacterA6 | Most cases are sporadic and not part of outbreaks |
| Ciprofloxacin (Cipro) resistance to Campylobacter increased from 12% in 1997 to 24% in 2011 | |
| Fluconazole- (Diflucan-) resistant Candida (fungus)A7 –A9 | Antifungal resistance in mucosal candidiasis varies by species |
| Extended spectrum β-lactamase–producing Enterobacteriaceae A10 ,A11 | Infections have become more common in recent years |
| Once confined largely to hospitals, these bacteria, especially Escherichia coli, are increasingly common in community-acquired infections, particularly urinary tract infections | |
| Vancomycin-resistant EnterococcusA12 –A14 | Enterococci are the fifth most common cause of health care–associated infections |
| Most likely to be found in urine and in wounds; may pose a risk for spreading in the outpatient setting | |
| Multidrug-resistant Pseudomonas aeruginosaA15 ,A16 | About 8% of all health care–associated infections are caused by P. aeruginosa; about 13% of severe P. aeruginosa health care–associated infections are multidrug resistant |
| P. aeruginosa may be isolated from outpatients with otitis, skin rash, and urinary tract infections | |
| Drug-resistant non-typhoidal SalmonellaA17 ,A18 | Estimated 1.2 million cases occur each year in the United States; most go unreported |
| About 100,000 cases (8%) are caused by drug-resistant Salmonella | |
| Outbreaks occur each year; some involve multiple states and/or national distribution | |
| Drug-resistant Salmonella serotype Typhi A19 | Estimated 5,700 cases annually in the United States |
| Most (up to 75%) are acquired during international travel | |
| Increasing resistance to antibiotics, especially fluoroquinolones | |
| Drug-resistant ShigellaA20 | High-risk groups include children in day care centers (younger than five years) and their caregivers, men who have sex with men, international travelers, and persons in custodial institutions |
| Increasing resistance to ciprofloxacin and azithromycin (Zithromax) is of particular concern | |
| Methicillin-resistant Staphylococcus aureusA21 –A23 | Although overall cases of invasive methicillin-resistant S. aureus are declining, the proportion of community-associated infections has increased |
| Should be considered in the differential diagnosis of skin and soft tissue infections | |
| Serious | |
| Drug-resistant Streptococcus pneumoniaeA24 | Increasing threat of antibiotic resistance makes vaccination according to the Advisory |
| Committee on Immunization Practices recommendations for children and adults more important | |
| Drug-resistant tuberculosis A25 ,A26 | Number of tuberculosis cases is declining in the United States 63% of tuberculosis cases in the United States occur among foreign-born persons |
| Proportion of primary multidrug-resistant tuberculosis cases occurring among foreign-born persons has been increasing | |
| Concerning | |
| Vancomycin-resistant S. aureusA27 | Rare; 13 cases have been identified in the United States since 2002 |
| Severity of the consequences of S. aureus resistance to vancomycin require continued vigilance for this pathogen | |
| Erythromycin-resistant group A StreptococcusA28 | Penicillin remains the drug of choice, but the resistance to other drugs needed for patients allergic to penicillin is worrisome |
| Of samples tested by the Centers for Disease Control and Prevention, 10% were erythromycin resistant and 3.4% were clindamycin resistant | |
| Clindamycin-resistant group B StreptococcusA29 | Neonates, pregnant women, and persons older than 65 years with underlying conditions are at highest risk |
| Penicillin remains the drug of choice, but the resistance to other drugs needed for patients allergic to penicillin is worrisome |
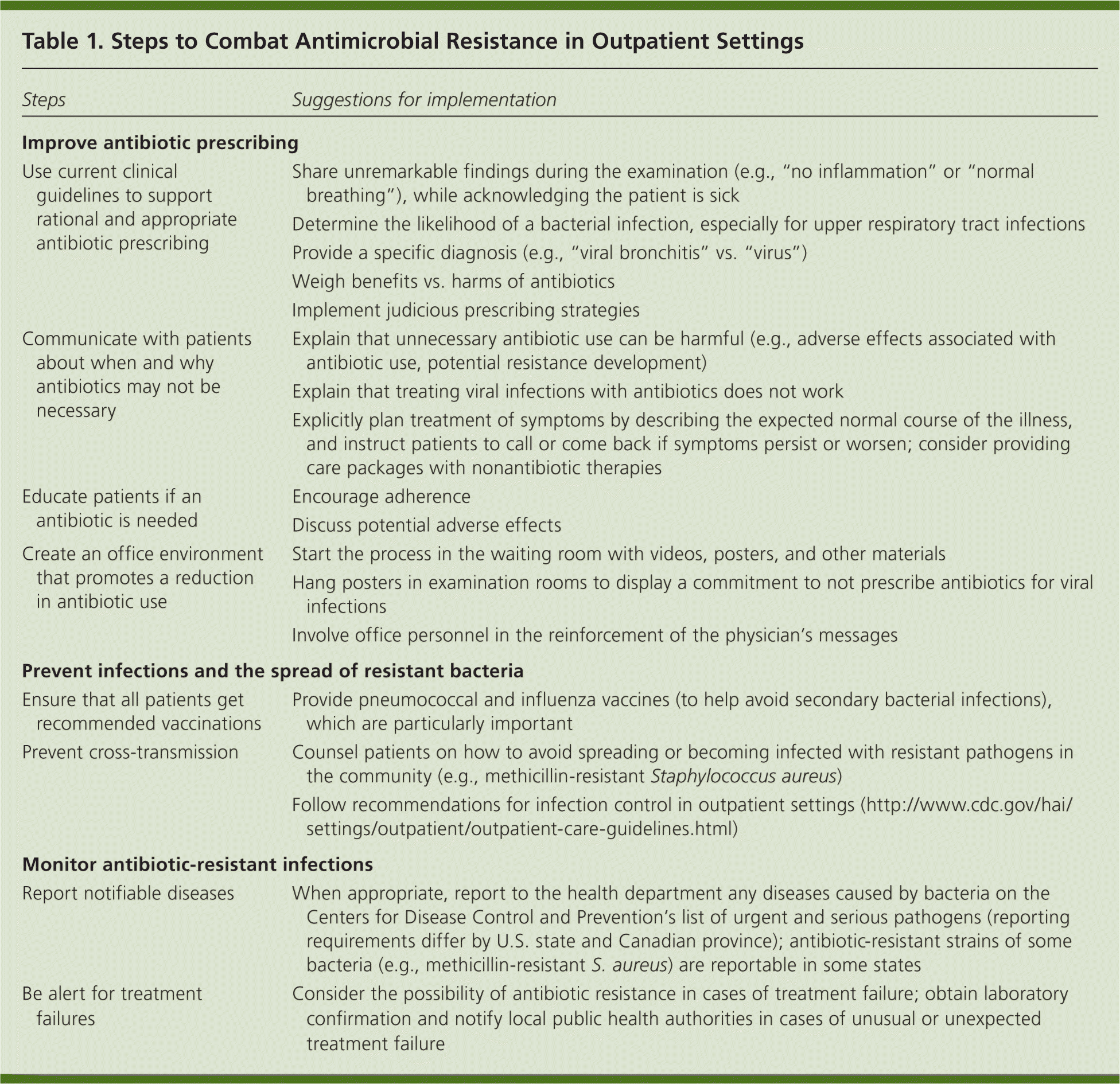
Prevention strategies can be effective, but the major goals of the CDC report are to (1) increase awareness of the magnitude and looming risk of untreatable infections, and (2) spur concerted action, both to prevent further spread of resistant pathogens and to preserve the effectiveness of existing antibiotics. Thinking of antibiotics as a precious and diminishing resource has engendered the concept of antimicrobial stewardship, a defined set of practices designed to improve the appropriate use of antimicrobial agents.2 Table 1 lists steps to help implement resistance prevention strategies. Family physicians have an important role in combating antibiotic resistance through carefully prescribing antibiotics, educating patients, and identifying and reporting unexpected treatment failures and suspected resistance.
