Am Fam Physician. 2014;90(5):312-318
Patient information: See related handout on genital warts, written by the authors of this article.
Author disclosure: No relevant financial affiliations.
Genital warts affect 1% of the sexually active U.S. population and are commonly seen in primary care. Human papillomavirus types 6 and 11 are responsible for most genital warts. Warts vary from small, flat-topped papules to large, cauliflower-like lesions on the anogenital mucosa and surrounding skin. Diagnosis is clinical, but atypical lesions should be confirmed by histology. Treatments may be applied by patients, or by a clinician in the office. Patient-applied treatments include topical imiquimod, podofilox, and sinecatechins, whereas clinician-applied treatments include podophyllin, bichloroacetic acid, and trichloroacetic acid. Surgical treatments include excision, cryotherapy, and electrosurgery. The quadrivalent human papillomavirus vaccine is active against virus subtypes that cause genital warts in men and women. Additionally, male circumcision may be effective in decreasing the transmission of human immunodeficiency virus, human papillomavirus, and herpes simplex virus.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Treatment of genital warts should be guided by patient preference, available resources, cost, and the experience of the physician. | C | 2 |
| Pregnant women should not be treated with podophyllin, and the safety of imiquimod (Aldara), sinecatechins (Veregen), and podofilox (Condylox) in pregnancy has not been established. | C | 2 |
| Administration of the quadrivalent human papillomavirus vaccine (Gardasil) in male and female adolescents prevents external genital warts. | A | 40 |
| Male circumcision may decrease the transmission of human immunodeficiency virus, human papillomavirus, and herpes simplex virus in heterosexuals. | A | 25, 42, 43 |
Epidemiology
Genital warts are clinically present in 1% of the sexually active U.S. population, with an estimated lifetime risk of about 10%.3 Prevalence varies with age; the highest prevalence is in sexually active women 20 to 24 years of age and in men 25 to 29 years of age.3 A study of privately insured patients found an average cost of $436 over three office visits for treatment of an episode of genital warts.4
Clinical Presentation
Genital warts vary from small, flat-topped papules to large, cauliflower-like lesions on the anogenital mucosa and surrounding skin (Figures 1 through 3). Lesions range from barely visible and asymptomatic (Figure 4) to large plaques that can interfere with toileting and sexual intercourse (Figure 5). Large warts are not associated with a higher risk of malignancy.
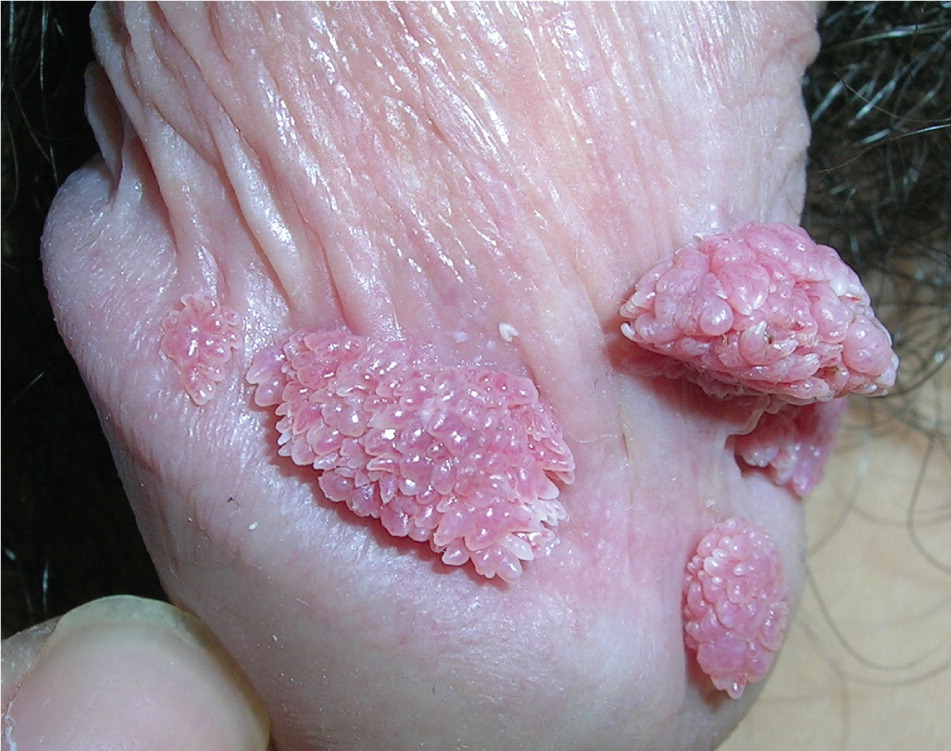
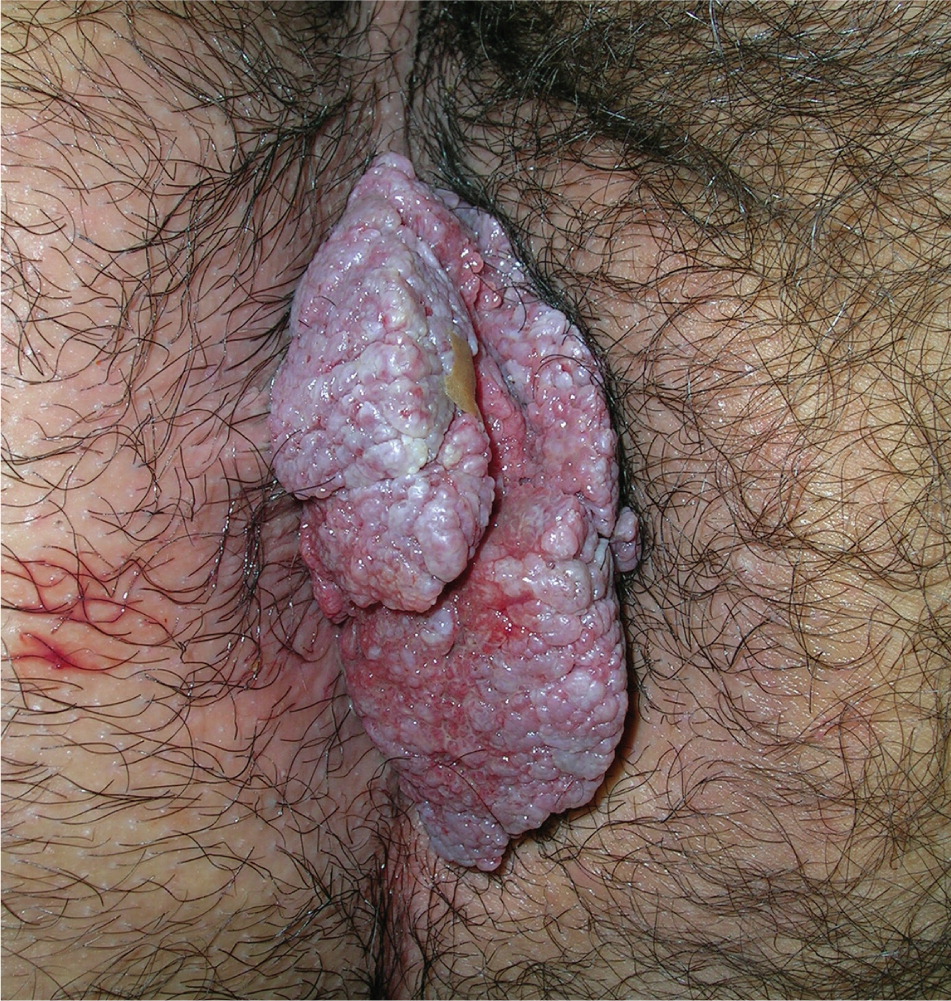
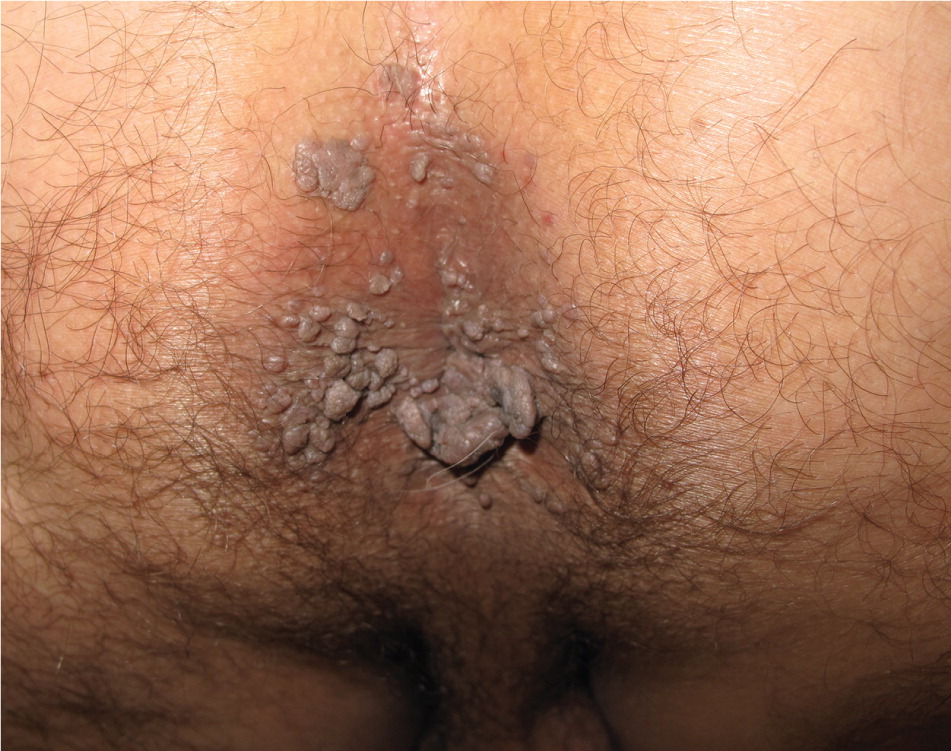
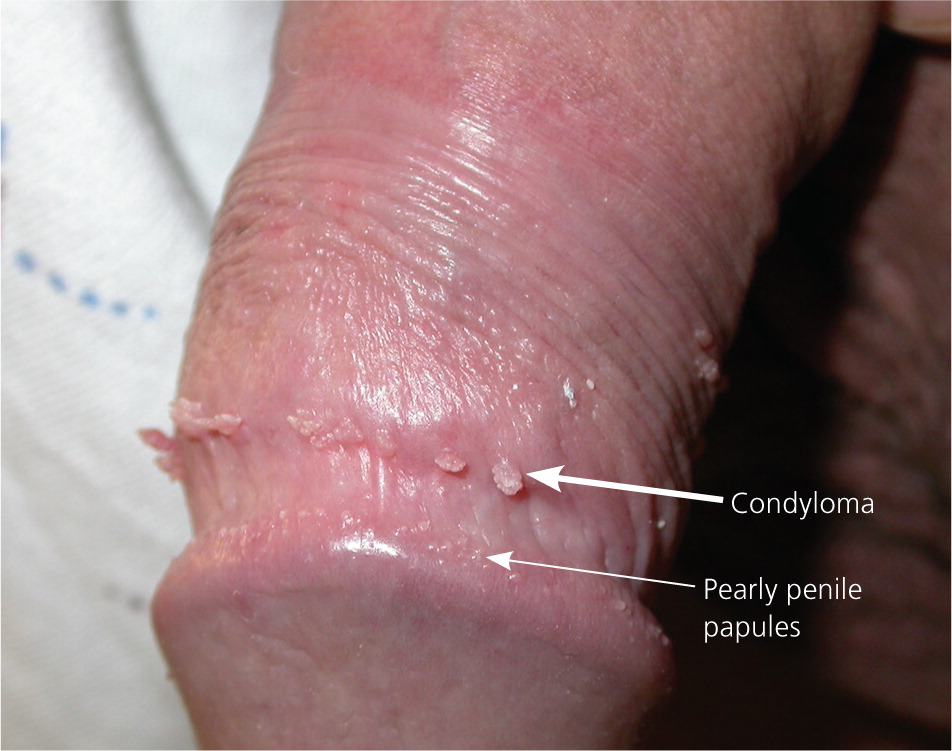
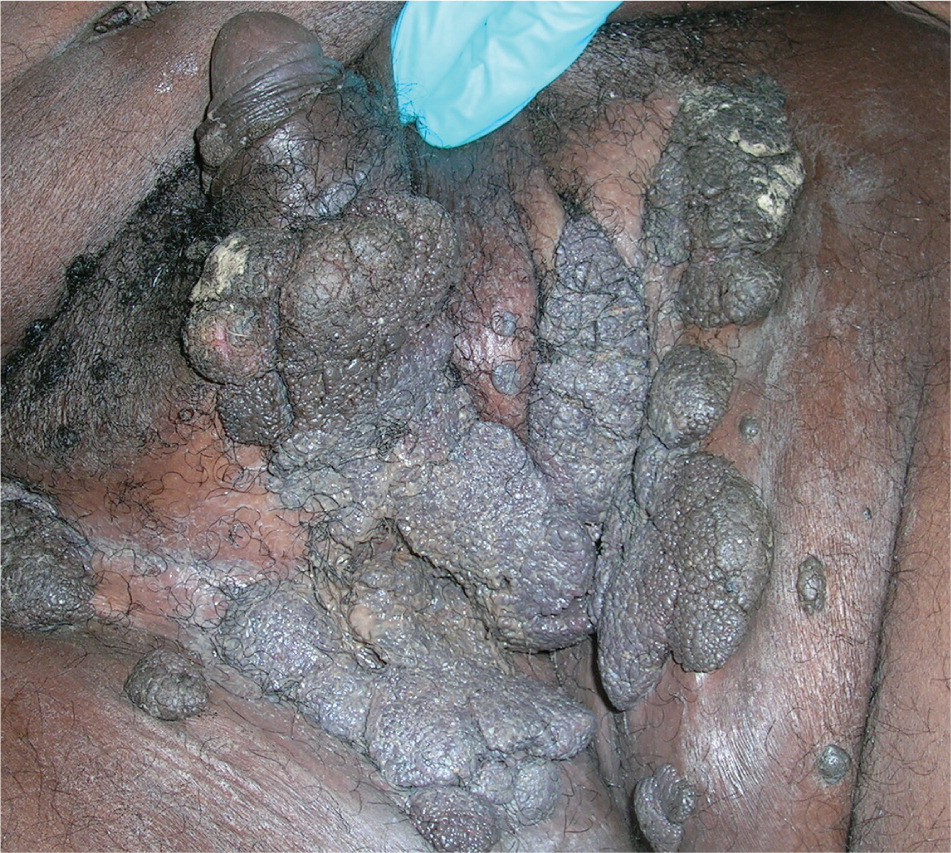
Diagnosis
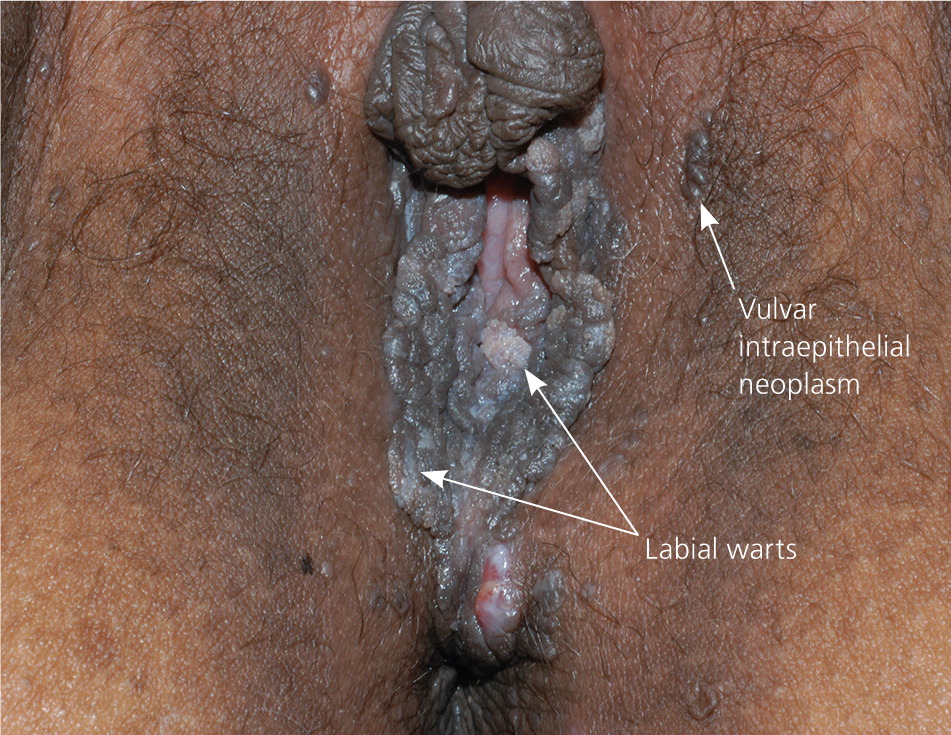
The diagnosis of anogenital warts is usually made clinically. Atypical lesions, however, should be confirmed by histology.5 Application of dilute acetic acid may reveal areas of subclinical HPV infection, but this method is nonspecific and not recommended in