| Steps | Suggestions for implementation |
|---|---|
| Improve antibiotic prescribing | |
| Use current clinical guidelines to support rational and appropriate antibiotic prescribing |
|
| Communicate with patients about when and why antibiotics may not be necessary |
|
| Educate patients if an antibiotic is needed |
|
| Create an office environment that promotes a reduction in antibiotic use |
|
| Prevent infections and the spread of resistant bacteria | |
| Ensure that all patients get recommended vaccinations |
|
| Prevent cross-transmission |
|
| Monitor antibiotic-resistant infections | |
| Report notifiable diseases |
|
| Be alert for treatment failures |
|
There are three main elements of preventing and controlling antibiotic resistance that are most applicable to outpatient practice. First, physicians must improve their antibiotic prescribing. Antibiotic use is the principal driver of antibacterial resistance. A considerable proportion of antibiotics in inpatient and outpatient settings are prescribed in cases when they are not needed or in which the choice of antibiotic, the dose, or the duration of therapy is inappropriate.3,4 Rates of outpatient antibiotic prescribing vary widely by region and state—a much greater variation than is likely explained by differences in patient populations or rates of bacterial diseases.5 It has been documented that inappropriate antibiotic prescribing, especially for viral upper respiratory tract infections, is common in ambulatory care.6 These illnesses are the most common reason for seeking medical attention in the United States and are associated with up to 75% of total antibiotic prescriptions each year.7 The causes of the overuse of antibiotics, which is a problem throughout the world, are complex and well described.8,9
The second main element is preventing infections and the spread of resistance. Preventing an infection eliminates the possibility that the infection could be drug resistant. Immunization and rigorous infection control, including hand washing, clearly reduce the likelihood of infection. On its website, the CDC provides information for patients on how to protect themselves from many types of infections, such as by ensuring safe food handling to prevent Salmonella and Campylobacter infections10 and by avoiding gonorrhea and other sexually transmitted infections.11 Counseling patients on how to avoid spreading or becoming infected with resistant pathogens in the community is an important role for physicians. For example, if an athlete is diagnosed with a methicillin-resistant Staphylococcus aureus infection, he or she should keep the wound properly covered, avoid whirlpools or therapy pools, shower after participation, clean uniforms and equipment after each use, and report infection to coaches and trainers.
The third main element is public health reporting. Gathering, analyzing, and disseminating information on resistant infections and the prevalence of resistant microorganisms is a critical strategy that informs clinical and public health decision making. A key component of detecting emerging and spreading resistance is identifying the cause of unexpected treatment failures. Patients who return with persistent or recurrent symptoms shortly after treatment should be retested by culture, and isolates should be submitted for antimicrobial susceptibility testing. Any case of unexplained treatment failure or a positive culture result after appropriate empiric treatment should be reported promptly to local or state health departments.12 Ultimately, public health surveillance is dependent on reporting by physicians and laboratories.
The nightmare scenario of the spread of pan-resistant bacteria is a real and frightening possibility. Cases of untreatable infections are already occurring. Preventing and controlling resistance requires the engagement of many different sectors of society. However, the physician's role in this effort is singularly important.12 As the threat becomes more urgent, the leadership of the medical community is the most critical factor to ensure a successful response.
The findings and conclusions of this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention. Mention of trade names or commercial products does not constitute endorsement or recommendation for use by the U.S. government.