the routine evaluation of external genital warts.6 The differential diagnosis includes benign conditions, infections, pearly penile papules, and seborrheic keratosis, as well as preinvasive disease (e.g., vulvar intraepithelial neoplasia [Figure 67]) and cancer (Table 1).8–15
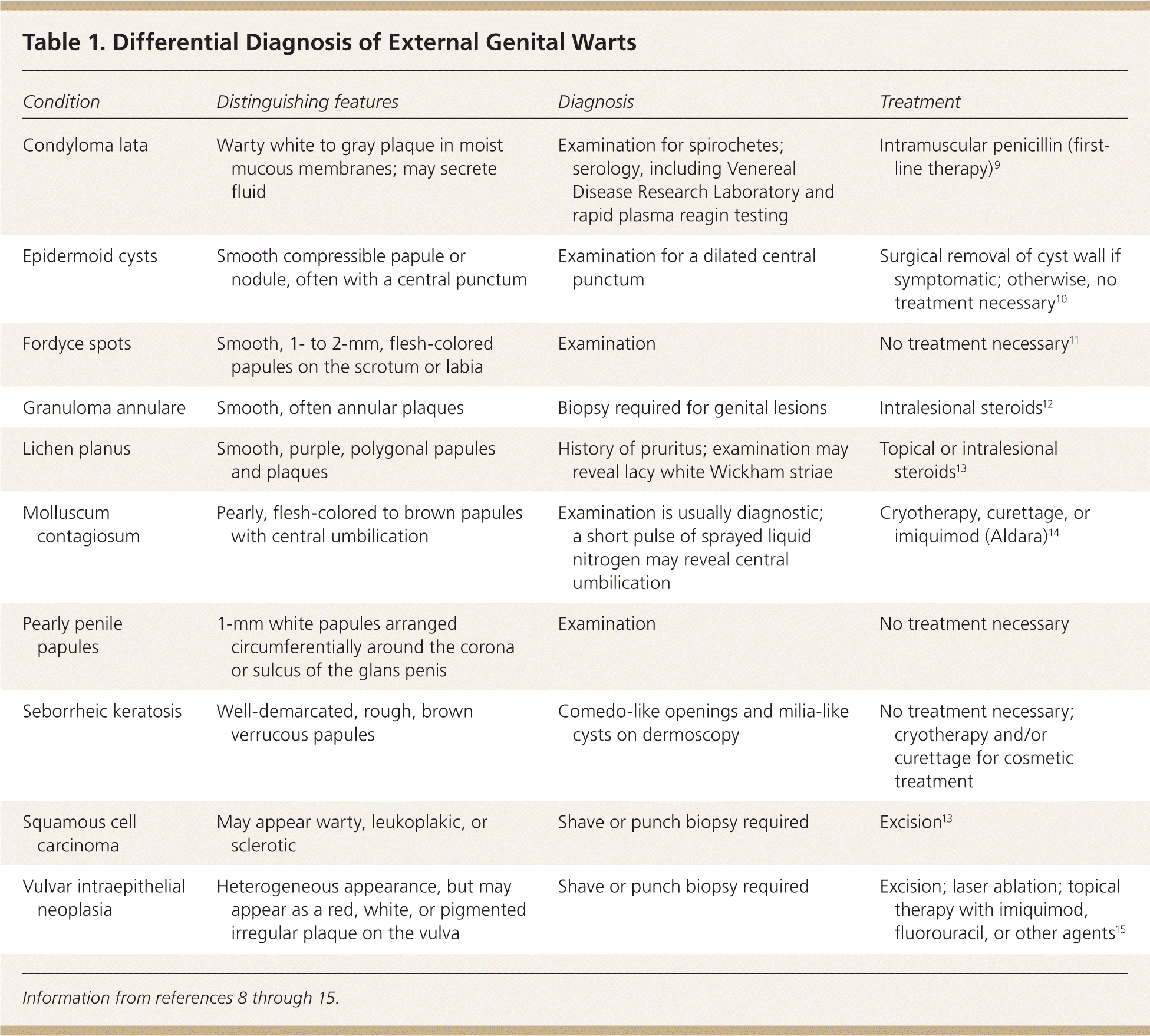
| Condition | Distinguishing features | Diagnosis | Treatment |
|---|---|---|---|
| Condyloma lata | Warty white to gray plaque in moist mucous membranes; may secrete fluid | Examination for spirochetes; serology, including Venereal Disease Research Laboratory and rapid plasma reagin testing | Intramuscular penicillin (first-line therapy)9 |
| Epidermoid cysts | Smooth compressible papule or nodule, often with a central punctum | Examination for a dilated central punctum | Surgical removal of cyst wall if symptomatic; otherwise, no treatment necessary10 |
| Fordyce spots | Smooth, 1- to 2-mm, flesh-colored papules on the scrotum or labia | Examination | No treatment necessary11 |
| Granuloma annulare | Smooth, often annular plaques | Biopsy required for genital lesions | Intralesional steroids12 |
| Lichen planus | Smooth, purple, polygonal papules and plaques | History of pruritus; examination may reveal lacy white Wickham striae | Topical or intralesional steroids13 |
| Molluscum contagiosum | Pearly, flesh-colored to brown papules with central umbilication | Examination is usually diagnostic; a short pulse of sprayed liquid nitrogen may reveal central umbilication | Cryotherapy, curettage, or imiquimod (Aldara)14 |
| Pearly penile papules | 1-mm white papules arranged circumferentially around the corona or sulcus of the glans penis | Examination | No treatment necessary |
| Seborrheic keratosis | Well-demarcated, rough, brown verrucous papules | Comedo-like openings and milia-like cysts on dermoscopy | No treatment necessary; cryotherapy and/or curettage for cosmetic treatment |
| Squamous cell carcinoma | May appear warty, leukoplakic, or sclerotic | Shave or punch biopsy required | Excision13 |
| Vulvar intraepithelial neoplasia | Heterogeneous appearance, but may appear as a red, white, or pigmented irregular plaque on the vulva | Shave or punch biopsy required | Excision; laser ablation; topical therapy with imiquimod, fluorouracil, or other agents15 |
Treatment
Common treatments for genital warts include patient- or clinician-applied topical therapies, as well as surgical and destructive approaches (Table 2).2,6,16–27 Effectiveness varies among treatments, and head-to-head studies of all available modalities are lacking. Recurrence rates range from 25% to 67%.28 Patients with asymptomatic lesions may prefer no treatment, and one-third of cases clear spontaneously.18 Patient-applied treatments include imiquimod (Aldara), podofilox (Condylox), and sinecatechins (Veregen). Clinician-applied methods include podophyllin, trichloroacetic and bichloroacetic acids, cryotherapy, electrosurgery, and surgical excision.
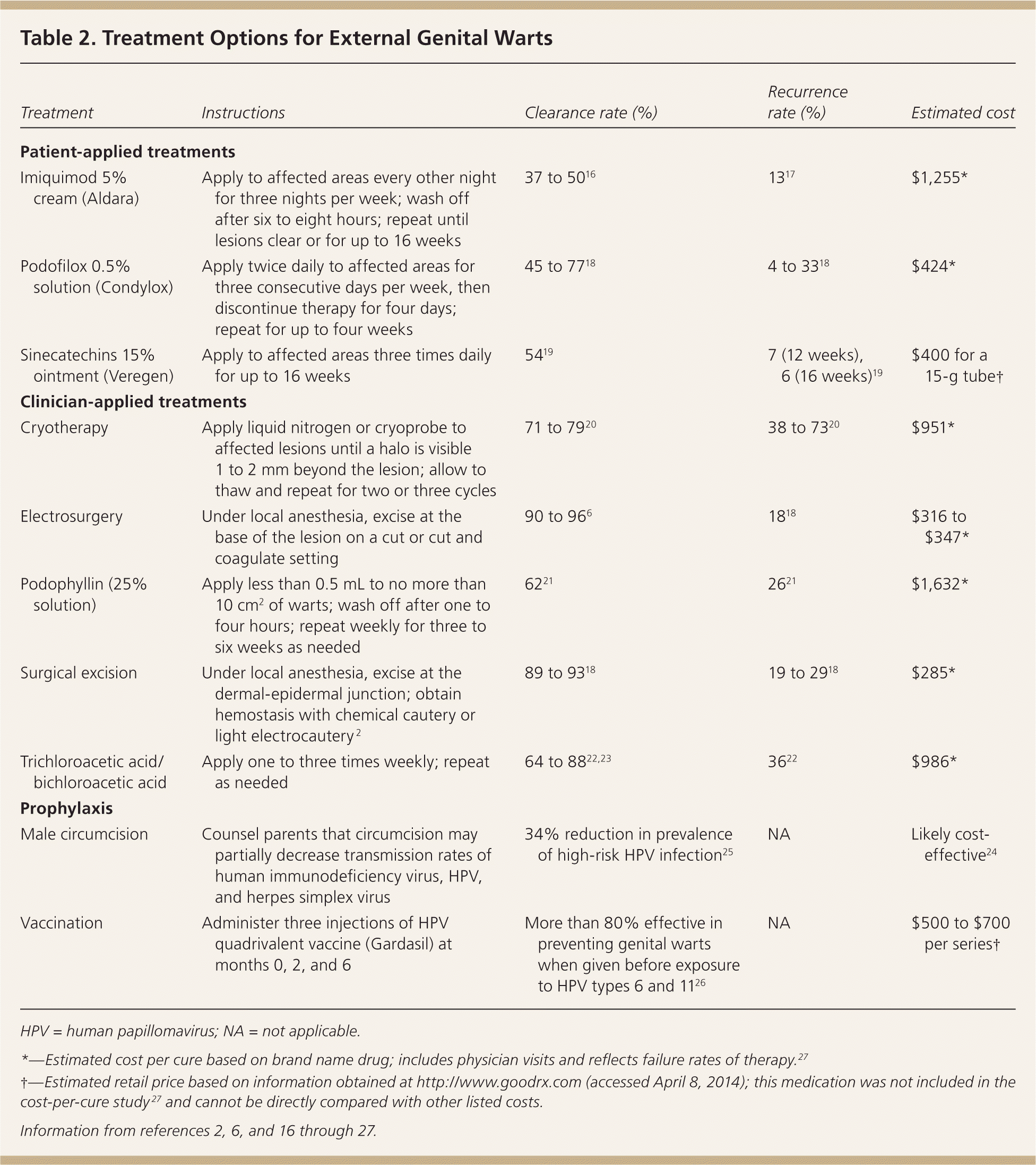
| Treatment | Instructions | Clearance rate (%) | Recurrence rate (%) | Estimated cost |
|---|---|---|---|---|
| Patient-applied treatments | ||||
| Imiquimod 5% cream (Aldara) | Apply to affected areas every other night for three nights per week; wash off after six to eight hours; repeat until lesions clear or for up to 16 weeks | 37 to 5016 | 1317 | $1,255* |
| Podofilox 0.5% solution (Condylox) | Apply twice daily to affected areas for three consecutive days per week, then discontinue therapy for four days; repeat for up to four weeks | 45 to 7718 | 4 to 3318 | $424* |
| Sinecatechins 15% ointment (Veregen) | Apply to affected areas three times daily for up to 16 weeks | 5419 | 7 (12 weeks), 6 (16 weeks)19 | $400 for a 15-g tube† |
| Clinician-applied treatments | ||||
| Cryotherapy | Apply liquid nitrogen or cryoprobe to affected lesions until a halo is visible 1 to 2 mm beyond the lesion; allow to thaw and repeat for two or three cycles | 71 to 7920 | 38 to 7320 | $951* |
| Electrosurgery | Under local anesthesia, excise at the base of the lesion on a cut or cut and coagulate setting | 90 to 966 | 1818 | $316 to $347* |
| Podophyllin (25% solution) | Apply less than 0.5 mL to no more than 10 cm2 of warts; wash off after one to four hours; repeat weekly for three to six weeks as needed | 6221 | 2621 | $1,632* |
| Surgical excision | Under local anesthesia, excise at the dermal-epidermal junction; obtain hemostasis with chemical cautery or light electrocautery 2 | 89 to 9318 | 19 to 2918 | $285* |
| Trichloroacetic acid/bichloroacetic acid | Apply one to three times weekly; repeat as needed | 64 to 8822,23 | 3622 | $986* |
| Prophylaxis | ||||
| Male circumcision | Counsel parents that circumcision may partially decrease transmission rates of human immunodeficiency virus, HPV, and herpes simplex virus | 34% reduction in prevalence of high-risk HPV infection25 | NA | Likely cost-effective24 |
| Vaccination | Administer three injections of HPV quadrivalent vaccine (Gardasil) at months 0, 2, and 6 | More than 80% effective in preventing genital warts when given before exposure to HPV types 6 and 1126 | NA | $500 to $700 per series† |
PATIENT-APPLIED TREATMENTS
Imiquimod. Imiquimod (U.S. Food and Drug Administration [FDA] pregnancy category C) is approved for treatment of genital warts and is available as a 5% cream.28 Imiquimod upregulates immune surveillance through cytokine induction and release of interferons.29 Patients should apply a thin layer onto affected areas every other night for three nights weekly (Monday, Wednesday, and Friday, or Tuesday, Thursday, and Saturday) until the warts resolve or for up to 16 weeks. The area should be washed six to eight hours after the cream is applied. In a randomized controlled trial (RCT), 50% of treated patients demonstrated clearance vs. 11% in the control group (number needed to treat = 2.6).17 Of the patients whose warts resolved, 13% had recurrence. In another RCT, 62% of treated patients had more than 80% reduction in total surface area of clinical warts, and 76% had more than 50% reduction.16 Only 37% of study participants achieved complete clearance. Although most of the data from RCTs assume a 12- to 16-week course of treatment, an eight-week course has also been successful.30 Adverse effects include itching, erythema, burning, irritation, and tenderness; ulceration, erosion, and pain are less common.
Podofilox. In the United States, podofilox (FDA pregnancy category C) is available as a 0.5% solution. It is purified from podophyllin, a clinician-applied option since the 1940s. Podofilox stops division of infected cells, causing tissue necrosis.6 Patients should apply the solution to affected areas twice daily for three days in a row, then discontinue therapy for four days. This weekly cycle should be repeated for up to four weeks. Clearance rates (45% to 77%) are similar to those of imiquimod and surgical approaches, and recurrence rates range from 4% to 33%.18 Common adverse effects include burning, pain, erosion, itching, and inflammation.31 A meta-analysis comparing imiquimod with podofilox did not show significant differences in clearance rates.32
Sinecatechins. Sinecatechins (FDA pregnancy category C) are extracts of green tea leaves that are compounded as a 15% ointment. Sinecatechins are thought to decrease viral replication. Patients should apply a 0.5-cm strand of ointment onto each wart three times daily for up to 16 weeks.2 In a 16-week trial of 604 patients, clearance rates were 53.6% in the treatment group and 35.3% in the control group (number needed to treat = 5.5).19 Recurrence rates of 6.8% and 5.8% were noted at 12 and 16 weeks, respectively, after complete clearance.19 Adverse effects included erythema, itching, burning, pain, erosion, ulceration, induration, and a vesicular rash.2 Up to 67% of patients experienced moderate to severe reactions, but only 2.3% discontinued therapy because of adverse effects.18,33
CLINICIAN-APPLIED TREATMENTS
Podophyllin. Podophyllin (FDA pregnancy category X) is an herbal extract compounded as a 25% solution. The preparation causes wart regression and necrosis by stopping mitosis. The solution is applied to warts up to once weekly for three to six weeks. Because of reported toxicity, including death, when podophyllin is overapplied or occluded, the Centers for Disease Control and Prevention recommends limiting the application area to less than 10 cm2 of warts per treatment, and limiting the amount applied to less than 0.5 mL per treatment.2 Application to mucosal areas increases the risk of systemic absorption; open ulcers or wounds should be avoided.2 The solution is left on for one to four hours, then washed off.2 In a small RCT, the clearance rate was 62%, with recurrence of 26% at 12 weeks.21 Podophyllin is contraindicated in pregnancy and can cause significant toxicity if not applied and removed properly. Because of the risks of podophyllin and the safety of its purified form, podofilox, some experts have recommended discontinuing its use as a treatment for genital warts.34 However, it is inexpensive, widely available, and is likely effective.18
Trichloroacetic and Bichloroacetic Acids. Trichloroacetic acid and dichloroacetic acid (also called bichloroacetic acid; both FDA pregnancy category N [not classified]) cause protein denaturing and cell death when applied to the skin. Trichloroacetic acid may be compounded in different strengths—generally 60% to 90%—whereas bichloroacetic acid comes in a standard strength. A thin amount is applied to each wart and allowed to turn white or “frost,” indicating precipitation of denatured proteins.2 The solution has low viscosity and can spread readily, thereby destroying unaffected tissue.2,20 The acids are applied in the office, with repeated applications up to three times weekly until the warts have resolved. There are no placebo-controlled trials with these acids, but small comparison trials with cryotherapy have shown similar clinical effectiveness (64% to 88% clearance rate, 36% recurrence rate).22,23
ABLATION AND EXCISIONAL PROCEDURES
Cryotherapy. Cryotherapy eliminates warts by thermolysis. Liquid nitrogen or a cryoprobe is applied centrally until a white halo of frozen tissue reaches 1 to 2 mm beyond the lesion (Figure 7). As the tissue thaws, cells are lysed. Often, a second or third cycle of freezing is necessary at each session. Pedunculated lesions may be treated by grasping with tweezers or a hemostat that has been cooled in liquid nitrogen. Treatment sessions often must be repeated every one to two weeks.2 Clearance rates range from 71% to 79%, with recurrence rates of 38% to 73% at six months.20 Although evidence is poor, cryotherapy has been used to treat external genital warts without adverse effects during pregnancy.35
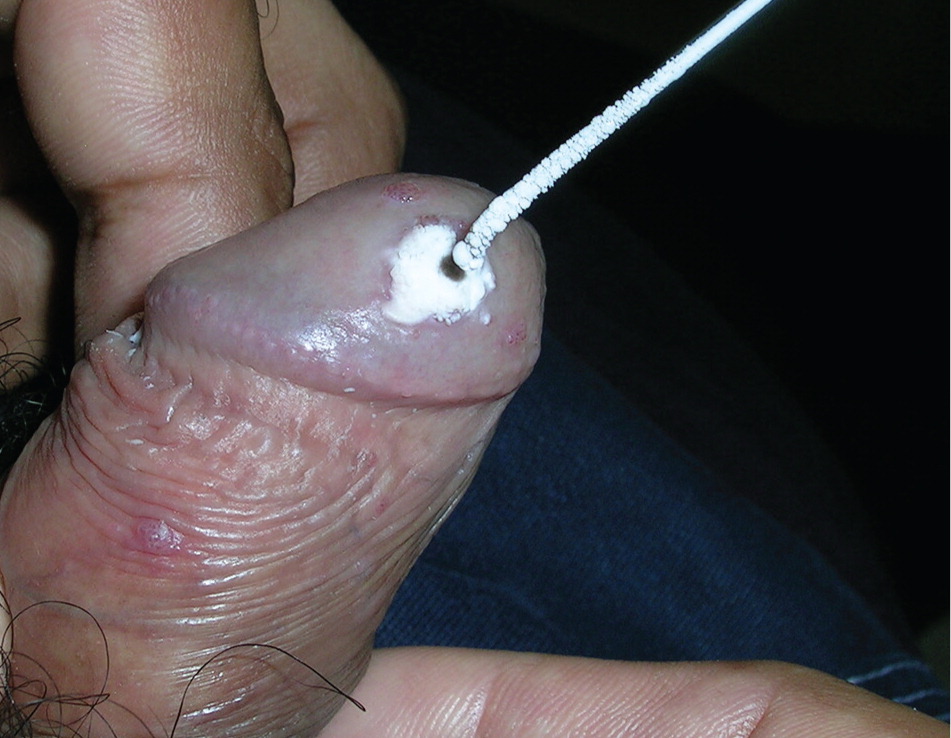
Electrosurgery. Electrosurgery effectively excises external genital warts—often in a single visit—but requires considerable technical skill and may result in pain and scarring if the excision is too deep.2,18 Local anesthesia is required. Lesions on the penis or anal verge require additional caution and skill. The smoke resulting from this procedure may contain HPV particles, similar to that from carbon dioxide laser surgery of warts.36 Transmission to the oropharynx is unlikely, but use of smoke evacuation equipment and a mask is recommended.36 Clearance rates with electrosurgery range from 90% to 96%,6 and recurrence rates of 18% have been reported.18
Surgical Excision. Surgical excision may be the most cost-effective treatment for genital warts.27 This method may be particularly helpful when warts are pedunculated or exophytic.2 After anesthesia is administered, lesions are excised tangentially at the base with a curved razor, scissors, scalpel, or curette.2 Hemostasis is obtained with aluminum chloride or light electrocautery.2 Sutures are rarely needed.2 This method preserves tissue for histologic examination and offers quick results. Pain, scarring, slow healing, and pigment changes are possible. Clearance rates with surgical excision range from 89% to 93%, and recurrence rates are 19% to 29% at 12 months.18
ALTERNATIVES
Additional treatment options are available for lesions that do not respond to one or more of the above therapies. These include topical and intralesional interferon alfa, topical and intravenous cidofovir (Vistide), photodynamic therapy, carbon dioxide laser therapy, and topical fluorouracil. Although it has been suggested that fluorouracil therapy be avoided because of adverse effects, a Cochrane review recommends further study.37 Carbon dioxide laser ablation is similar to electrosurgery, but requires additional training and equipment.
Special Situations
PREGNANCY
There is little evidence to guide treatment of genital warts during pregnancy. A Danish retrospective study found that infants born to women with external genital warts during pregnancy had a more than 200-fold risk of respiratory papillomatosis.38 However, only seven infants out of 1,000 in mothers with external genital warts developed respiratory papillomatosis, and cesarean delivery did not reduce this risk.38 The Centers for Disease Control and Prevention does not recommend prophylactic cesarean delivery or routine treatment of external genital warts in pregnant women unless lesions will cause obstructed labor or significant bleeding during delivery.2 The safety of imiquimod, sinecatechins, and podofilox in pregnancy has not been established.
IMMUNOSUPPRESSION
Patients with significant immunosuppression (e.g., from human immunodeficiency virus [HIV] infection, immunosuppressive therapy to suppress transplant rejection, or other concomitant disease) are at increased risk of squamous cell carcinoma, which may be clinically similar or identical to genital warts.2 Lesions that ulcerate, grow rapidly, or are atypical should be biopsied to rule out squamous cell carcinoma.2,39
REFRACTORY LESIONS
Warts that do not respond to one or more treatments should prompt the physician to review the differential diagnosis and consider a biopsy or referral to a subspecialist.28
Prevention
VACCINATION
Developed primarily to prevent cervical cancer, two available HPV vaccines target the L1 proteins of high-risk HPV subtypes 16 and 18, which are associated with cervical, penile, and oropharyngeal cancers.40 The quadrivalent vaccine (Gardasil) also targets low-risk HPV types 6 and 11, which are responsible for 90% of cases of external genital warts.2 In population-wide data from Australia, a 59% reduction in external genital warts was observed in the vaccinated population in the second year of implementation.41 A recent double-blind RCT involving more than 4,000 males 16 to 26 years of age from 18 countries demonstrated an 83.8% decrease in the incidence of external genital warts at 36 months.26
MALE CIRCUMCISION
Three recent RCTs in sub-Saharan Africa demonstrated that male circumcision decreased heterosexual transmission of HIV, high-risk types of HPV, and herpes simplex virus.25,42,43 Based on current data, it is not clear if these findings can be generalized to men who have sex with men or to populations with lower HIV prevalence. However, the World Health Organization and the Joint United Nations Programme on HIV/AIDS recommend circumcision to prevent HIV infection in heterosexuals.2
Data Sources: A PubMed and Google Scholar search was completed using the terms genital warts, human papillomavirus, and HPV. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. Additional searches were performed using the Cochrane database and BMJ Clinical Evidence. Search date: May 2014.